Abstract
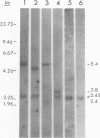
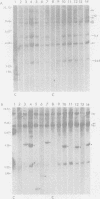
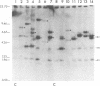
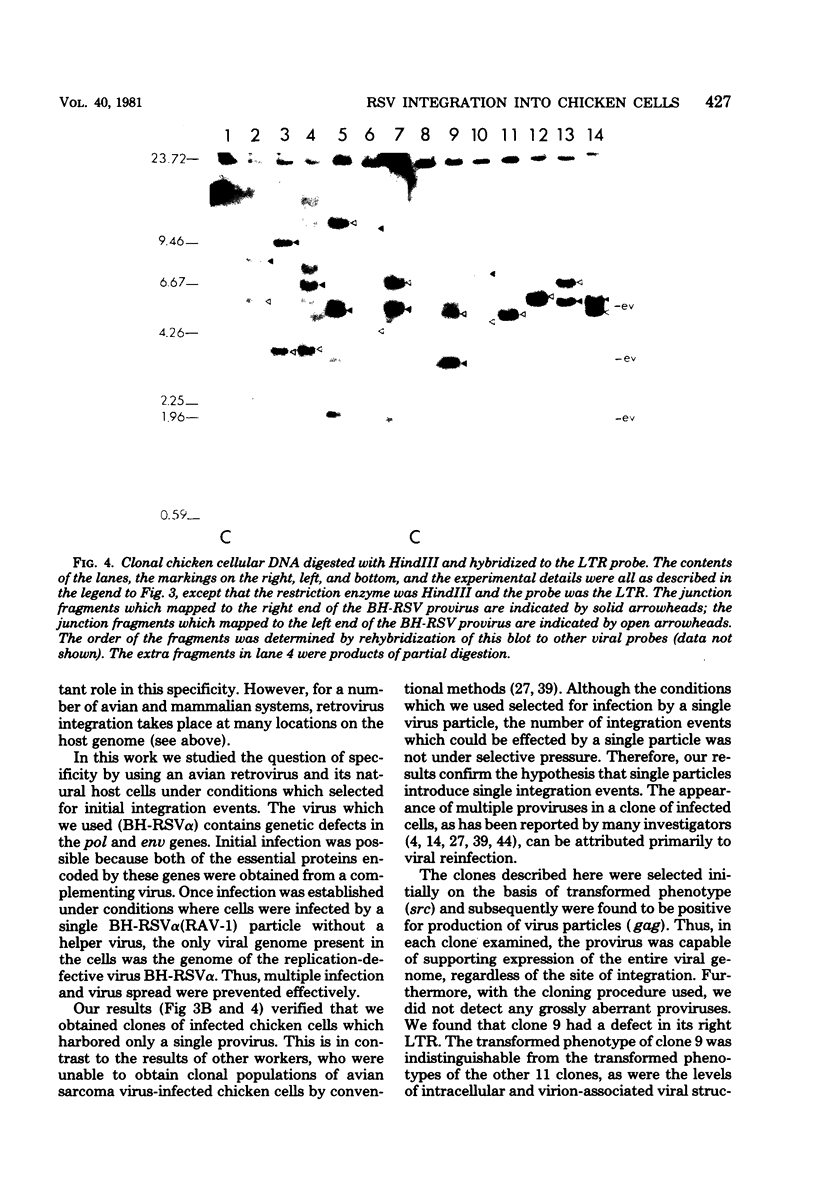
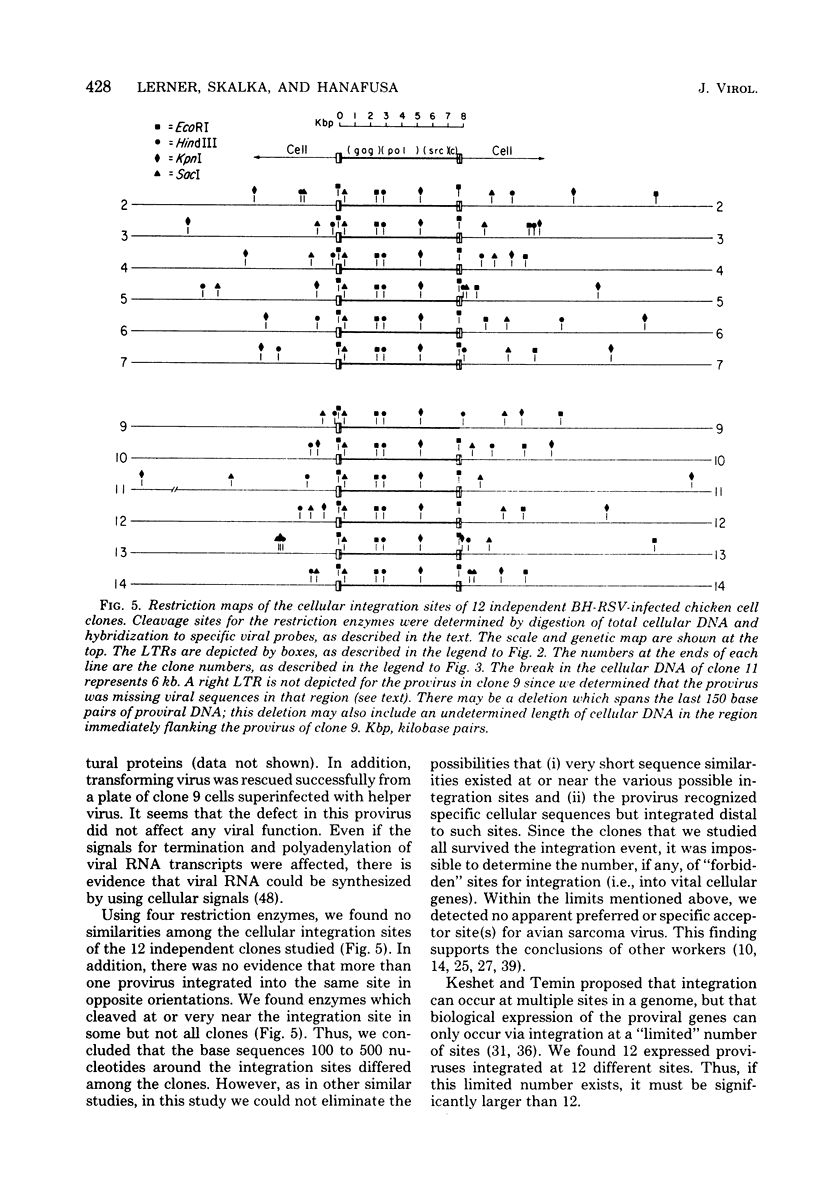
We analyzed retroviral integration into a host genome by using avian sarcoma virus infection of natural target cells under conditions where secondary integration via virus spread was inhibited. This was accomplished by using the noninfectious pol- env- alpha variant of the Bryan high-titer strain of Rous sarcoma virus. A total of 12 independent Bryan high-titer Rous sarcoma virus-transformed chicken embryo fibroblast clones were obtained and mapped by using restriction endonucleases. Provirus-cell junction fragments were identified with appropriate hybridization probes. We found that expression of the viral genes could occur after proviral integration at many sites on the chicken genome and that there was no apparent preference for specific integration sites.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Astrin S. M. Endogenous viral genes of the White Leghorn chicken: common site of residence and sites associated with specific phenotypes of viral gene expression. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5941–5945. doi: 10.1073/pnas.75.12.5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astrin S. M., Robinson H. L., Crittenden L. B., Buss E. G., Wyban J., Hayward W. S. Ten genetic loci in the chicken that contain structural genes for endogenous avian leukosis viruses. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):1105–1109. doi: 10.1101/sqb.1980.044.01.119. [DOI] [PubMed] [Google Scholar]
- Bacheler L. T., Fan H. Multiple integration sites for Moloney murine leukemia virus in productively infected mouse fibroblasts. J Virol. 1979 Jun;30(3):657–667. doi: 10.1128/jvi.30.3.657-667.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann D. G., Baluda M. A. DNA of avian myeloblastosis-associated virus type 2 integrates at multiple sites in the chicken genome. J Virol. 1980 Sep;35(3):968–971. doi: 10.1128/jvi.35.3.968-971.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone L. R., Skalka A. M. Viral DNA synthesized in vitro by avian retrovirus particles permeabilized with melittin. I. Kinetics of synthesis and size of minus- and plus-strand transcripts. J Virol. 1981 Jan;37(1):109–116. doi: 10.1128/jvi.37.1.109-116.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botchan M., Topp W., Sambrook J. The arrangement of simian virus 40 sequences in the DNA of transformed cells. Cell. 1976 Oct;9(2):269–287. doi: 10.1016/0092-8674(76)90118-5. [DOI] [PubMed] [Google Scholar]
- Coffin J. M., Champion M., Chabot F. Nucleotide sequence relationships between the genomes of an endogenous and an exogenous avian tumor virus. J Virol. 1978 Dec;28(3):972–991. doi: 10.1128/jvi.28.3.972-991.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. C., Shank P. R., Morris V. L., Cardiff R., Varmus H. E. Integration of the DNA of mouse mammary tumor virus in virus-infected normal and neoplastic tissue of the mouse. Cell. 1979 Feb;16(2):333–345. doi: 10.1016/0092-8674(79)90010-2. [DOI] [PubMed] [Google Scholar]
- Collins C. J., Boettiger D., Green T. L., Burgess M. B., Devlin H., Parsons J. T. Arrangement of integrated avian sarcoma virus DNA sequences within the cellular genomes of transformed and revertant mammalian cells. J Virol. 1980 Feb;33(2):760–768. doi: 10.1128/jvi.33.2.760-768.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins C. J., Parsons J. T. Integration of avian sarcoma virus DNA sequences in transformed mammalian cells. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4301–4305. doi: 10.1073/pnas.74.10.4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLorbe W. J., Luciw P. A., Goodman H. M., Varmus H. E., Bishop J. M. Molecular cloning and characterization of avian sarcoma virus circular DNA molecules. J Virol. 1980 Oct;36(1):50–61. doi: 10.1128/jvi.36.1.50-61.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H., Kawai S., Wang L. H., Vogt P. K., Murphy H. M., Hanafusa H. RNA of replication-defective strains of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1569–1573. doi: 10.1073/pnas.72.4.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmer T. M., Parsons J. T. Analysis of cellular integration sites in avian sarcoma virus infected duck embryo cells. J Virol. 1979 Dec;32(3):762–769. doi: 10.1128/jvi.32.3.762-769.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groner B., Hynes N. E. Number and location of mouse mammary tumor virus proviral DNA in mouse DNA of normal tissue and of mammary tumors. J Virol. 1980 Mar;33(3):1013–1025. doi: 10.1128/jvi.33.3.1013-1025.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafusa H., Baltimore D., Smoler D., Watson K. F., Yaniv A., Spiegelman S. Absence of polymerase protein in virions of alpha-type rous sarcoma virus. Science. 1972 Sep 29;177(4055):1188–1191. doi: 10.1126/science.177.4055.1188. [DOI] [PubMed] [Google Scholar]
- Hanafusa H., Hanafusa T. Further studies on RSV production form transformed cells. Virology. 1968 Apr;34(4):630–636. doi: 10.1016/0042-6822(68)90084-6. [DOI] [PubMed] [Google Scholar]
- Hanafusa H., Hanafusa T. Noninfectious RSV deficient in DNA polymerase. Virology. 1971 Jan;43(1):313–316. doi: 10.1016/0042-6822(71)90251-0. [DOI] [PubMed] [Google Scholar]
- Hanafusa H. Rapid transformation of cells by Rous sarcoma virus. Proc Natl Acad Sci U S A. 1969 Jun;63(2):318–325. doi: 10.1073/pnas.63.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseltine W. A., Maxam A. M., Gilbert W. Rous sarcoma virus genome is terminally redundant: the 5' sequence. Proc Natl Acad Sci U S A. 1977 Mar;74(3):989–993. doi: 10.1073/pnas.74.3.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward W. S., Braverman S. B., Astrin S. M. Transcriptional products and DNA structure of endogenous avian proviruses. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):1111–1121. doi: 10.1101/sqb.1980.044.01.120. [DOI] [PubMed] [Google Scholar]
- Highfield P. E., Rafield L. F., Gilmer T. M., Parsons J. T. Molecular cloning of avian sarcoma virus closed circular DNA: structural and biological characterization of three recombinant clones. J Virol. 1980 Oct;36(1):271–279. doi: 10.1128/jvi.36.1.271-279.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hishinuma F., DeBona P. J., Astrin S., Skalka A. M. Nucleotide sequence of acceptor site and termini of integrated avian endogenous provirus ev1: integration creates a 6 bp repeat of host DNA. Cell. 1981 Jan;23(1):155–164. doi: 10.1016/0092-8674(81)90280-4. [DOI] [PubMed] [Google Scholar]
- Hsu T. W., Sabran J. L., Mark G. E., Guntaka R. V., Taylor J. M. Analysis of unintegrated avian RNA tumor virus double-stranded DNA intermediates. J Virol. 1978 Dec;28(3):810–818. doi: 10.1128/jvi.28.3.810-818.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S. H., Shank P. R., Spector D. H., Kung H. J., Bishop J. M., Varmus H. E., Vogt P. K., Breitman M. L. Proviruses of avian sarcoma virus are terminally redundant, co-extensive with unintegrated linear DNA and integrated at many sites. Cell. 1978 Dec;15(4):1397–1410. doi: 10.1016/0092-8674(78)90064-8. [DOI] [PubMed] [Google Scholar]
- Hughes S. H., Toyoshima K., Bishop J. M., Varmus H. E. Organization of the endogenous proviruses of chickens: implications for origin and expression. Virology. 1981 Jan 15;108(1):189–207. doi: 10.1016/0042-6822(81)90538-9. [DOI] [PubMed] [Google Scholar]
- Hughes S. H., Vogt P. K., Stubblefield E., Bishop J. M., Varmus H. E. Integration of avian sarcoma virus DNA in chicken cells. Virology. 1981 Jan 15;108(1):208–221. doi: 10.1016/0042-6822(81)90539-0. [DOI] [PubMed] [Google Scholar]
- Jenkins N. A., Cooper G. M. Integration, expression, and infectivity of exogenously acquired proviruses of Rous-associated virus-O. J Virol. 1980 Dec;36(3):684–691. doi: 10.1128/jvi.36.3.684-691.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju G., Boone L., Skalka A. M. Isolation and characterization of recombinant DNA clones of avian retroviruses: size heterogeneity and instability of the direct repeat. J Virol. 1980 Mar;33(3):1026–1033. doi: 10.1128/jvi.33.3.1026-1033.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai S. Transformation of rat cells by fusion-infection with Rous sarcoma virus. J Virol. 1980 Jun;34(3):772–776. doi: 10.1128/jvi.34.3.772-776.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshet E., Temin H. M. Sites of integration of reticuloendotheliosis virus DNA in chicken DNA. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3372–3376. doi: 10.1073/pnas.75.7.3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketner G., Kelly T. J., Jr Integrated simian virus 40 sequences in transformed cell DNA: analysis using restriction endonucleases. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1102–1106. doi: 10.1073/pnas.73.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landy A., Ross W. Viral integration and excision: structure of the lambda att sites. Science. 1977 Sep 16;197(4309):1147–1160. doi: 10.1126/science.331474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neel B. G., Hayward W. S., Robinson H. L., Fang J., Astrin S. M. Avian leukosis virus-induced tumors have common proviral integration sites and synthesize discrete new RNAs: oncogenesis by promoter insertion. Cell. 1981 Feb;23(2):323–334. doi: 10.1016/0092-8674(81)90128-8. [DOI] [PubMed] [Google Scholar]
- O'Rear J. J., Mizutani S., Hoffman G., Fiandt M., Temin H. M. Infectious and noninfectious recombinant clones of the provirus of SNV differ in cellular DNA and are apparently the same in viral DNA. Cell. 1980 Jun;20(2):423–430. doi: 10.1016/0092-8674(80)90628-5. [DOI] [PubMed] [Google Scholar]
- Panet A., Baltimore D., Hanafusa T. Quantitation of avian RNA tumor virus reverse transcriptase by radioimmunoassay. J Virol. 1975 Jul;16(1):146–152. doi: 10.1128/jvi.16.1.146-152.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettenmier C. W., Hanafusa H. Structural protein markers in the avian oncoviruses. J Virol. 1977 Dec;24(3):850–864. doi: 10.1128/jvi.24.3.850-864.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabran J. L., Hsu T. W., Yeater C., Kaji A., Mason W. S., Taylor J. M. Analysis of integrated avian RNA tumor virus DNA in transformed chicken, duck and quail fibroblasts. J Virol. 1979 Jan;29(1):170–178. doi: 10.1128/jvi.29.1.170-178.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shank P. R., Hughes S. H., Kung H. J., Majors J. E., Quintrell N., Guntaka R. V., Bishop J. M., Varmus H. E. Mapping unintegrated avian sarcoma virus DNA: termini of linear DNA bear 300 nucleotides present once or twice in two species of circular DNA. Cell. 1978 Dec;15(4):1383–1395. doi: 10.1016/0092-8674(78)90063-6. [DOI] [PubMed] [Google Scholar]
- Shank P. R., Hughes S. H., Varmus H. E. Restriction endonuclease mapping of the DNA of Rous-associated virus O reveals extensive homology in structure and sequence with avian sarcoma virus DNA. Virology. 1981 Jan 15;108(1):177–188. doi: 10.1016/0042-6822(81)90537-7. [DOI] [PubMed] [Google Scholar]
- Shimotohno K., Temin H. M. No apparent nucleotide sequence specificity in cellular DNA juxtaposed to retrovirus proviruses. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7357–7361. doi: 10.1073/pnas.77.12.7357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Steffen D., Weinberg R. A. The integrated genome of murine leukemia virus. Cell. 1978 Nov;15(3):1003–1010. doi: 10.1016/0092-8674(78)90284-2. [DOI] [PubMed] [Google Scholar]
- Taylor J. M., Hsu T. W., Lai M. M. Restriction enzyme sites on the avian RNA tumor virus genome. J Virol. 1978 May;26(2):479–484. doi: 10.1128/jvi.26.2.479-484.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. M., Hsu T. W., Yeater C., Mason W. S. Synthesis and integration of avian sarcoma virus DNA. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):1091–1096. doi: 10.1101/sqb.1980.044.01.117. [DOI] [PubMed] [Google Scholar]
- Weissbach A., Bolden A., Muller R., Hanafusa H., Hanafusa T. Deoxyribonucleic acid polymerase activities in normal and leukovirus-infected chicken embryo cells. J Virol. 1972 Sep;10(3):321–327. doi: 10.1128/jvi.10.3.321-327.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Jay G., Pastan I. Unusual features in the nucleotide sequence of a cDNA clone derived from the common region of avian sarcoma virus messenger RNA. Proc Natl Acad Sci U S A. 1980 Jan;77(1):176–180. doi: 10.1073/pnas.77.1.176. [DOI] [PMC free article] [PubMed] [Google Scholar]