Abstract
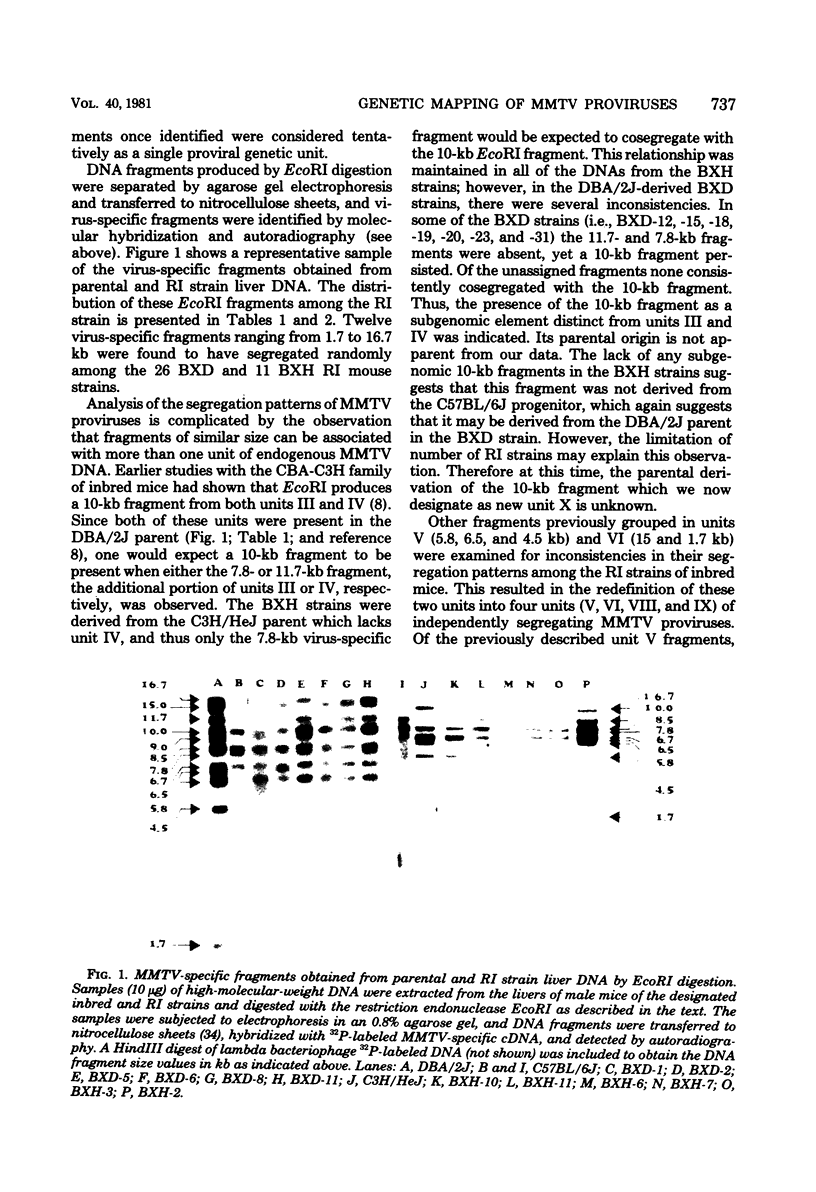
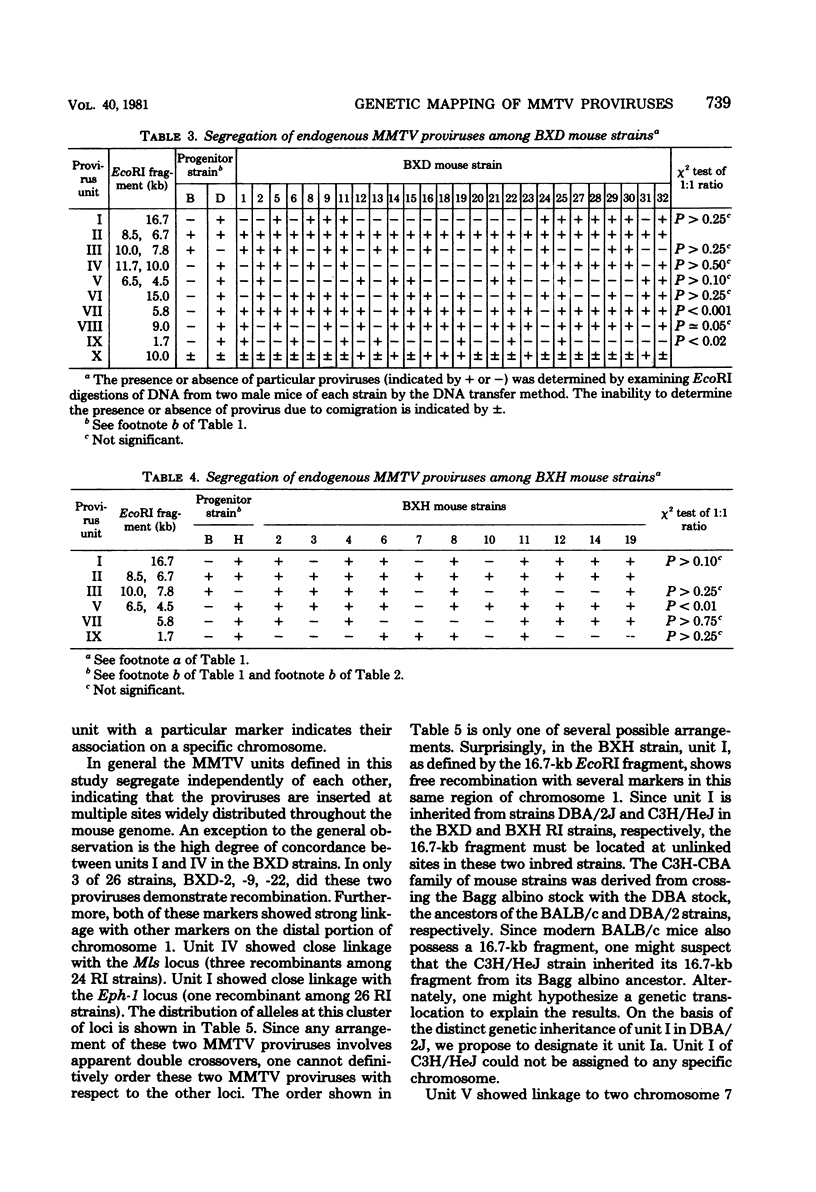
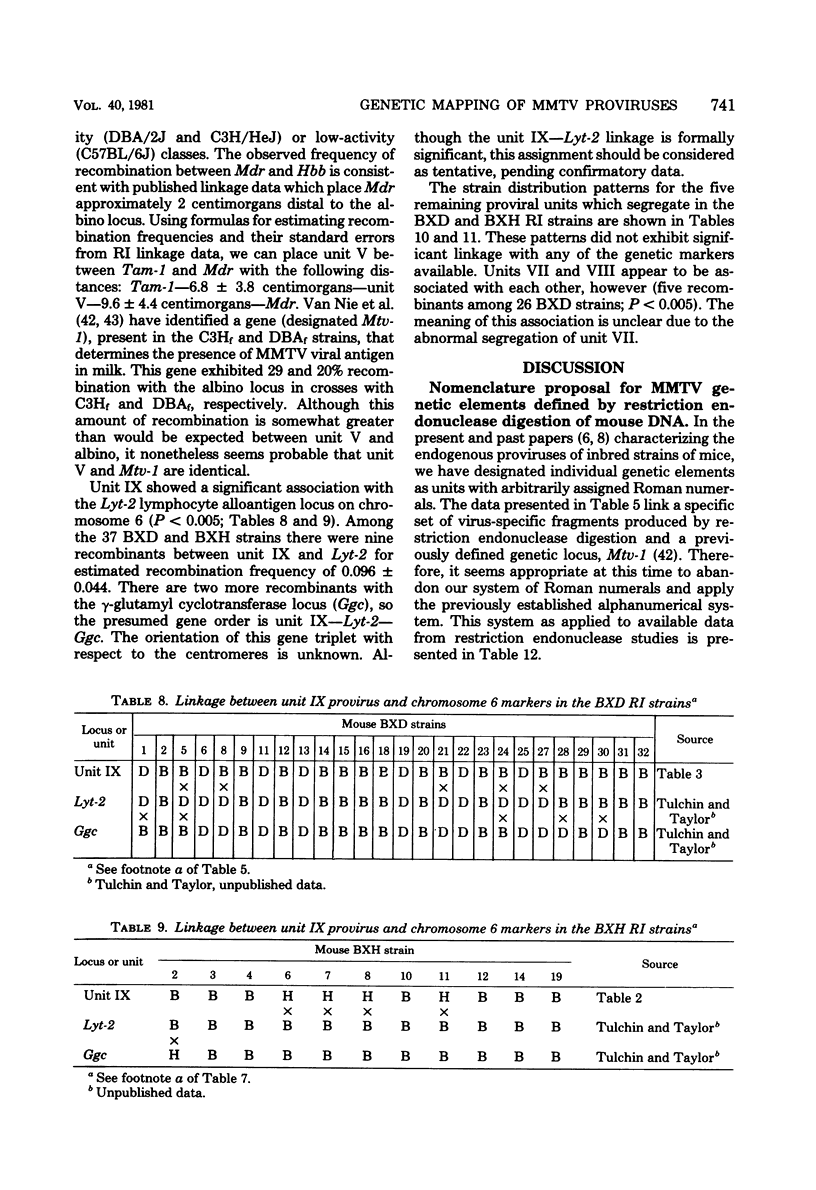
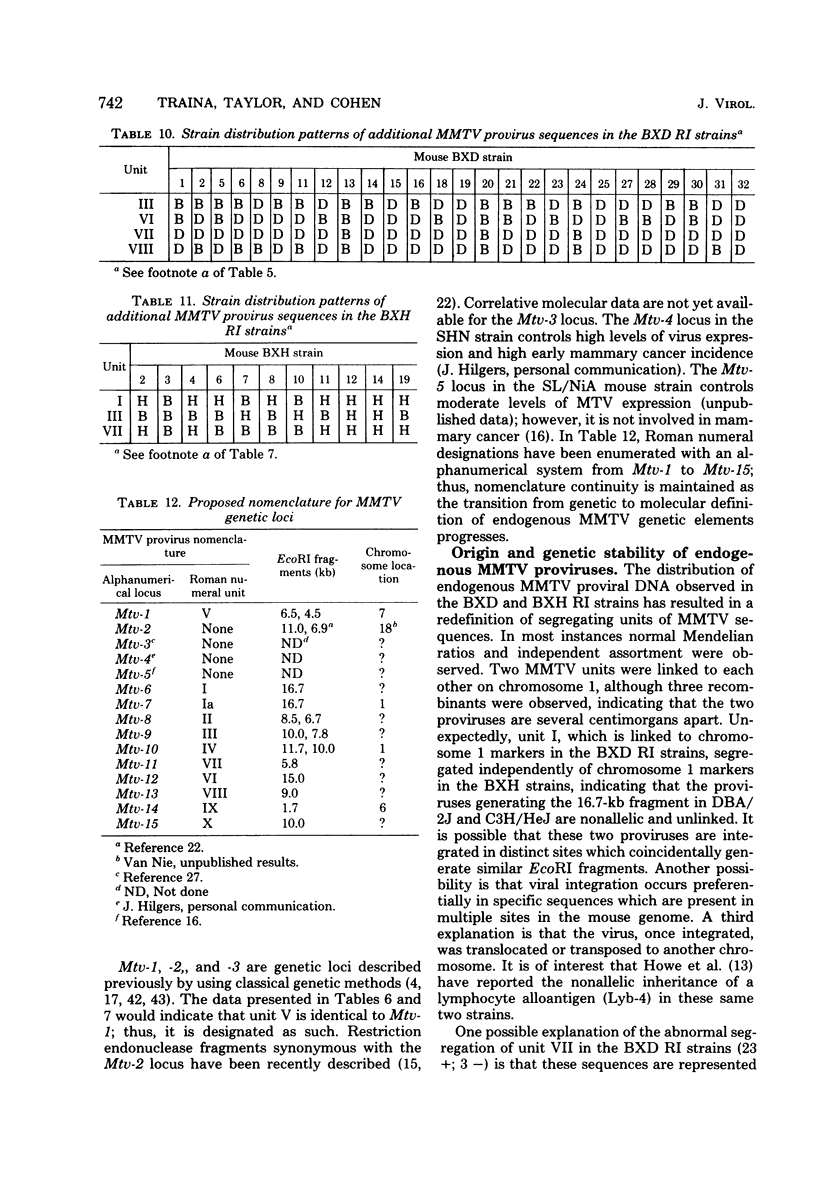
The restriction endonuclease EcoRI has been used to study the inheritance of strain difference in endogenous mouse mammary tumor virus DNA sequences. This enzyme, which cleaves at only one site within the nondefective viral genome, generates DNA fragments containing mouse mammary tumor virus sequences which vary in size according to the locations of EcoRI restriction sites in the flanking mouse sequences, thereby defining unique integration sites of the viral genome. Recombinant inbred strains of mice have been used to study the inheritance of these DNA fragments which hybridize to mouse mammary tumor virus cDNA sequences. The results define 11 segregating units consisting of 1 or 2 fragments. These units were shown to segregate among the recombinant inbred strains, and in some instances linkage was established. Two units were shown to be linked on chromosome 1. Another unit was mapped to chromosome 7, which is presumably identical to the previously defined genetic locus Mtv- 1. One other mouse mammary tumor virus locus was tentatively assigned to chromosome 6. The results are consistent with the view that integration of mouse mammary tumor virus can take place at numerous sites within the genome, and once inserted, these proviruses appear to be relatively stable genetic entities.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Astrin S. M., Buss E. G., Haywards W. S. Endogenous viral genes are non-essential in the chicken. Nature. 1979 Nov 15;282(5736):339–341. doi: 10.1038/282339a0. [DOI] [PubMed] [Google Scholar]
- Bentvelzen P., Brinkhof J., Haaijman J. J. Genetic control of endogenous murine mammary tumour viruses reinvestigated. Eur J Cancer. 1978 Oct;14(10):1137–1147. doi: 10.1016/0014-2964(78)90070-1. [DOI] [PubMed] [Google Scholar]
- Bentvelzen P. Host-virus interactions in murine mammary carcinogenesis. Biochim Biophys Acta. 1974 Dec 31;355(3-4):236–259. doi: 10.1016/0304-419x(74)90012-2. [DOI] [PubMed] [Google Scholar]
- Bernstein A. H. Current status of the law of consent to treatment. Hospitals. 1979 Feb 1;53(3):83-5, 87. [PubMed] [Google Scholar]
- Cohen J. C., Majors J. E., Varmus H. E. Organization of mouse mammary tumor virus-specific DNA endogenous to BALB/c mice. J Virol. 1979 Nov;32(2):483–496. doi: 10.1128/jvi.32.2.483-496.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. C., Shank P. R., Morris V. L., Cardiff R., Varmus H. E. Integration of the DNA of mouse mammary tumor virus in virus-infected normal and neoplastic tissue of the mouse. Cell. 1979 Feb;16(2):333–345. doi: 10.1016/0092-8674(79)90010-2. [DOI] [PubMed] [Google Scholar]
- Cohen J. C., Varmus H. E. Endogenous mammary tumour virus DNA varies among wild mice and segregates during inbreeding. Nature. 1979 Mar 29;278(5703):418–423. doi: 10.1038/278418a0. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Gillespie D., Gillespie S., Gallo R. C., East J. L., Dmochowski L. Genetic origin of RD114 and other RNA tumour viruses assayed by molecular hybridization. Nat New Biol. 1973 Jul 11;244(132):51–54. doi: 10.1038/newbio244051a0. [DOI] [PubMed] [Google Scholar]
- Helling R. B., Goodman H. M., Boyer H. W. Analysis of endonuclease R-EcoRI fragments of DNA from lambdoid bacteriophages and other viruses by agarose-gel electrophoresis. J Virol. 1974 Nov;14(5):1235–1244. doi: 10.1128/jvi.14.5.1235-1244.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes N. E., Groner B., Diggelmann H., Van Nie R., Michalides R. Genomic location of mouse mammary tumor virus proviral DNA in normal mouse tissue and in mammary tumors. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):1161–1168. doi: 10.1101/sqb.1980.044.01.125. [DOI] [PubMed] [Google Scholar]
- Imai S., Hilgers J. Levels of mammary tumor virus proteins (MTVp27 and MTVgp52) in the milk of low and high mammary cancer mouse strains of Japanese origin compared with European and American strains. Int J Cancer. 1979 Sep 15;24(3):359–364. doi: 10.1002/ijc.2910240315. [DOI] [PubMed] [Google Scholar]
- Imai S., Hilgers J., Van Nie R., Verstraeten R. Mammary tumor virus expression in the GR mouse strain: efficiency of chromosomal and extrachromosomal transmission. Gan. 1978 Oct;69(5):607–611. [PubMed] [Google Scholar]
- Keshet E., Temin H. M. Sites of integration of reticuloendotheliosis virus DNA in chicken DNA. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3372–3376. doi: 10.1073/pnas.75.7.3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyman S. D., Poland A., Taylor B. A. Genetic polymorphism of microsomal epoxide hydrolase activity in the mouse. J Biol Chem. 1980 Sep 25;255(18):8650–8654. [PubMed] [Google Scholar]
- Michalides R., Schlom J. Relationship in nucleic acid sequences between mouse mammary tumor virus variants. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4635–4639. doi: 10.1073/pnas.72.11.4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalides R., van Nie R., Nusse R., Hynes N. E., Groner B. Mammary tumor induction loci in GR and DBAf mice contain one provirus of the mouse mammary tumor virus. Cell. 1981 Jan;23(1):165–173. doi: 10.1016/0092-8674(81)90281-6. [DOI] [PubMed] [Google Scholar]
- Morris V. L., Kozak C., Cohen J. C., Shank P. R., Jolicoeur P., Ruddle F., Varmus H. E. Endogenous mouse mammary tumor virus DNA is distributed among multiple mouse chromosomes. Virology. 1979 Jan 15;92(1):46–55. doi: 10.1016/0042-6822(79)90213-7. [DOI] [PubMed] [Google Scholar]
- Morris V. L., Medeiros E., Ringold G. M., Bishop J. M., Varmus H. E. Comparison of mouse mammary tumor virus-specific DNA in inbred, wild and Asian mice, and in tumors and normal organs from inbred mice. J Mol Biol. 1977 Jul;114(1):73–91. doi: 10.1016/0022-2836(77)90284-4. [DOI] [PubMed] [Google Scholar]
- Nusse R., de Moes J., Hilkens J., van Nie R. Localization of a gene for expression of mouse mammary tumor virus antigens in the GR/Mtv-2- mouse strain. J Exp Med. 1980 Sep 1;152(3):712–719. doi: 10.1084/jem.152.3.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine R. R., Langley C. H. Genetic analysis of protein variations in Mus musculus using two-dimensional electrophoresis. Biochem Genet. 1980 Feb;18(1-2):185–197. doi: 10.1007/BF00504368. [DOI] [PubMed] [Google Scholar]
- Ringold G. M., Shank P. R., Varmus H. E., Ring J., Yamamoto K. R. Integration and transcription of mouse mammary tumor virus DNA in rat hepatoma cells. Proc Natl Acad Sci U S A. 1979 Feb;76(2):665–669. doi: 10.1073/pnas.76.2.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringold G. M., Yamamoto K. R., Shank P. R., Varmus H. E. Mouse mammary tumor virus DNA in infected rat cells: characterization of unintegrated forms. Cell. 1977 Jan;10(1):19–26. doi: 10.1016/0092-8674(77)90135-0. [DOI] [PubMed] [Google Scholar]
- Rowe W. P., Kozak C. A. Germ-line reinsertions of AKR murine leukemia virus genomes in Akv-1 congenic mice. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4871–4874. doi: 10.1073/pnas.77.8.4871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shank P. R., Cohen J. C., Varmus H. E., Yamamoto K. R., Ringold G. M. Mapping of linear and circular forms of mouse mammary tumor virus DNA with restriction endonucleases: evidence for a large specific deletion occurring at high frequency during circularization. Proc Natl Acad Sci U S A. 1978 May;75(5):2112–2116. doi: 10.1073/pnas.75.5.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shank P. R., Hughes S. H., Kung H. J., Majors J. E., Quintrell N., Guntaka R. V., Bishop J. M., Varmus H. E. Mapping unintegrated avian sarcoma virus DNA: termini of linear DNA bear 300 nucleotides present once or twice in two species of circular DNA. Cell. 1978 Dec;15(4):1383–1395. doi: 10.1016/0092-8674(78)90063-6. [DOI] [PubMed] [Google Scholar]
- Skow L. C. Genetic variation at a locus (TAM-1) for submaxillary gland protease in the mouse and its location on chromosome No. 7. Genetics. 1978 Dec;90(4):713–724. doi: 10.1093/genetics/90.4.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Staats J. Standardized nomenclature for inbred strains of mice: seventh listing for the International Committee on Standardized Genetic Nomenclature for Mice. Cancer Res. 1980 Jul;40(7):2083–2128. [PubMed] [Google Scholar]
- Stern R. H., Russell E. S., Taylor B. A. Strain distribution and linkage relationship of a mouse embryonic hemoglobin variant. Biochem Genet. 1976 Apr;14(3-4):373–381. doi: 10.1007/BF00484775. [DOI] [PubMed] [Google Scholar]
- Swanstrom R., Shank P. R. X-Ray Intensifying Screens Greatly Enhance the Detection by Autoradiography of the Radioactive Isotopes 32P and 125I. Anal Biochem. 1978 May;86(1):184–192. doi: 10.1016/0003-2697(78)90333-0. [DOI] [PubMed] [Google Scholar]
- Taylor B. A., Bedigian H. G., Meier H. Genetic studies of the Fv-1 locus of mice: linkage with Gpd-1 in recombinant inbred lines. J Virol. 1977 Jul;23(1):106–109. doi: 10.1128/jvi.23.1.106-109.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya A. B., Lasfargues E. Y., Heubel G., Lasfargues J. C., Moore D. H. Murine mammary tumor virus: characterization of infection of nonmurine cells. J Virol. 1976 Jun;18(3):911–917. doi: 10.1128/jvi.18.3.911-917.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Nie R., Verstraeten A. A., De Moes J. Genetic transmission of mammary tumour virus by GR mice. Int J Cancer. 1977 Mar 15;19(3):383–390. doi: 10.1002/ijc.2910190316. [DOI] [PubMed] [Google Scholar]
- Verstraeten A. A., van Nie R. Genetic transmission of mammary tumour virus in the DBAf mouse strain. Int J Cancer. 1978 Apr 15;21(4):473–475. doi: 10.1002/ijc.2910210412. [DOI] [PubMed] [Google Scholar]
- Wood A. W., Taylor B. A. Genetic regulation of coumarin hydroxylase activity in mice. Evidence for single locus control on chromosome. J Biol Chem. 1979 Jul 10;254(13):5647–5651. [PubMed] [Google Scholar]
- van Nie R., Verstraeten A. A. Studies of genetic transmission of mammary tumour virus by C3Hf mice. Int J Cancer. 1975 Dec 15;16(6):922–931. doi: 10.1002/ijc.2910160606. [DOI] [PubMed] [Google Scholar]



