Abstract
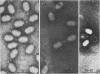
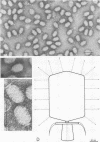
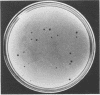
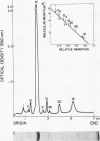
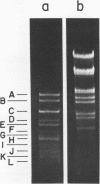
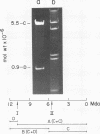
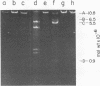
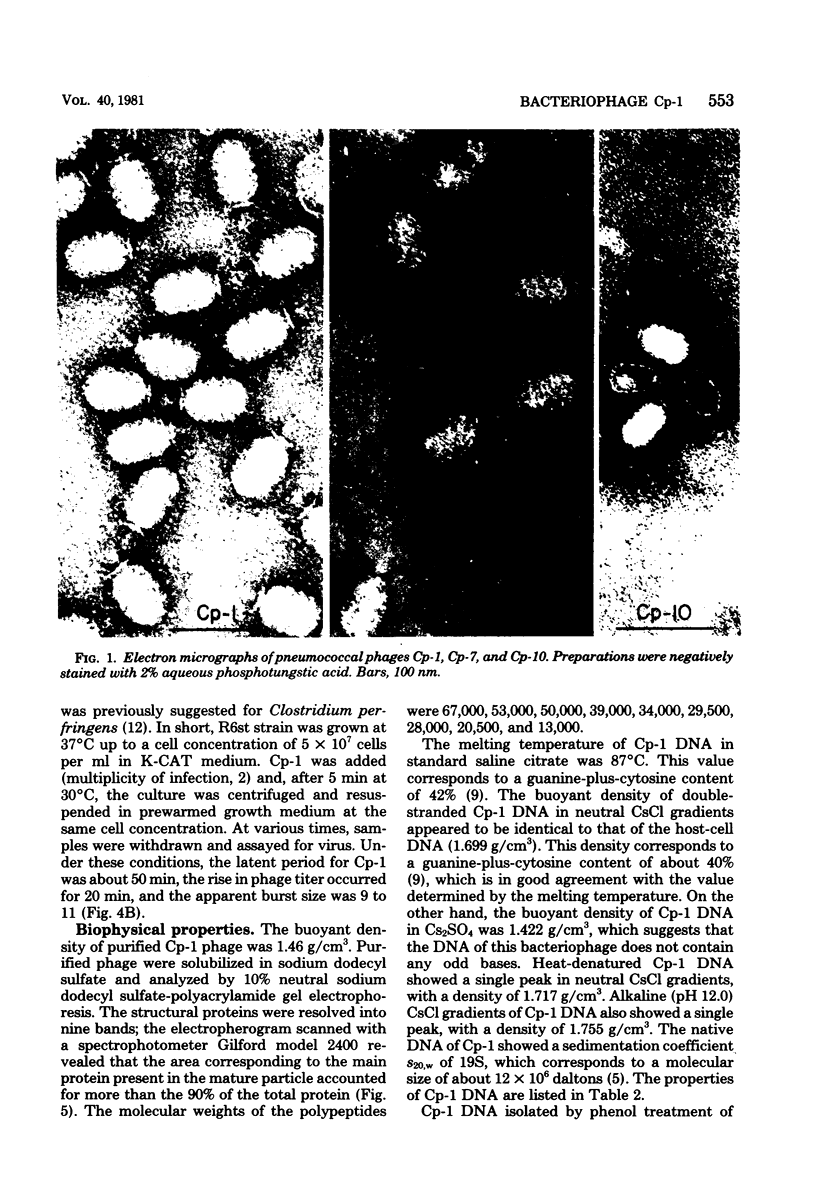
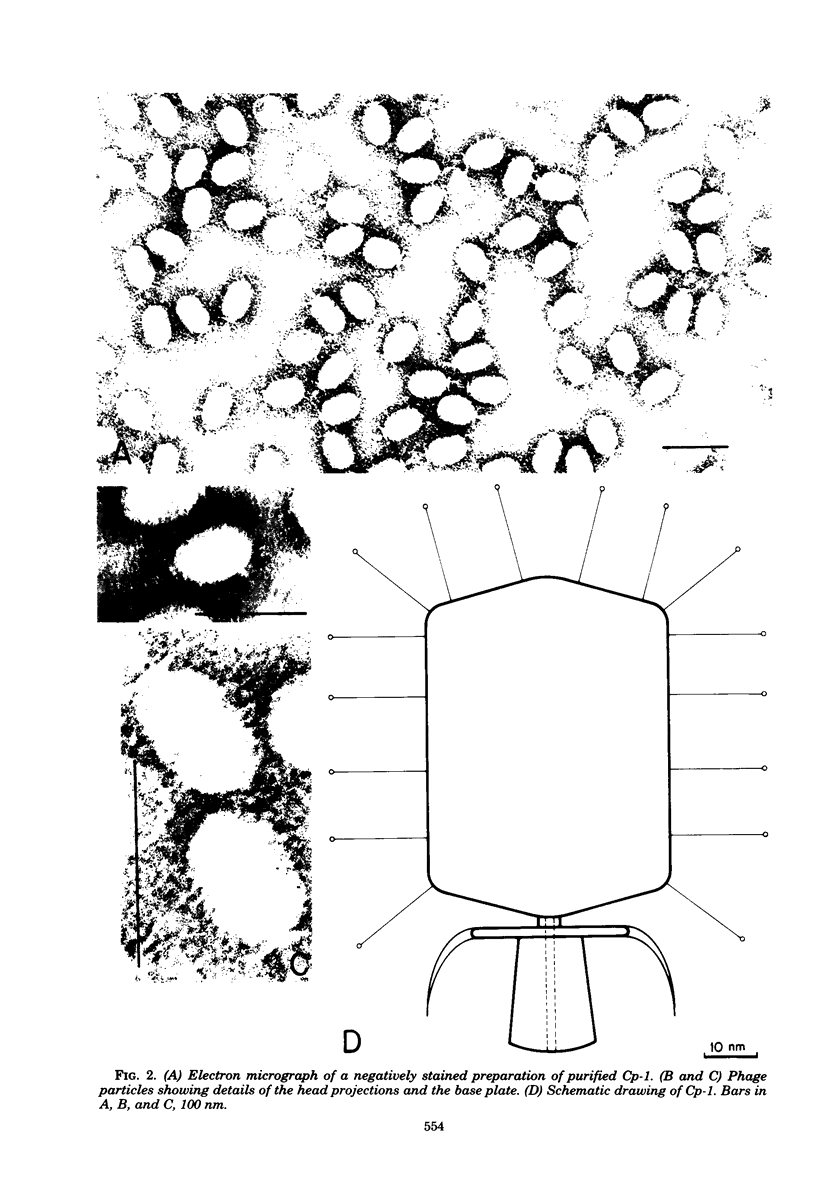
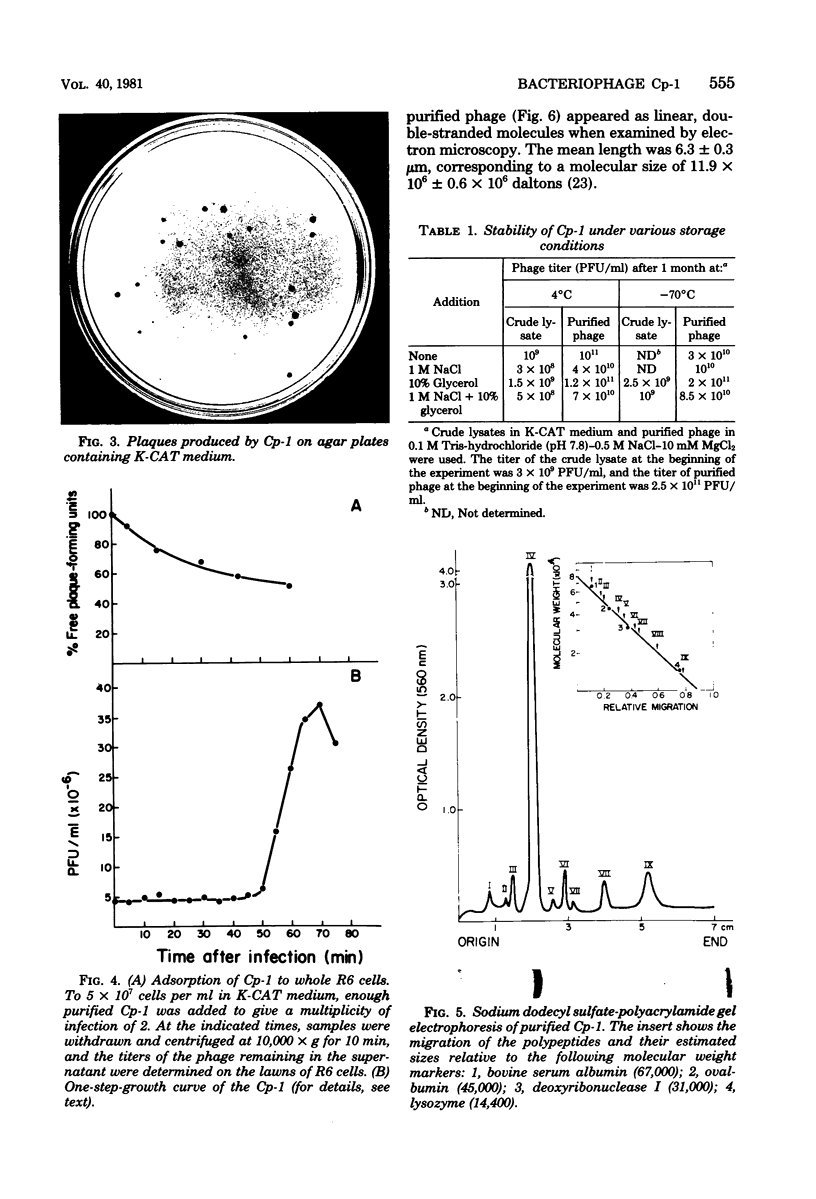
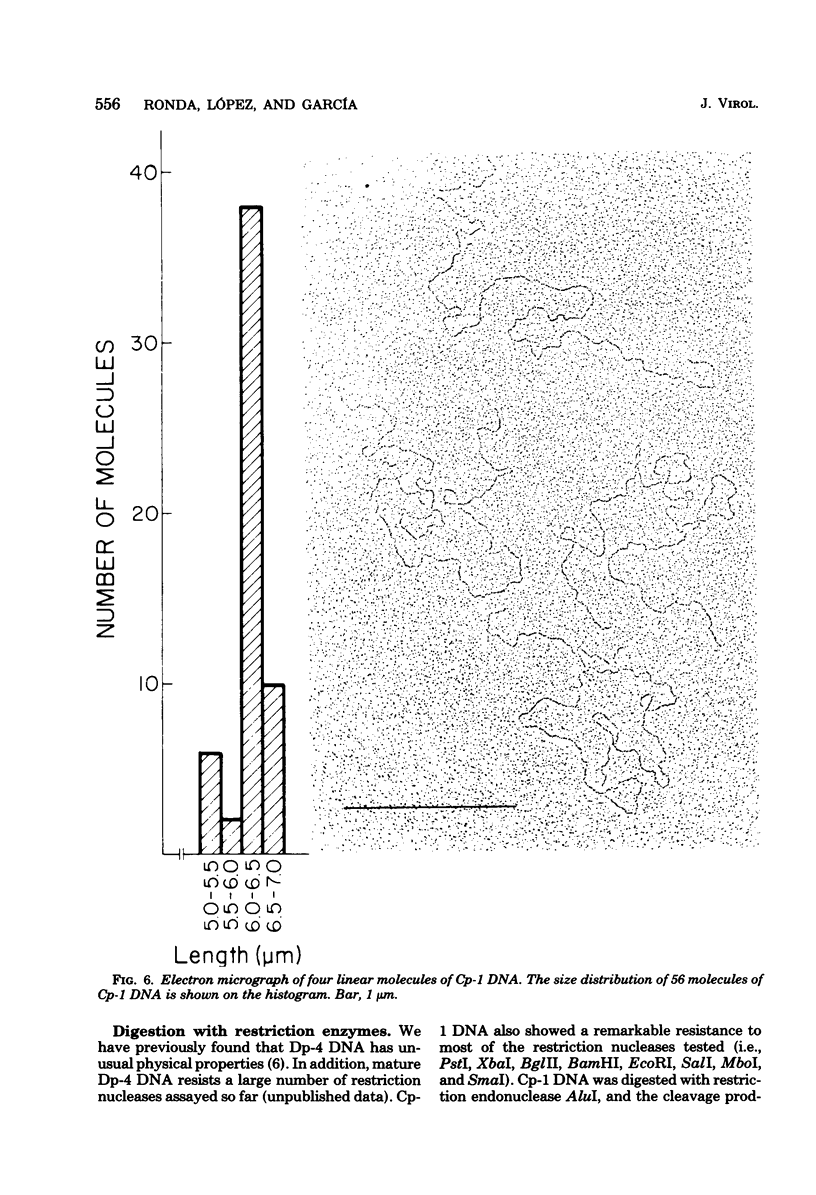
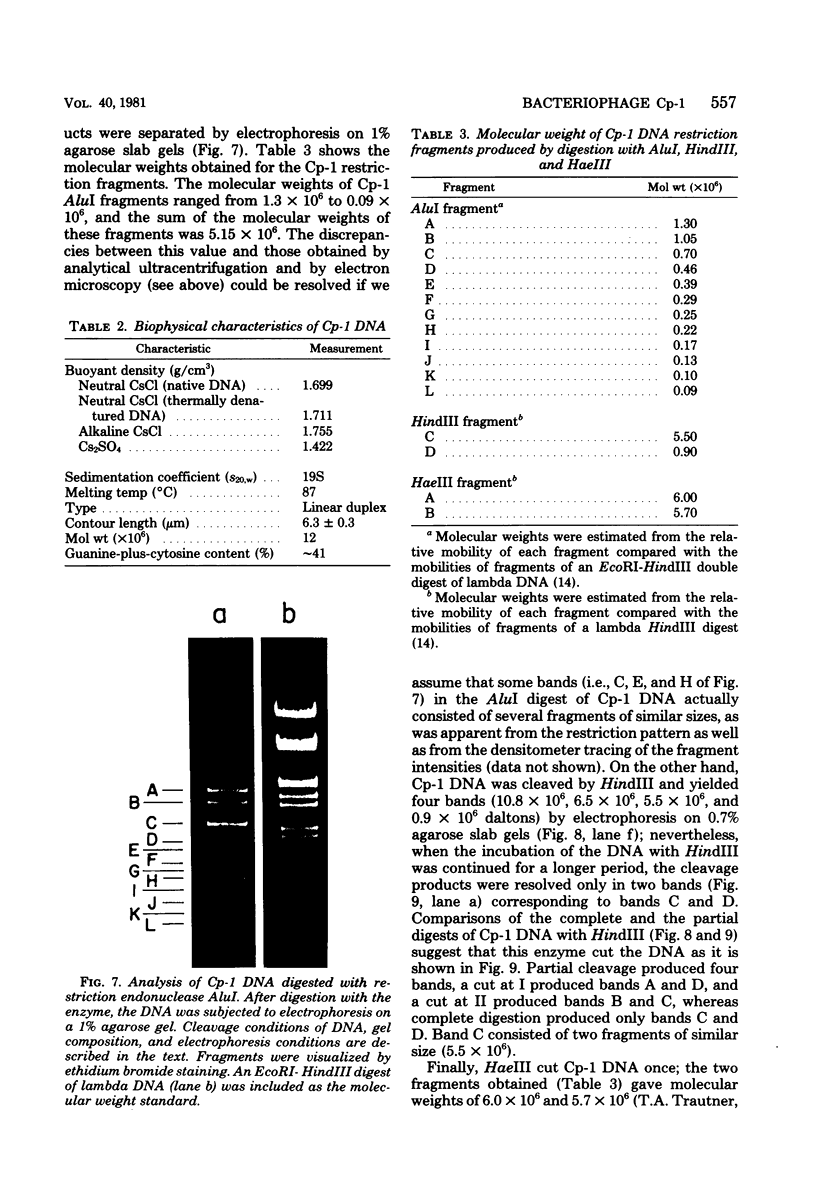
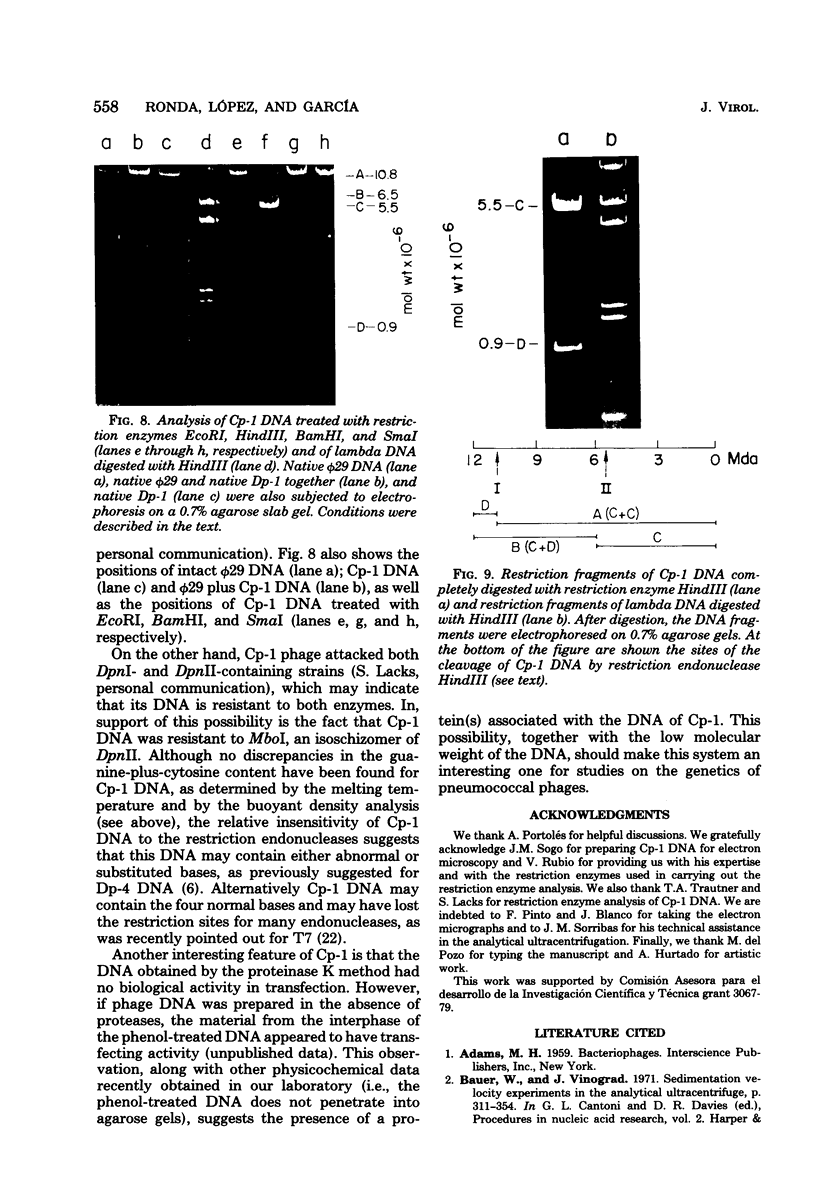
Several pneumococcal phages showing a morphology completely different from those of all other previously found pneumococcal bacteriophages have been isolated. Bacteriophage Cp-1, one of the phages isolated, showed an irregular hexagonal structure and a short tail of 20 nm. The virion density was 1.46 g/cm3. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis revealed the presence of nine polypeptides. The polypeptide showing a molecular weight of 39,000 accounted for more than the 90% of the total protein. The nucleic acid of Cp-1 was linear, double-stranded DNA with a mean length of 6.3 microns and a guanine-plus-cytosine content of 41%; its buoyant density was 1.699 and 1.422 g/cm3 in CsCl and CS2SO4, respectively. Its sedimentation coefficient (S20,w) was 19S. Cp-1 DNA showed a remarkable resistance to a large number of restriction endonucleases. A total of 12 fragments, ranging in molecular weight from 1.3 X 10(6) to 0.09 X 10(6), were produced by AluI, two fragments (molecular weight, 5.5 X 10(6) and 0.9 X 10(6)) were generated by HindIII, and two fragments (molecular weight, 6.0 X 10(6) and 5.7 X 10(6)) were produced by HaeIII. The easy visualization of th plaques produced by Cp-1, the small size of Cp-1 DNA (12 X 10(6) daltons), and other biological and physiochemical properties make this phage potentially useful for genetic studies.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernheimer H. P. Lysogenic pneumococci and their bacteriophages. J Bacteriol. 1979 May;138(2):618–624. doi: 10.1128/jb.138.2.618-624.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freifelder D. Molecular weights of coliphages and coliphage DNA. IV. Molecular weights of DNA from bacteriophages T4, T5 and T7 and the general problem of determination of M. J Mol Biol. 1970 Dec 28;54(3):567–577. doi: 10.1016/0022-2836(70)90127-0. [DOI] [PubMed] [Google Scholar]
- García E., Ronda C., López R. Bacteriophages of Streptococcus pneumoniae. Physicochemical properties of bacteriophage Dp-4 and its transfecting DNA. Eur J Biochem. 1979 Nov 1;101(1):59–64. doi: 10.1111/j.1432-1033.1979.tb04216.x. [DOI] [PubMed] [Google Scholar]
- García E., Ronda C., López R. Replication of bacteriophage Dp-4 DNA in Streptococcus pneumoniae. Virology. 1980 Sep;105(2):405–414. doi: 10.1016/0042-6822(80)90041-0. [DOI] [PubMed] [Google Scholar]
- Gross-Bellard M., Oudet P., Chambon P. Isolation of high-molecular-weight DNA from mammalian cells. Eur J Biochem. 1973 Jul 2;36(1):32–38. doi: 10.1111/j.1432-1033.1973.tb02881.x. [DOI] [PubMed] [Google Scholar]
- Kropinski A. M. Bacteriophage DNA: correlation of buoyant density, melting temperature, and the chemically determined base composition. J Virol. 1974 Mar;13(3):753–756. doi: 10.1128/jvi.13.3.753-756.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez R., Ronda C., Tomasz A., Portoles A. Properties of "diplophage": a lipid-containing bacteriophage. J Virol. 1977 Oct;24(1):201–210. doi: 10.1128/jvi.24.1.201-210.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López R., García E., Ronda C. Selective replication of diplophage Dp-4 deoxyribonucleic acid in 6-(p-hydroxyphenylazo)-uracil treated Streptococcus pneumoniae. FEBS Lett. 1980 Feb 25;111(1):66–68. doi: 10.1016/0014-5793(80)80762-9. [DOI] [PubMed] [Google Scholar]
- Mahony D. E., Easterbrook K. B. Intracellular development of a bacteriophage of Clostridium perfringens. Can J Microbiol. 1970 Oct;16(10):983–988. doi: 10.1139/m70-167. [DOI] [PubMed] [Google Scholar]
- Mcdonnell M., Lain R., Tomasz A. "Diplophage": a bacteriophage of Diplococcus pneumoniae. Virology. 1975 Feb;63(2):577–582. doi: 10.1016/0042-6822(75)90329-3. [DOI] [PubMed] [Google Scholar]
- Murray K., Murray N. E. Phage lambda receptor chromosomes for DNA fragments made with restriction endonuclease III of Haemophilus influenzae and restriction endonuclease I of Escherichia coli. J Mol Biol. 1975 Nov 5;98(3):551–564. doi: 10.1016/s0022-2836(75)80086-6. [DOI] [PubMed] [Google Scholar]
- Nugent K. M., Cole R. M. Characterization of group H streptococcal temperate bacteriophage phi 227. J Virol. 1977 Mar;21(3):1061–1073. doi: 10.1128/jvi.21.3.1061-1073.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter R. D., Guild W. R. Characterization of some pneumococcal bacteriophages. J Virol. 1976 Aug;19(2):659–667. doi: 10.1128/jvi.19.2.659-667.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter R. D., Guild W. R. Transfection in pneumococcus: single-strand intermediates in the formation of infective centers. J Virol. 1978 Jan;25(1):60–72. doi: 10.1128/jvi.25.1.60-72.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter R. D., Shoemaker N. B., Rampe G., Guild W. R. Bacteriophage-associated gene transfer in pneumococcus: transduction or pseudotransduction? J Bacteriol. 1979 Jan;137(1):556–567. doi: 10.1128/jb.137.1.556-567.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronda-Lain C., Lopez R., Tapia A., Tomasz A. Role of the pneumococcal autolysin (murein hydrolase) in the release of progeny bacteriophage and in the bacteriophage-induced lysis of the host cells. J Virol. 1977 Jan;21(1):366–374. doi: 10.1128/jvi.21.1.366-374.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronda C., Lopez R., Tomasz A., Portoles A. Transfection of Streptococcus pneumoniae with bacteriophage DNA. J Virol. 1978 May;26(2):221–225. doi: 10.1128/jvi.26.2.221-225.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg A. H., Simon M. N., Studier F. W., Roberts R. J. Survey and mapping of restriction endonuclease cleavage sites in bacteriophage T7 DNA. J Mol Biol. 1979 Dec 25;135(4):907–915. doi: 10.1016/0022-2836(79)90519-9. [DOI] [PubMed] [Google Scholar]
- Sogo J. M., Inciarte M. R., Corral J., Viñuela E., Salas M. RNA polymerase binding sites and transcription map of the DNA of Bacillus subtilis phage phi29. J Mol Biol. 1979 Feb 5;127(4):411–436. doi: 10.1016/0022-2836(79)90230-4. [DOI] [PubMed] [Google Scholar]
- Tiraby J. G., Tiraby E., Fox M. S. Pneumococcal bacteriophages. Virology. 1975 Dec;68(2):566–569. doi: 10.1016/0042-6822(75)90300-1. [DOI] [PubMed] [Google Scholar]