Abstract
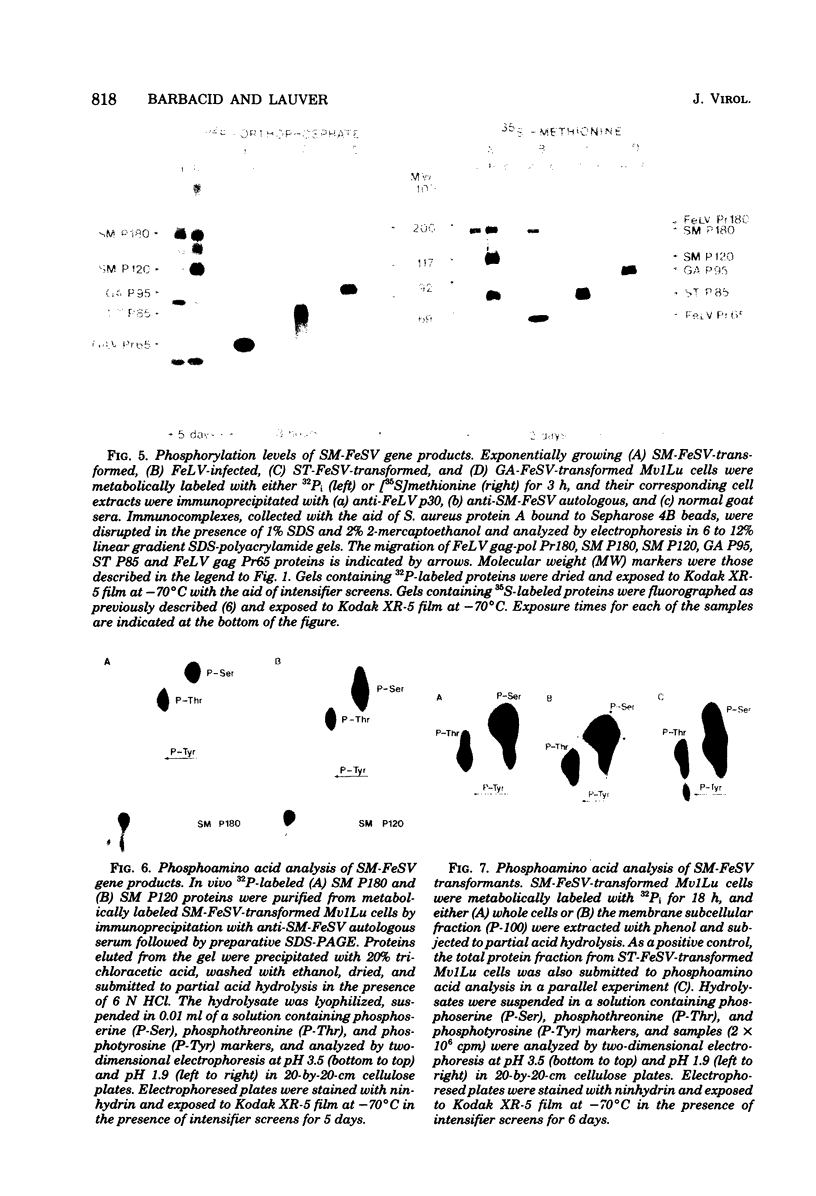
The primary translational product of the McDonough (SM) strain of feline sarcoma virus (FeSV) is a 180,000-dalton molecule, SM P180, that contains the p15-p12-p30 region of the FeLV gag gene-coded precursor protein and a sarcoma virus-specific polypeptide. In addition, cells transformed by SM-FeSV express a 120,000-dalton molecule, SM P120, that is highly related to the non-helper virus domain of SM P180. Both SM-FeSV gene products were found to be intimately associated with the membrane fraction of SM-FeSV-transformed cells. Immunoprecipitates containing SM P180 and SM P120 exhibited a protein kinase activity capable of phosphorylating tyrosine residues of both viral gene products but not immune immunoglobulin G molecules. By independently immunoprecipitating each of the two SM-FeSV proteins we found that most of the tyrosine-specific phosphorylating activity was associated with the SM P120 molecule. In vivo analysis of 32P-labeled SM P180 and SM P120 revealed their phosphoprotein nature; however, both molecules exhibited low levels of phosphorylation and did not contain phosphotyrosine residues. Finally, we did not detect any significant elevation in the levels of phosphotyrosine in the protein fraction of SM-FeSV transformants. Thus, if SM-FeSV were to induce malignant transformation by a mechanism involving phosphorylation of tyrosine residues, the viral gene products must interact with a small subset of cellular proteins that do not represent a significant fraction of the total cellular protein content.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbacid M., Beemon K., Devare S. G. Origin and functional properties of the major gene product of the Snyder-Theilen strain of feline sarcoma virus. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5158–5162. doi: 10.1073/pnas.77.9.5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbacid M., Donner L., Ruscetti S. K., Sherr C. J. Transformation-defective mutants of Snyder-Theilen feline sarcoma virus lack tyrosine-specific protein kinase activity. J Virol. 1981 Jul;39(1):246–254. doi: 10.1128/jvi.39.1.246-254.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbacid M., Lauver A. V., Devare S. G. Biochemical and immunological characterization of polyproteins coded for by the McDonough, Gardner-Arnstein, and Snyder-Theilen strains of feline sarcoma virus. J Virol. 1980 Jan;33(1):196–207. doi: 10.1128/jvi.33.1.196-207.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beemon K. Transforming proteins of some feline and avian sarcoma viruses are related structurally and functionally. Cell. 1981 Apr;24(1):145–153. doi: 10.1016/0092-8674(81)90510-9. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Burr J. G., Dreyfuss G., Penman S., Buchanan J. M. Association of the src gene product of Rous sarcoma virus with cytoskeletal structures of chicken embryo fibroblasts. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3484–3488. doi: 10.1073/pnas.77.6.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Erikson R. L. Protein kinase activity associated with the avian sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2021–2024. doi: 10.1073/pnas.75.4.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtneidge S. A., Levinson A. D., Bishop J. M. The protein encoded by the transforming gene of avian sarcoma virus (pp60src) and a homologous protein in normal cells (pp60proto-src) are associated with the plasma membrane. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3783–3787. doi: 10.1073/pnas.77.7.3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H. Transforming genes of retroviruses. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):13–29. doi: 10.1101/sqb.1980.044.01.005. [DOI] [PubMed] [Google Scholar]
- Eckhart W., Hutchinson M. A., Hunter T. An activity phosphorylating tyrosine in polyoma T antigen immunoprecipitates. Cell. 1979 Dec;18(4):925–933. doi: 10.1016/0092-8674(79)90205-8. [DOI] [PubMed] [Google Scholar]
- Fedele L. A., Even J., Garon C. F., Donner L., Sherr C. J. Recombinant bacteriophages containing the integrated transforming provirus of Gardner--Arnstein feline sarcoma virus. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4036–4040. doi: 10.1073/pnas.78.7.4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R. A., Hanafusa T., Hanafusa H. Characterization of protein kinase activity associated with the transforming gene product of Fujinami sarcoma virus. Cell. 1980 Dec;22(3):757–765. doi: 10.1016/0092-8674(80)90552-8. [DOI] [PubMed] [Google Scholar]
- Frankel A. E., Gilbert J. H., Porzig K. J., Scolnick E. M., Aaronson S. A. Nature and distribution of feline sarcoma virus nucleotide sequences. J Virol. 1979 Jun;30(3):821–827. doi: 10.1128/jvi.30.3.821-827.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner M. B., Rongey R. W., Arnstein P., Estes J. D., Sarma P., Huebner R. J., Rickard C. G. Experimental transmission of feline fibrosarcoma to cats and dogs. Nature. 1970 May 30;226(5248):807–809. doi: 10.1038/226807a0. [DOI] [PubMed] [Google Scholar]
- Garma P. S., Log T. Defectiveness of a feline sarcoma virus demonstrable by the acquisition of a new envelope antigenic type by phenotypic mixing. Virology. 1977 May 1;78(1):336–339. doi: 10.1016/0042-6822(77)90106-4. [DOI] [PubMed] [Google Scholar]
- Hanafusa T., Mathey-Prevot B., Feldman R. A., Hanafusa H. Mutants of Fujinami sarcoma virus which are temperature sensitive for cellular transformation and protein kinase activity. J Virol. 1981 Apr;38(1):347–355. doi: 10.1128/jvi.38.1.347-355.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafusa T., Wang L. H., Anderson S. M., Karess R. E., Hayward W. S., Hanafusa H. Characterization of the transforming gene of Fujinami sarcoma virus. Proc Natl Acad Sci U S A. 1980 May;77(5):3009–3013. doi: 10.1073/pnas.77.5.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay A. J. Studies on the formation of the influenza virus envelope. Virology. 1974 Aug;60(2):398–418. doi: 10.1016/0042-6822(74)90335-3. [DOI] [PubMed] [Google Scholar]
- Henderson I. C., Lieber M. M., Todaro G. J. Mink cell line Mv 1 Lu (CCL 64). Focus formation and the generation of "nonproducer" transformed cell lines with murine and feline sarcoma viruses. Virology. 1974 Jul;60(1):282–287. doi: 10.1016/0042-6822(74)90386-9. [DOI] [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. Cellular location of viral transforming proteins. Cell. 1980 Oct;21(3):601–602. doi: 10.1016/0092-8674(80)90421-3. [DOI] [PubMed] [Google Scholar]
- Kawai S., Yoshida M., Segawa K., Sugiyama H., Ishizaki R., Toyoshima K. Characterization of Y73, an avian sarcoma virus: a unique transforming gene and its product, a phosphopolyprotein with protein kinase activity. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6199–6203. doi: 10.1073/pnas.77.10.6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzyzek R. A., Mitchell R. L., Lau A. F., Faras A. J. Association of pp60src and src protein kinase activity with the plasma membrane of nonpermissive and permissive avian sarcoma virus-infected cells. J Virol. 1980 Dec;36(3):805–815. doi: 10.1128/jvi.36.3.805-815.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W. H., Bister K., Pawson A., Robins T., Moscovici C., Duesberg P. H. Fujinami sarcoma virus: an avian RNA tumor virus with a unique transforming gene. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2018–2022. doi: 10.1073/pnas.77.4.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson A. D., Oppermann H., Levintow L., Varmus H. E., Bishop J. M. Evidence that the transforming gene of avian sarcoma virus encodes a protein kinase associated with a phosphoprotein. Cell. 1978 Oct;15(2):561–572. doi: 10.1016/0092-8674(78)90024-7. [DOI] [PubMed] [Google Scholar]
- McDonough S. K., Larsen S., Brodey R. S., Stock N. D., Hardy W. D., Jr A transmissible feline fibrosarcoma of viral origin. Cancer Res. 1971 Jul;31(7):953–956. [PubMed] [Google Scholar]
- Neil J. C., Breitman M. L., Vogt P. K. Characterization of a 105,000 molecular weight gag-related phosphoprotein from cells transformed by the defective avian sarcoma virus PRCII. Virology. 1981 Jan 15;108(1):98–110. doi: 10.1016/0042-6822(81)90530-4. [DOI] [PubMed] [Google Scholar]
- Neil J. C., Ghysdael J., Vogt P. K. Tyrosine-specific protein kinase activity associated with p105 of avian sarcoma virus PRCII. Virology. 1981 Feb;109(1):223–228. doi: 10.1016/0042-6822(81)90493-1. [DOI] [PubMed] [Google Scholar]
- Pawson T., Guyden J., Kung T. H., Radke K., Gilmore T., Martin G. S. A strain of Fujinami sarcoma virus which is temperature-sensitive in protein phosphorylation and cellular transformation. Cell. 1980 Dec;22(3):767–775. doi: 10.1016/0092-8674(80)90553-x. [DOI] [PubMed] [Google Scholar]
- Porzig K. J., Barbacid M., Aaronson S. A. Biological properties and translational products of three independent isolates of feline sarcoma virus. Virology. 1979 Jan 15;92(1):91–107. doi: 10.1016/0042-6822(79)90217-4. [DOI] [PubMed] [Google Scholar]
- Reynolds F. H., Jr, Van de Ven W. J., Blomberg J., Stephenson J. R. Differences in mechanisms of transformation by independent feline sarcoma virus isolates. J Virol. 1981 Jun;38(3):1084–1089. doi: 10.1128/jvi.38.3.1084-1089.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds F. H., Jr, Van de Ven W. J., Blomberg J., Stephenson J. R. Involvement of a high-molecular-weight polyprotein translational product of Snyder-Theilen Feline sarcoma virus in malignant transformation. J Virol. 1981 Feb;37(2):643–653. doi: 10.1128/jvi.37.2.643-653.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds F. H., Jr, Van de Ven W. J., Stephenson J. R. Abelson murine leukemia virus transformation-defective mutants with impaired P120-associated protein kinase activity. J Virol. 1980 Nov;36(2):374–386. doi: 10.1128/jvi.36.2.374-386.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins K. C., Barbacid M., Porzig K. J., Aaronson S. A. Involvement of different exogenous feline leukemia virus subgroups in the generation of independent feline sarcoma virus isolates. Virology. 1979 Aug;97(1):1–11. doi: 10.1016/0042-6822(79)90367-2. [DOI] [PubMed] [Google Scholar]
- Ruscetti S. K., Turek L. P., Sherr C. J. Three independent isolates of feline sarcoma virus code for three distinct gag-x polyproteins. J Virol. 1980 Jul;35(1):259–264. doi: 10.1128/jvi.35.1.259-264.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton B. M., Hunter T., Beemon K., Eckhart W. Evidence that the phosphorylation of tyrosine is essential for cellular transformation by Rous sarcoma virus. Cell. 1980 Jul;20(3):807–816. doi: 10.1016/0092-8674(80)90327-x. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Hunter T., Raschke W. C. Evidence that the Abelson virus protein functions in vivo as a protein kinase that phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1552–1556. doi: 10.1073/pnas.78.3.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. E., Smith R., Griffin B., Fried M. Protein kinase activity associated with polyoma virus middle T antigen in vitro. Cell. 1979 Dec;18(4):915–924. doi: 10.1016/0092-8674(79)90204-6. [DOI] [PubMed] [Google Scholar]
- Snyder S. P., Theilen G. H. Transmissible feline fibrosarcoma. Nature. 1969 Mar 15;221(5185):1074–1075. doi: 10.1038/2211074a0. [DOI] [PubMed] [Google Scholar]
- Van de Ven W. J., Reynolds F. H., Jr, Nalewaik R. P., Stephenson J. R. Characterization of a 170,000-dalton polyprotein encoded by the McDonough strain of feline sarcoma virus. J Virol. 1980 Jul;35(1):165–175. doi: 10.1128/jvi.35.1.165-175.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Ven W. J., Reynolds F. H., Jr, Stephenson J. R. The nonstructural components of polyproteins encoded by replication-defective mammalian transforming retroviruses are phosphorylated and have associated protein kinase activity. Virology. 1980 Feb;101(1):185–197. doi: 10.1016/0042-6822(80)90495-x. [DOI] [PubMed] [Google Scholar]
- Witte O. N., Dasgupta A., Baltimore D. Abelson murine leukaemia virus protein is phosphorylated in vitro to form phosphotyrosine. Nature. 1980 Feb 28;283(5750):826–831. doi: 10.1038/283826a0. [DOI] [PubMed] [Google Scholar]
- Witte O. N., Goff S., Rosenberg N., Baltimore D. A transformation-defective mutant of Abelson murine leukemia virus lacks protein kinase activity. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4993–4997. doi: 10.1073/pnas.77.8.4993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte O. N., Rosenberg N., Paskind M., Shields A., Baltimore D. Identification of an Abelson murine leukemia virus-encoded protein present in transformed fibroblast and lymphoid cells. Proc Natl Acad Sci U S A. 1978 May;75(5):2488–2492. doi: 10.1073/pnas.75.5.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]