Abstract
There is conflicting evidence as to whether cavities in proteins that are nonpolar and large enough to accommodate solvent are empty or are occupied by disordered water molecules. Here, we use multiple-wavelength x-ray data collected from crystals of the selenomethionine-substituted L99A/M102L mutant of T4 lysozyme to obtain a high-resolution electron density map free of bias that is unavoidably associated with conventional model-based structure determination and refinement. The mutant, L99A/M102L, has four cavities, two being polar in character and the other two nonpolar. Cavity 1 (polar, volume 45.2 Å3) was expected to contain two well ordered water molecules, and this is confirmed in the experimental electron density map. Likewise, cavity 2 (polar, 16.9 Å3) is confirmed to contain a single water molecule. Cavity 3 (nonpolar, 21.4 Å3) was seen to be empty in conventional x-ray refinement, and this is confirmed in the experimental map. Unexpectedly, however, cavity 4 (nonpolar, volume 133.5 Å3) was seen to contain diffuse electron density equivalent to ≈1.5 water molecules. Although cavity 4 is largely nonpolar, it does have some polar character, and this apparently contributes to the presence of solvent. The cavity is large enough to accommodate four to five water molecules, and it appears that a hydrogen-bonded chain of three or more solvent molecules could occupy the cavity at a given time. The results are consistent with theoretical predictions that cavities in proteins that are strictly nonpolar will not contain solvent until the volume is large enough to permit mutually satisfying water–water hydrogen bonds.
Keywords: bound water, hydrogen bonding, protein cavity
The interiors of globular proteins are generally well packed. At the same time, some internal cavities, both polar and nonpolar, with volume large enough to hold one or more water molecules are found in many proteins (1, 2). Buried water that is hydrogen-bonded and well ordered within polar cavities has been observed by many physical techniques including x-ray crystallography, neutron diffraction, NMR, and ultrafast UV fluorescence (3, 4). However, hydration in nonpolar protein cavities is more controversial (3, 5–9). Support for such solvent comes from some thermodynamic analyses and molecular dynamics simulations (6, 10, 11) as well as some physical techniques including NMR, femtosecond UV fluorescence and, in some cases, x-ray crystallography (3, 4, 6). On the other hand, other theoretical and crystallographic studies suggest that nonpolar cavities have little if any bound solvent (12–14).
Recent developments in crystallographic phasing allow direct observation of protein solvation based on a set of complete and accurate experimental phases (15). Such phases are free from the inherent bias that is associated with “solvent flattening” and related procedures in conventional crystallographic refinement. Experimental phases were recently used to demonstrate that the nonpolar cavity in interleukin-1β (IL-1β) contains little if any bound water (9). Here, the same strategy is used to examine two nonpolar or largely nonpolar cavities in the engineered Leu-99 to Ala (L99A) mutant of T4 lysozyme (16). Two other cavities that are polar in nature and contain ordered water molecules are used as internal controls.
Results
Experimental Phasing.
The L99A cavity in T4 lysozyme is, in many respects, the prototypical nonpolar cavity in proteins. It was one of the first such cavities to be engineered (16) and has been the subject of thermodynamic, structural (16), ligand-binding (17–19), NMR (20–22), pressure-dependent, and theoretical studies (23, 24). With a volume of ≈120 Å3, it is larger than one typically obtains from a “large-to-small” substitution (e.g., Leu → Ala or Phe → Ala) in the core of a protein (16, 25).
Conventional crystallographic x-ray refinement suggests that the L99A cavity is empty, i.e., essentially free of solvent (16). At sufficiently high pressure, however (≈200–300 MPa), the cavity appears to be occupied by two to four water molecules (23, 24). This behavior is also suggested by theory (23). The question as to whether apolar cavities in proteins are empty at ambient pressure and temperature has, however, remained controversial. In particular, it is not easy to exclude the possibility that solvent molecules might occupy apolar cavities but, in the absence of hydrogen bonding, be highly mobile and difficult to detect by using standard crystallographic structure determination.
To address this question, we have attempted to obtain an accurate electron density map of the L99A cavity that is based strictly on experimentally determined phase angles. By using a selenomethionine-substituted protein and measuring high-resolution data at multiple wavelengths, it is, in principle, possible to obtain such a high-quality electron density map (26).
Pseudowild-type T4 lysozyme contains five methionines. One of these (Met-102) is located in the wall of the L99A cavity. It was replaced by leucine because an electron-dense selenium atom close to the cavity might perturb the electron density in the vicinity. It has previously been shown that the M102L mutant has structure and stability fairly close to that of the wild type (27).
The selenomethionine-containing L99A/M102L mutant was constructed and purified (see Methods). It crystallized isomorphously with the wild type. To minimize possible systematic variations and to optimize phase determination to the highest possible resolution, all of the data at four wavelengths were collected at 100 K from a single rod-shaped crystal measuring 0.6 × 0.4 × 0.4 mm. The crystal was mounted with the longest dimension parallel to the spindle axis and translated when there was apparent radiation decay. Data to 1.20-Å resolution were collected but truncated at 1.25 Å to maintain a high level of data completeness (Table 1). Care was taken to ensure that all of the low-resolution reflections were included (see Methods).
Table 1.
X-ray data collection statistics for T4 lysozyme M102L/L99A mutant
| Dataset | λ, Å | Resolution, Å | Redundancy | Completeness, % | I/σ(I) | Rmerge, % |
|---|---|---|---|---|---|---|
| Peak | 0.97958 | ∞-1.25 (1.27–1.25) | 9.5 (7.2) | 99.9 (100) | 21.0 (7.4) | 12.2 (35.5) |
| Inflection | 0.97970 | ∞-1.25 (1.27–1.25) | 9.6 (7.2) | 99.9 (100) | 23.2 (13.9) | 11.5 (21.4) |
| High | 0.97420 | ∞-1.25 (1.27–1.25) | 9.7 (7.2) | 99.9 (100) | 21.2 (12.6) | 13.1 (21.3) |
| Low | 0.98060 | ∞-1.25 (1.27–1.25) | 9.6 (7.2) | 99.9 (100) | 32.2 (9.9) | 9.5 (26.6) |
Data in parentheses refer to the highest-resolution shell. Redundancy and completeness were calculated with each reflection of the Friedel pairs treated separately.
Structure factor analysis (Table 2) indicated strong anomalous signals for the “peak” and “high” datasets in particular. The “inflection” dataset was used as the “native” structure amplitudes, and “isomorphous” differences were calculated relative to these. Combining all four datasets, the figure of merit (FOM) to 1.25-Å resolution was 0.81 (Table 3).
Table 2.
Structure factor statistics for anomalous and isomorphous differences
| Dataset | Peak, % | Inflection, % | High, % | Low, % |
|---|---|---|---|---|
| Peak | 10.45 | 5.22 | 6.37 | 5.57 |
| Inflection | 5.90 | 6.55 | 5.62 | |
| High | 6.74 | 5.12 | ||
| Low | 2.36 |
The values in italics along the diagonal give the average anomalous difference (i.e. the difference between Friedel pairs) for each of the datasets collected at different wavelengths. The values off the diagonal give the average isomorphous difference for the same reflection measured at two different wavelengths.
Table 3.
X-ray phasing statistics to 1.25-Å resolution
| Dataset | Phasing power |
Figure of merit |
||
|---|---|---|---|---|
| Isomorphous | Anomalous | Isomorphous | Anomalous | |
| Peak | 1.54 | 2.75 | 0.43 | 0.51 |
| Inflection | – | 1.91 | – | 0.38 |
| High | 1.23 | 2.05 | 0.25 | 0.39 |
| Low | 2.69 | 2.89 | 0.47 | 0.48 |
The isomorphous phasing power refers to phasing information obtained from the same reflection measured at different wavelengths. The anomalous phasing is derived from the Friedel-related reflections measured at a given wavelength. The overall figure of merit is 0.81.
Cavities in L99A/M102L Lysozyme.
The L99A/M102L lysozyme structure contains four cavities that appear to be large enough to accommodate one or more water molecules (Table 4). The first two cavities (1 and 2) are polar and contain ordered solvent. They serve as controls for the other two cavities (3 and 4), which are essentially nonpolar.
Table 4.
Electron density within cavities in T4 lysozyme L99A/M102L
| Cavity | Volume of cavity, Å3 | Resolution of density map*, Å | Experimental electron density within cavity |
Experimental integrated density within cavity (electrons) | Inferred water molecules in cavity | Model integrated density within cavity (electrons)† | |||
|---|---|---|---|---|---|---|---|---|---|
| Min, e/Å3 | Max, e/Å3 | Mean, e/Å3 | rmsd, e/Å3 | ||||||
| 1 | 45.2 | 1.25 | −0.52 | 4.37 | 0.36 | 0.71 | 16.1 | 2 | 16.8 (2) |
| 1.50 | −0.61 | 3.75 | 0.36 | 0.66 | 16.4 | ||||
| 2 | 16.9 | 1.25 | −0.54 | 3.85 | 0.46 | 0.73 | 7.7 | 1 | 8.0 (1) |
| 1.50 | −0.40 | 3.37 | 0.48 | 0.69 | 8.0 | ||||
| 3 | 21.4 | 1.25 | −0.94 | 0.91 | −0.04 | 0.35 | −0.8 | 0 | 0.7 (0) |
| 1.50 | −0.77 | 0.78 | −0.01 | 0.32 | −0.3 | ||||
| 4 | 133.5 | 1.25 | −0.71 | 1.05 | 0.12 | 0.28 | 16.2 | 1.5 | 3.7 (0) |
| 1.50 | −0.65 | 0.93 | 0.13 | 0.27 | 17.6 | ||||
*Phasing at 1.50-Å resolution was performed independently from that at 1.25 Å.
†The model calculations were to 1.25-Å resolution based on the structure factors and phases calculated from the refined structure of L99A/M102L. The value in parentheses is the number of water molecules in the cavity included in the model calculation.
Cavity 1 is located close to the interface between the N- and C-terminal domains of T4 lysozyme and is adjacent to the long α-helix that connects the two domains. It was seen in the original structure determination of the protein (28, 29) to contain two ordered solvent molecules, and these solvent molecules are also clearly seen in the present electron density map (Fig. 1A). There are a total of five hydrogen bonds between the two water molecules and the surrounding main-chain or backbone atoms of Gly-28, Ile-29, Ala-63, and Asp-70. The water molecules also hydrogen-bond to each other (2.8 Å). With the two water molecules removed, the calculated volume of the cavity is 45.2 Å3.
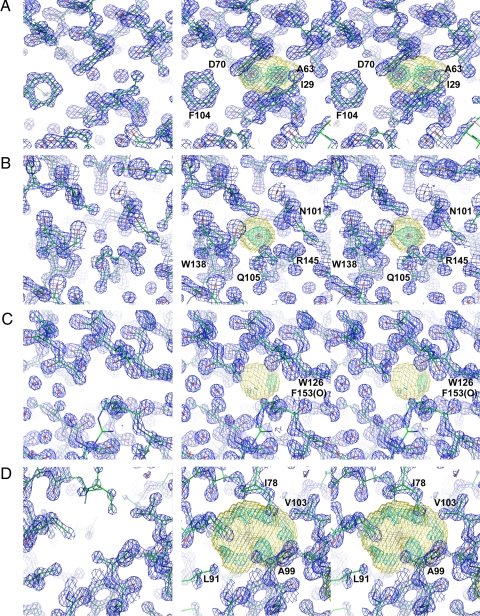
Fig. 1.
Electron density for hydrated and nonhydrated cavities in M102L/L99A lysozyme. (Left) The mono image shows the electron density corresponding to the crystallographically refined model. Coefficients are (2Fo−Fc)exp iαc, where Fc and αc are the amplitude and phase calculated from the refined model with solvent molecules included and marked with a red cross. The resolution is 1.25 Å, and the blue contours are drawn at 1.0e/Å3. (Right) The stereo pair shows the 1.25-Å resolution experimental electron density. Coefficients are mFo exp iα, where m is the figure of merit and α the experimental phase angle, and the map was scaled as described in Methods. The shape of the cavity is shown in yellow. Outside the cavity the electron density is contoured in blue at 1.0e/Å3 (the same as in Left). Inside the cavity the electron density is contoured in cyan at 0.4e/Å3 and in red at 3.0e/Å3. (A) Cavity 1 has two well ordered water molecules seen in conventional crystallographic refinement (Left) and in the experimentally phased map (Right). (B) Cavity 2 has a single well ordered water molecule. (C) Cavity 3 appears to lack solvent in conventional crystallographic refinement (Left) and also has essentially zero electron density in the experimentally phased map (Right). (D) Cavity 4. In the experimental map there is weak electron density distributed around the periphery of the disk-shaped cavity. One of the three highest peaks is 2.4 Å from the carbonyl oxygen of Ala-99, another is close to the β-carbon of Ala-99, and the third is within the elongated peak close to the side chain of Leu-91. This figure was prepared with PyMOL (43).
Cavity 2, the second polar cavity, contains a single well ordered water molecule (Fig. 1B). It has a volume of 16.9 Å3 and is surrounded by Asn-101, Leu-102, Gln-105, Trp-138, and Val-149. The principal interactions with the water molecule are with OE1 of Gln-105 (2.8 Å) and OD1 of Asn-101 (2.9 Å).
Cavity 3, the first nonpolar cavity, has a volume of 21.4 Å3 and is present in the wild-type lysozyme as well as the L99A/M102L mutant structure. It is located in the C-terminal domain and is surrounded by Trp-126, Ala-130, Phe-153, and Arg-154. Conventional crystallographic refinement (29) did not reveal any solvent within the cavity.
Cavity 4 is the larger nonpolar space created by the L99A mutation. It is surrounded by the nonpolar residues Leu-84, Val-87, Tyr-88, Leu-91, Ala-99, Val-203, Val-111, Leu-118, and Leu-102. In the original L99A mutant (16), residue 102 was methionine. In the present case, a leucine was substituted to avoid the presence of the electron-dense selenium atom. Methionine and leucine have side chains of similar volume and related shape, and Leu-102 in the L99A/M102L structure occupies essentially the same space as Met-102 in the original L99A mutant. Correspondingly, the overall size and shape of cavity 4 is similar. After refinement of the L99A/M102L structure with the present high-resolution data, the volume of the cavity was calculated to be 133.5 Å3, ≈10 Å3 larger than in L99A.
Electron Density Within Cavities.
Maps showing the electron density from the experimentally phased map in the vicinity of cavities 1–4 are shown in Fig. 1. The analysis of density within each cavity is summarized in Table 4. Table 4 includes results obtained for the electron density map calculated at 1.25-Å resolution and for a second map that was independently phased at 1.5-Å resolution (see Methods). In the following discussion, we will focus on the higher-resolution map while noting that the lower-resolution calculation gives similar results (Table 4).
Cavity 1 has two distinct electron density peaks with maxima of 4.37 electrons (e)/Å3 and 3.78 e/Å3, respectively. These correspond to the two previously identified water molecules. The minimum density of −0.52 e/Å3 is located on the van der Waals surface of the cavity. This is exactly as might be expected. (In the independently refined model for L99A/M102L, the B-factors for these water molecules are 8.5 Å2 and 9.9 Å2, respectively.) The integrated electron density is 16.1 e, which is somewhat less than the expected value of 20 e for two water molecules.
The results for cavity 2 are similar. There is a well defined peak of height 3.85 e/Å3 corresponding to the expected single water molecule. The minimum density (−0.54 e/Å3) is, again, on the van der Waals surface of the cavity. The integrated electron density (7.7 e) is, again, slightly less than that expected for a single fully occupied water site (10 e). In the refined model, there is a single water molecule at this site with a B-factor of 10.5 Å2.
Cavity 3, which is small and nonpolar, has electron density ranging from -0.94 to 0.91 e/Å3 and integrated electron density of -0.8 e. There is no distinct peak as in cavities 1 and 2, and the experimental evidence strongly suggests that there is no bound solvent in this cavity (Table 4).
Cavity 4 is quite large (volume 133.5 Å3) and essentially nonpolar. Its highest density is about a quarter that of the well ordered water molecules in cavities 1 and 2 (Table 4). This indicates that there are no high-occupancy well localized solvent molecules in this cavity. On the other hand, the integrated density is 16.2 e, which indicates that the cavity contains ≈1.5 water molecules, on average. Inspection of the electron density in the cavity (Fig. 1D) shows that the point of second-highest density (0.86 e/Å3) is within a broad peak and ≈2.4 Å from the carbonyl oxygen of Ala-99. Other peaks are distributed around the periphery of the cavity (see Discussion).
Discussion
Accuracy of the Estimation of Hydration.
Two different factors can introduce uncertainties into the present estimation of hydration within protein cavities. First, there are limitations in the accuracy of the experimental electron density map. The second limitation is due to possible “spillover” of electron density, either into or out of a given cavity (9). We discuss these below.
Accuracy of the electron density map.
In general terms, the experimental electron density map is expected to be of high quality. The x-ray data are >99.9% complete to high resolution (1.25 Å) (Table 1). Also, care was taken to include all low-order reflections. The average FOM (0.81) is also high (Table 3), indicating that the phase angles should be reliable.
The value of the zero-order structure amplitude F(000) cannot be measured experimentally. To determine whether the estimated value (see Methods) was appropriate, we determined the electron density in the region of the unit cell corresponding to the bulk solvent. Including only those parts of the unit cell that are at least 8 Å away from any atom of the protein, the average experimental electron density is 0.411 ± 0.254 e/Å3. This can be compared with the value of 0.391 e/Å3 based on chemical composition (see Methods). There is a discrepancy of 0.020 e/Å3. If we were to adjust F(000) to eliminate this discrepancy, the integrated density in the largest cavity would decrease by 133.5 × 0.020 = 2.7 e and in the smallest cavity by 0.3 e. These changes are modest and would not alter the main conclusions of this analysis.
In another test, we truncated the data to 1.5-Å resolution, rerefined the selenium sites, and calculated an electron density map at this resolution. In this map, which has ≈42% of the total reflections, the accuracy of the phases will be higher but the resolution lower. The analysis (Table 4) gives values very close to those for the 1.25-Å resolution map.
Uncertainties due to “spillover” of electron density.
The volume occupied by a single molecule in bulk water is 29.9 Å3. In contrast, the calculated volume for cavity 2, which contains a single well ordered water molecule, is 16.9 Å3 (Table 4), which is far less. How can a water molecule fit into such a small cavity? The discrepancy occurs because cavity volume is determined by placing a probe atom inside the cavity and calculating the region where it can make van der Waals contacts with atoms in the walls of the cavity. The water molecule in cavity 2, however, makes two hydrogen bonds (2.8 and 2.9 Å) to surrounding atoms, approaches that are less than the allowed van der Waals contacts. Under these circumstances, some of the 10 electrons associated with the water molecule will spill over outside the calculated boundaries of the cavity. It is presumably for this reason that the value of 7.7 e for the integrated electron density in cavity 2 (Table 4) is less than the expected value of 10 e. It is also possible, however, that the electron density could spill into the cavity from surrounding atoms. To investigate this possibility, we carried out a model calculation at 1.25-Å resolution based on the structure factors and phases from the refined structure of the L99A/M102L mutant, with a single water molecule (B = 10.5 Å2) in cavity 2. This model calculation has integrated electron density in cavity 2 of 8.0 e (Table 4) in remarkably good agreement with the experimental value of 7.7 e. Thus, both the experimental and model calculations suggest that the occupancy of the water in cavity 2 is 100% and that the electron density for two of the 10 electrons is outside the boundaries of the 16.9-Å3 region.
A similar situation occurs for cavity 1, in which two water molecules (total volume 59.8 Å3) occupy a cavity with calculated volume of 45.2 Å3. There are multiple hydrogen bonds between the water molecules and surrounding polar atoms. In this case, the integrated electron density in the cavity is 16.1 e, somewhat less than the expected value of 20 e for two water molecules. A model calculation, as above, with two water molecules in the cavity (B values of 8.5 and 9.9 Å2) gave integrated density of 16.8 e, again in good agreement with the experimental value of 16.1 e. As for cavity 1, there appears to be spillover of density from the inside to the outside of the cavity.
In cases where a cavity appears to be empty, or nearly so, it is likely that there may be some net spillover of electron density into the cavity. This possibility for cavities 3 and 4 was explored by model calculation, as above, in which no water molecules were located in these cavities. Because no water molecule is in the cavity, the respective values of 0.7 and 3.7 e (Table 4) give the estimated spillover of outside density into cavity 3 and cavity 4. For cavity 3, the amount is negligible. For cavity 4, it could be that the experimental value of 16.2 e for the density in the cavity includes spillover density of up to three to four electrons.
Overall Conclusions.
The experimental electron density map clearly shows that the expected water molecules bind and are well localized within the two interior polar cavities (cavities 1 and 2) in T4 lysozyme. These results serve, in part, as internal controls for the overall experiment.
The small nonpolar cavity (cavity 3) is clearly empty. It might be noted that, under pressure, this cavity will bind xenon, which has a volume of 45.3 Å3 (19). There is no evidence that a major change in conformation is required to allow the ligand to reach the binding site (22). Halle and coworkers (30–32) have also shown that exchange of water at internal sites in proteins is rapid.
The results for the larger nonpolar cavity (cavity 4) are more interesting and were, at first, unexpected. In this case, the experimental map indicates that there is electron density corresponding to ≈1.5 water molecules in the cavity. The density is quite diffuse, indicating that these solvent molecules are not well ordered. One of the peaks in the electron density is at hydrogen-bonding distance from the carbonyl oxygen of Ala-99. Ala-99 is within the α-helix that extends from residues 93 to 106 and runs through the interior of the C-terminal domain. The L99A mutation truncates the leucine side chain and, in effect, exposes the adjacent carbonyl oxygen. Although this carbonyl group continues to receive a hydrogen bond from the backbone amide of Val-103 in the next turn of the helix, it is also available to accept a hydrogen bond from solvent. Clearly the cavity is not strictly nonpolar, and the bound water molecule(s) have an apparent preference for the more hydrophilic region.
The cavity is disk-like rather than spherical in shape, and the electron density is concentrated around the perimeter (Fig. 1D), suggesting that this is where the solvent is preferentially located. This is in contrast to pressure-induced binding by xenon and by water, in which cases the preferred binding sites are essentially in the center of the cavity (19, 23). These two studies were, however, at room temperature, whereas the present is at 100 K. The three strongest electron density peaks in the L99A/M102L cavity have approximately equal height (0.85–1.05 e/Å3) and correspond to the three peaks that are located across the “bottom” of the cavity in Fig. 1D. The right-most peak is the one that is 2.4 Å from the carbonyl oxygen of Ala-99. The central electron density peak in the cavity (Fig. 1D) is 2.8 Å from the first. The left-most of the three peaks is elongated, extending ≈1.6–3.7 Å from the central peak. Taken together, the peaks suggest a string of three water molecules, hydrogen-bonded to each other and to the carbonyl oxygen of Ala-99. If a single water molecule were to enter the cavity, it could be expected to move about rather freely but to preferentially hydrogen-bond to the carbonyl oxygen of Ala-99. A second water would also be rather mobile but might preferentially hydrogen-bond to the first, and so on. Under this scenario, the occupancy would be highest for the first binding site and successively lower for the second and third sites. This is not observed. Rather, the electron density is roughly the same for all three sites, with the hypothetical second site being highest. This could suggest that the three water molecules enter the cavity together (i.e., as a hydrogen-bonded cluster).
It might also be noted that in the present experiment, the x-ray data were collected at 100 K. Because the crystals were flash- frozen, it is possible that the experiment traps any water molecules that happen to be in the cavity at room temperature. On the other hand, molecular dynamics simulations suggest that water molecules can escape from the cavity in less than a picosecond (24) whereas the estimated time taken to freeze a crystal of the size used here is on the order of a second (33). Assuming that equilibrium is established, the entropy cost of localizing solvent molecules at 100 K will be less than at room temperature. In any event, any solvent molecules will presumably be less mobile and better located than at higher temperatures.
Vaitheeswaran et al. (33) have argued that water molecules will only bind stably within hydrophobic cavities if the cavity is large enough to admit enough water molecules to form multiple hydrogen bonds to each other. That a single water molecule will not occupy a hydrophobic cavity is clearly supported by the lack of electron density in cavity 3. The minimum number of water molecules required for a stable cluster is not yet clear, and presumably depends on the geometry, size, and chemical composition of the cavity wall (34). For the L99A/M102L mutant of T4 lysozyme, the size of the engineered cavity is, in principle, sufficient to accommodate 4.4 water molecules. Also, the cavity walls have a limited amount of polar character, which presumably promotes solvent retention to some degree. Nevertheless, the cavity does not contain 4.4 water molecules, suggesting that the minimum number of water molecules to form a stable cluster in a strictly hydrophobic environment may be five or more.
Methods
Protein Expression, Purification, and Crystallization.
The starting point for the experiments described here was cysteine-free pseudowild-type T4 lysozyme (35) with the cavity-containing mutation L99A (16). This protein contains five methionines, one of which (Met-102) is adjacent to the L99A cavity. To avoid possible artifacts in the electron density due to a selenomethionine close to the cavity, Met-102 was replaced by leucine. The protein was prepared by using the feedback inhibitor method (36). This mutant, which was used for all subsequent experiments, is identified as L99A/M102L.
Purification of the mutant protein followed the standard method for T4 lysozymes (37) with 15 mM l-methionine added to the buffers at each step to prevent Se-Met from oxidation. The protein was concentrated to 25 mg/ml in the harvesting buffer (50 mM sodium phosphate (pH 6.2), 0.55 M sodium chloride, 0.02% sodium azide, and 15 mM l-methionine) and used for crystallization experiments.
The standard vapor-diffusion hanging-drop method was used to crystallize the mutant protein. The reservoir wells were filled with 2.0 M sodium-potassium phosphate at pH 6.5 with both 50 mM 2-mercaptoethanol and 50 mM hydroxylethyl disulfide. The crystallization drops were mixed from 5 μl of protein solution and 5 μl of reservoir solution. Crystals were grown at 4°C in the dark for ≈3 weeks to reach their maximum dimension. Crystals of regular shape and of size 0.6 × 0.4 × 0.4 mm were selected for data collection.
Data Collection and Processing.
Diffraction data were recorded on a Quantum 4 CCD detector (Area Detection Systems Corporation) at beamline 8.2.1 of the Advanced Light Source, Lawrence Berkeley National Laboratory, Berkeley, CA. Diffraction data were collected at 100 K, and crystals were cryoprotected by paraton-N (Hampton Research).
Data were measured at four wavelengths (Table 1). At each wavelength, one sweep of data was collected for low-resolution (∞-2.0 Å) reflections and another for high-resolution (∞-1.25 Å) with exposure times of 1 and 5 s, respectively. [The (1, 0, 0) reflection corresponds to a resolution of 52.2 Å.] Each sweep consisted of images with oscillation of 1° per image. To reduce the systematic error in the measurement of anomalous differences within each sweep, the inverse-beam strategy was used for each 20° wedge. Diffraction data were integrated and reduced with HKL2000 (38). Statistics are listed in Table 1. The crystals are isomorphous with the wild type, with space group P3221 and cell dimensions a = b = 60.23 Å, c = 96.68 Å. Datasets at the same four wavelengths were collected for two different crystals, A and B. The data for crystal A were to a higher resolution and substantially superior to those for crystal B. These were therefore used for the present analysis. Dataset A, however, did not include the low-resolution reflections (0, 0, 3), (1, 0, 2), and (0, 0, 6), although these were included in dataset B. We therefore scaled the two datasets together and transferred the data for these three missing reflections. Because the cell dimensions for the two datasets did not differ by >0.10 Å, and because these are low-resolution reflections, there should be essentially no error due to nonisomorphism. Overall, ≈100 reflections remained absent, none lower than 10-Å resolution.
Experimental Phasing.
The four selenium sites in L99A/M102L (SeMet1, SeMet6, SeMet106, and SeMet120) were located by using SHELXD (39) and confirmed with anomalous Patterson maps (data not shown). The refinement of the selenium positions was executed with CNS (26). Use of the high-resolution (1.25 Å) data showed that SeMet1 occupied two subsites 1.4 Å apart. (These two sites correspond to alternative conformations of the SeMet1 side chain.) Among the four x-ray datasets (Table 1), the one collected at the inflection wavelength was used as “native” because it has the lowest f′ contribution from the Se atoms. The structure factor statistics generated by CNS (26) are shown in Table 2. Based on the refined selenium positions and the x-ray data (Tables 1 and 2) the phases for the protein were then calculated (40). To avoid introduction of any model-related artifacts, no phase- or density-modification techniques such as solvent flattening were performed. The statistics for the experimental phases are shown in Table 3.
Electron Density Analysis.
For the purpose of model calculations and to define the location and size of the different cavities, the L99A/M102L structure was independently refined with Refmac within CCP4 (39). In this refinement, the cell constants and structure amplitudes measured at the inflection wavelength were used. The crystallographic R and Rfree were 15.5% and 17.3%, respectively. The protein structure, with all water and solvent molecules removed, was then used for cavity calculation by MSP version 3.7 (41). The van der Waals radii for hydroxyl and carbonyl oxygen was 1.54 Å, for carboxyl oxygen was 1.45 Å, and for peptide nitrogen was 1.57 Å (9). The map was generated on a 0.2-Å grid and analyzed by using SLICED (9). The procedure for calculation of electron density within cavities was as described by Quillin et al. (9).
In principle, F (000) is given by the total number of electrons in the unit cell, but in practice, one has to allow for the fact that the FOM is <100%, and also a small fraction of the structure amplitudes could not be measured (Table 1). We therefore proceeded as follows. First, we note that the unit cell volume (303,700 Å3) is made up of three components; (i) the volume occupied by the protein, (ii) a region of “bound water” surrounding the protein, and (iii) the remaining space between the protein molecules that is occupied by the mother liquor with which the protein crystals are equilibrated. The protein molecules occupy 45.6% of the unit cell volume. The bound water surrounding these protein molecules was assumed to correspond to 25% of the mass of the protein (ref. 42 and references therein). This corresponds to 15.3% of the unit cell volume, leaving 39.1% of the cell occupied by bulk solvent. The bulk solvent (measured density 1.20 g/cc) consists of 1.0 M NaH2PO4, 1.0 M K2HPO4, and 50.33 M H2O. Its average electron density is 0.391 e/Å3. Thus, the overall value of F (000) is 121,700 e, comprising 59,700 e from the protein, 15,580 e from the bound water, and 46,400 e from the bulk solvent.
For the electron density map to 1.25-Å resolution, the mean FOM was 0.81, and the completeness of data included in the calculation was 99.9%. Therefore, to bring the map to an absolute scale, we multiplied all of the reflections except F (000) by 1/(0.81 × 0.999). For the map to 1.5-Å resolution, the scaling factor was 1/(0.85 × 0.999).
Acknowledgments.
We thank Andy Fields for protein preparation and crystallization; Drs. Corie Ralston and Nathan Smith for help with data collection at Beamline 8.2.1 at the Advanced Light Source, Berkeley, CA; and Marcus Collins for comments on the manuscript. This work was supported in part by National Institutes of Health Grant GM21967 (to B.W.M.).
Footnotes
The authors declare no conflict of interest.
Data Deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.pdb.org (PDB ID Code 3DKE).
References
- 1.Rashin AA, Iofin M, Honig B. Internal cavities and buried waters in globular proteins. Biochemistry. 1986;25:3619–3625. doi: 10.1021/bi00360a021. [DOI] [PubMed] [Google Scholar]
- 2.Williams MA, Goodfellow JM, Thornton JM. Buried waters and internal cavities in monomeric proteins. Protein Sci. 1994;3:1224–1235. doi: 10.1002/pro.5560030808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levitt M, Park BH. Water: Now you see it, now you don't. Structure (London) 1993;1:223–226. doi: 10.1016/0969-2126(93)90011-5. [DOI] [PubMed] [Google Scholar]
- 4.Schoenborn BP, Garcia A, Knott R. Hydration in protein crystallography. Prog Biophys Mol Biol. 1995;64:105–119. doi: 10.1016/0079-6107(95)00012-7. [DOI] [PubMed] [Google Scholar]
- 5.Muller N. Does hydrophobic hydration destabilize protein native structures? Trends Biochem Sci. 1992;17:459–463. doi: 10.1016/0968-0004(92)90488-u. [DOI] [PubMed] [Google Scholar]
- 6.Ernst JA, Clubb RT, Zhou H-X, Gronenborn AM, Clore GM. Demonstration of positionally disordered water within a protein hydrophobic cavity by NMR. Science. 1995;267:1813–1817. doi: 10.1126/science.7892604. [DOI] [PubMed] [Google Scholar]
- 7.Matthews BW, Morton AG, Dahlquist FW. Use of NMR to detect water within nonpolar cavities. Science. 1995;270:1847–1849. doi: 10.1126/science.270.5243.1847. [DOI] [PubMed] [Google Scholar]
- 8.Yu B, Blaber M, Gronenborn AM, Clore GM, Caspar DLD. Disordered water within a hydrophobic protein cavity visualized by x-ray crystallography. Proc Natl Acad Sci USA. 1999;96:103–108. doi: 10.1073/pnas.96.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quillin ML, Wingfield PT, Matthews BW. Determination of solvent content in cavities in IL-1β using experimentally phased electron density. Proc Natl Acad Sci USA. 2006;103:18148–18153. doi: 10.1073/pnas.0609442104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roux B, Nina M, Pomes R, Smith JC. Thermodynamic stability of water molecules in the bacteriorhodopsin proton channel: a molecular dynamics free energy perturbation study. Biophys J. 1996;71:670–681. doi: 10.1016/S0006-3495(96)79267-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olano LR, Rick SW. Hydration free energies and entropies for water in protein interiors. J Am Chem Soc. 2004;126:7991–8000. doi: 10.1021/ja049701c. [DOI] [PubMed] [Google Scholar]
- 12.Wade RC, Mazor MH, McCammon JA, Quiocho FA. A molecular dynamics study of thermodynamic and structural aspects of the hydration of cavities in proteins. Biopolymers. 1991;31:919–931. doi: 10.1002/bip.360310802. [DOI] [PubMed] [Google Scholar]
- 13.Wolfenden R, Radzicka A. On the probability of finding a water molecule in a nonpolar cavity. Science. 1994;265:936–937. doi: 10.1126/science.8052849. [DOI] [PubMed] [Google Scholar]
- 14.Zhang L, Hermans J. Hydrophilicity of cavities in proteins. Proteins. 1996;24:433–438. doi: 10.1002/(SICI)1097-0134(199604)24:4<433::AID-PROT3>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 15.Burling FT, Weis WI, Flaherty KM, Brunger AT. Direct observation of protein solvation and discrete disorder with experimental crystallographic phases. Science. 1996;271:72–77. doi: 10.1126/science.271.5245.72. [DOI] [PubMed] [Google Scholar]
- 16.Eriksson AE, et al. Response of a protein structure to cavity-creating mutations and its relation to the hydrophobic effect. Science. 1992;255:178–183. doi: 10.1126/science.1553543. [DOI] [PubMed] [Google Scholar]
- 17.Eriksson AE, Baase WA, Wozniak JA, Matthews BW. A cavity-containing mutant of T4 lysozyme is stabilized by buried benzene. Nature. 1992;355:371–373. doi: 10.1038/355371a0. [DOI] [PubMed] [Google Scholar]
- 18.Morton A, Baase WA, Matthews BW. Energetic origins of specificity of ligand binding in an interior nonpolar cavity of T4 lysozyme. Biochemistry. 1995;34:8564–8575. doi: 10.1021/bi00027a006. [DOI] [PubMed] [Google Scholar]
- 19.Quillin ML, Breyer WA, Griswold IJ, Matthews BW. Size versus polarizability in protein–ligand interactions: Binding of noble gases within engineered cavities in phage T4 lysozyme. J Mol Biol. 2000;302:955–977. doi: 10.1006/jmbi.2000.4063. [DOI] [PubMed] [Google Scholar]
- 20.Mulder FAA, Hon B, Muhandiram DR, Dahlquist FW, Kay LE. Flexibility and ligand exchange in a buried cavity mutant of T4 lysozyme studied by multinuclear NMR. Biochemistry. 2000;39:12614–12622. doi: 10.1021/bi001351t. [DOI] [PubMed] [Google Scholar]
- 21.Mulder FAA, Hon B, Mittermaier A, Dahlquist FW, Kay LE. Slow internal dynamics in proteins: Application of NMR relaxation dispersion spectroscopy to methyl groups in a cavity mutant of T4 lysozyme. J Am Chem Soc. 2002;124:1443–1451. doi: 10.1021/ja0119806. [DOI] [PubMed] [Google Scholar]
- 22.Desvaux H, et al. Dynamics of xenon binding inside the hydrophobic cavity of pseudo-wild-type bacteriophage T4 lysozyme explored through xenon-based NMR spectroscopy. J Am Chem Soc. 2005;127:11676–11683. doi: 10.1021/ja053074p. [DOI] [PubMed] [Google Scholar]
- 23.Collins MD, Hummer G, Quillin ML, Matthews BW, Gruner SM. Cooperative water filling of a nonpolar protein cavity observed by high-pressure crystallography and simulation. Proc Natl Acad Sci USA. 2005;102:16668–16671. doi: 10.1073/pnas.0508224102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Collins MD, Quillin ML, Hummer G, Matthews BW, Gruner SM. Structural rigidity of a large cavity-containing protein revealed by high-pressure crystallography. J Mol Biol. 2007;367:752–763. doi: 10.1016/j.jmb.2006.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu J, Baase WA, Baldwin E, Matthews BW. The response of T4 lysozyme to large-to-small substitutions within the core and its relation to the hydrophobic effect. Protein Sci. 1998;7:158–177. doi: 10.1002/pro.5560070117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brunger AT, et al. Crystallography and NMR system (CNS): A new software system for macromolecular structure determination. Acta Crystallogr D. 1998;54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- 27.Hurley JH, Baase WA, Matthews BW. Design and structural analysis of alternative hydrophobic core packing arrangements in bacteriophage T4 lysozyme. J Mol Biol. 1992;224:1143–1159. doi: 10.1016/0022-2836(92)90475-y. [DOI] [PubMed] [Google Scholar]
- 28.Remington SJ, et al. Structure of lysozyme from bacteriophage T4: An electron density map at 2.4 Å resolution. J Mol Biol. 1978;118:81–98. doi: 10.1016/0022-2836(78)90245-0. [DOI] [PubMed] [Google Scholar]
- 29.Weaver LH, Matthews BW. Structure of bacteriophage T4 lysozyme refined at 1.7Å resolution. J Mol Biol. 1987;193:189–199. doi: 10.1016/0022-2836(87)90636-x. [DOI] [PubMed] [Google Scholar]
- 30.Denisov VP, Peters J, Hörlein HD, Halle B. Using buried water molecules to explore the energy landscape of proteins. Nat Struct Biol. 1996;3:505–509. doi: 10.1038/nsb0696-505. [DOI] [PubMed] [Google Scholar]
- 31.Wiesner S, Kurian E, Prendergast FG, Halle B. Water molecules in the binding cavity of intestinal fatty acid binding protein: Dynamic characterization by water 17O and 2H magnetic relaxation dispersion. J Mol Biol. 1999;286:233–246. doi: 10.1006/jmbi.1998.2490. [DOI] [PubMed] [Google Scholar]
- 32.Modig K, Rademacher M, Lucke C, Halle B. Water dynamics in the large cavity of three lipid-binding proteins monitored by 17O magnetic relaxation dispersion. J Mol Biol. 2003;332:965–977. doi: 10.1016/s0022-2836(03)00968-9. [DOI] [PubMed] [Google Scholar]
- 33.Kriminski S, Kazmierczak M, Thorne RE. Heat transfer from protein crystals: Implications for flash-cooling and X-ray beam heating. Acta Crystallogr D. 2003;59:697–708. doi: 10.1107/s0907444903002713. [DOI] [PubMed] [Google Scholar]
- 34.Vaitheeswaran S, Yin H, Rasaiah JC, Hummer G. Water clusters in nonpolar cavities. Proc Natl Acad Sci USA. 2004;101:17002–17005. doi: 10.1073/pnas.0407968101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsumura M, Matthews BW. Control of enzyme activity by an engineered disulfide bond. Science. 1989;243:792–794. doi: 10.1126/science.2916125. [DOI] [PubMed] [Google Scholar]
- 36.Van Duyne GD, Standaert RF, Karplus PA, Schreiber SL, Clardy J. Atomic structures of the human immunophilin FKBP-12 complexes with FK506 and rapamycin. J Mol Biol. 1993;229:105–124. doi: 10.1006/jmbi.1993.1012. [DOI] [PubMed] [Google Scholar]
- 37.Eriksson AE, Baase WA, Matthews BW. Similar hydrophobic replacements of Leu 99 and Phe 153 within the core of T4 lysozyme have different structural and thermodynamic consequences. J Mol Biol. 1993;229:747–769. doi: 10.1006/jmbi.1993.1077. [DOI] [PubMed] [Google Scholar]
- 38.Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 39.Uson I, Sheldrick GM. Advances in direct methods for protein crystallography. Curr Opin Struct Biol. 1999;9:643–648. doi: 10.1016/s0959-440x(99)00020-2. [DOI] [PubMed] [Google Scholar]
- 40.Collaborative Computational Project No. 4. The CCP4 suite: Programs for protein crystallography. Acta Crystallogr D. 1994;50:760–763. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- 41.Connolly ML. The molecular surface package. J Mol Graphics. 1993;11:139–141. doi: 10.1016/0263-7855(93)87010-3. [DOI] [PubMed] [Google Scholar]
- 42.Matthews BW. Determination of molecular weight from protein crystals. J Mol Biol. 1974;82:513–526. doi: 10.1016/0022-2836(74)90245-9. [DOI] [PubMed] [Google Scholar]
- 43.DeLano WL. The PyMOL Molecular Graphics System. San Carlos, CA.: DeLano Scientific; 2002. [Google Scholar]