Abstract
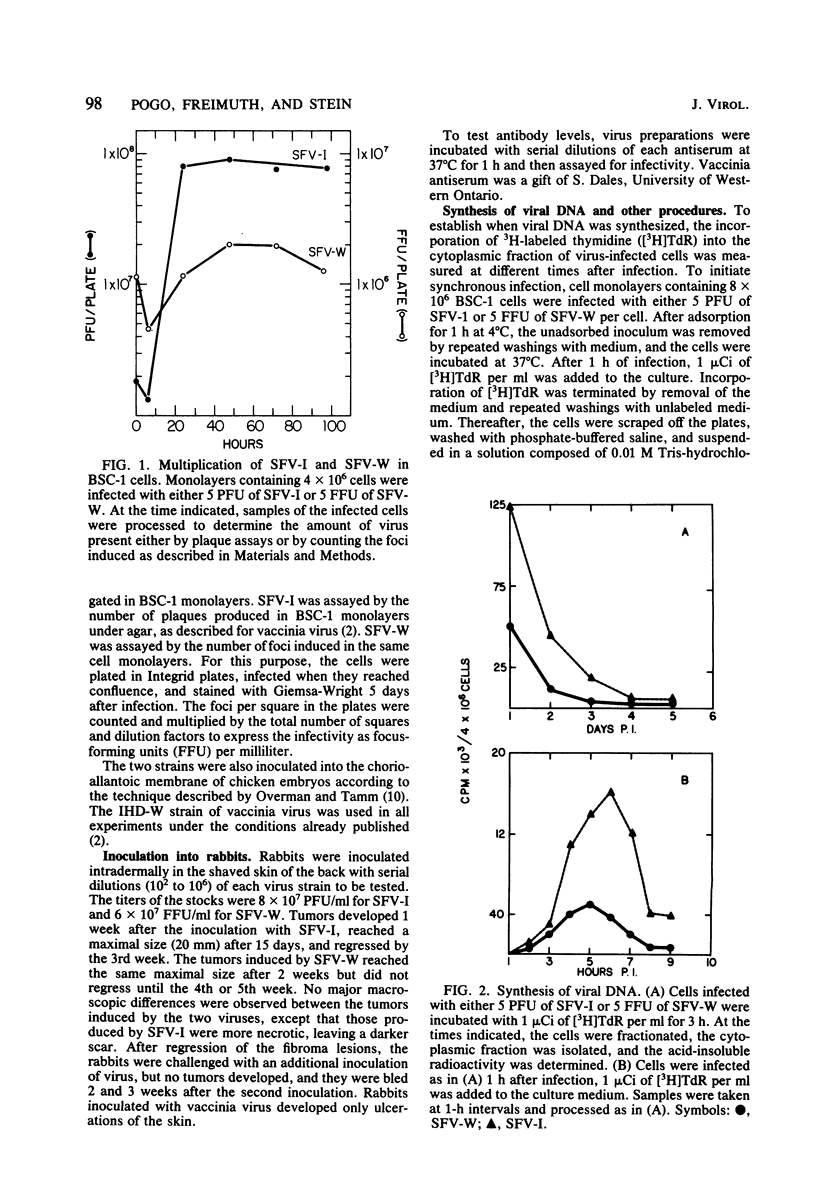
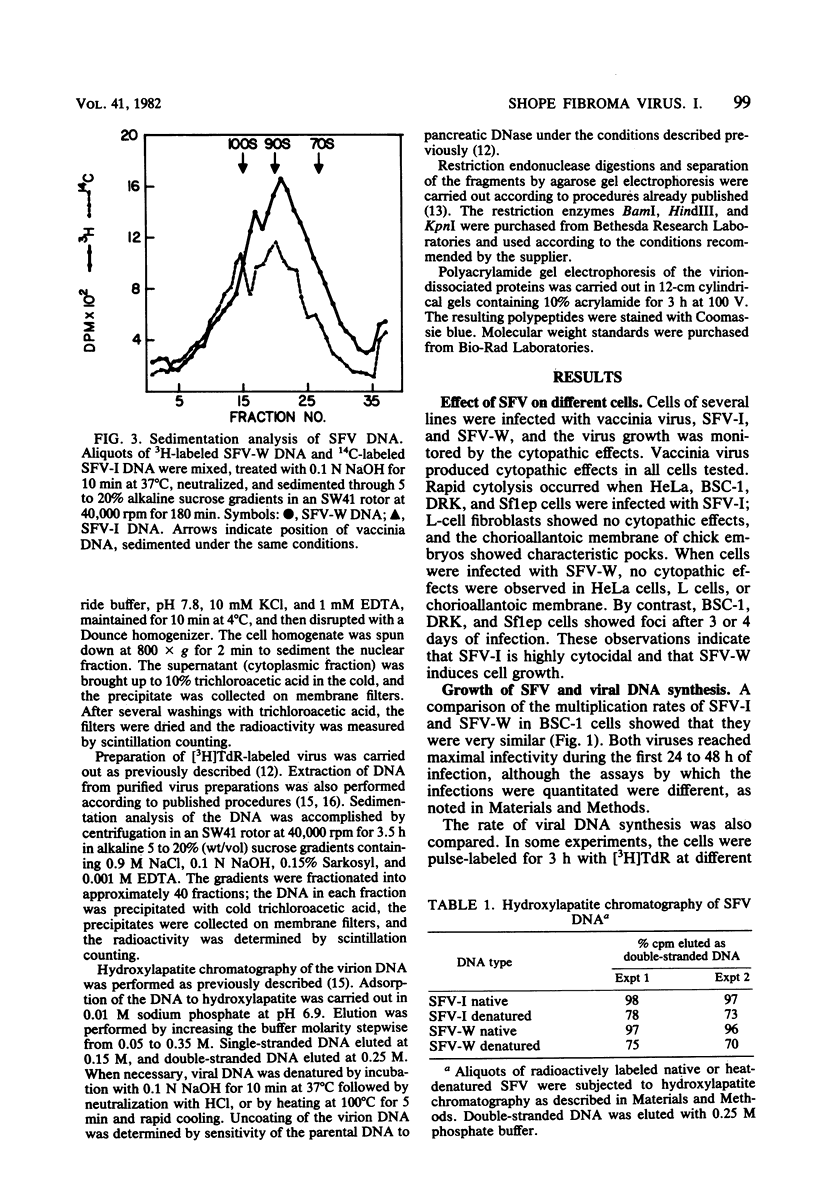
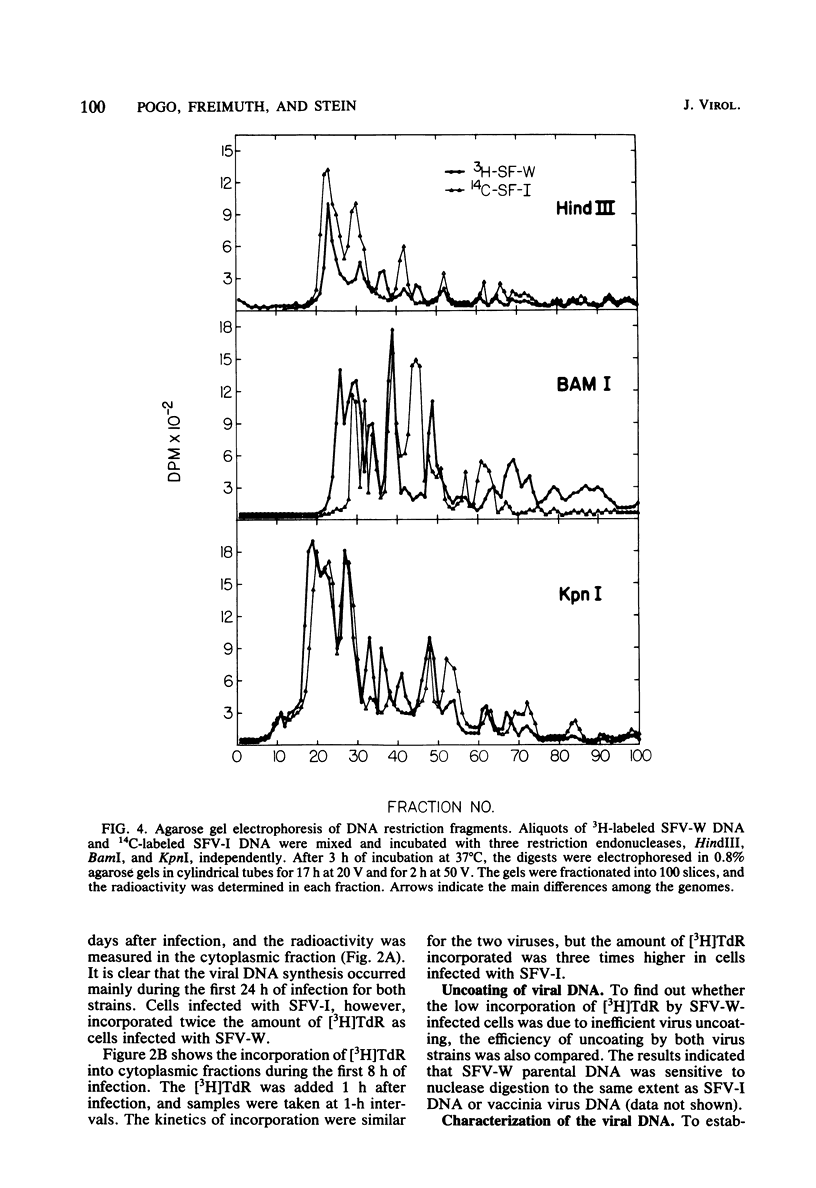
The biological and molecular properties of two strains of Shope fibroma virus (SFV) were compared. SFV-I was highly cytocidal to most of the cell lines tested and produced pocks in the chorioallantoic membrane of chick embryos. By contrast, SFV-W did not produce cytopathic effects in any of the cell lines or in the chorioallantoic membrane, but it induced characteristic foci 3 to 4 days after infection. Both strains produced tumors when inoculated into the skin of susceptible rabbits. Maximal infectivity in BSC-1 cells was reached by both strains between 24 to 48 h after inoculation. Viral DNA synthesis also took place at the same time, although cells infected with SFV-I incorporated three times more [3H]thymidine than cells infected with SFV-W. Sedimentation analysis and hydroxylapatite chromatography of the two viral DNAs indicated that their molecular weights were similar and that both were naturally cross-linked. Digestion with three restriction endonucleases, however, revealed that they had different restriction sites. When SFV-I and vaccinia DNA were compared, the restriction patterns were more alike. Analysis of the virion structural proteins by gel electrophoresis indicated that SFV-I, SFV-W, and vaccinia virus had many polypeptides in common, although there were distinctive differences among the three viruses. Finally, the results of plaque neutralization tests with different antisera showed that SFV-I and SFV-W shared common antigens and that vaccinia antiserum inhibited SFV-I but not SFV-W. We conclude that the SFV-I genome contains information for both cytolysis and tumorigenesis. This unusual virus may be a recombinant between an orthopoxvirus and a leporipoxvirus.
Full text
PDF
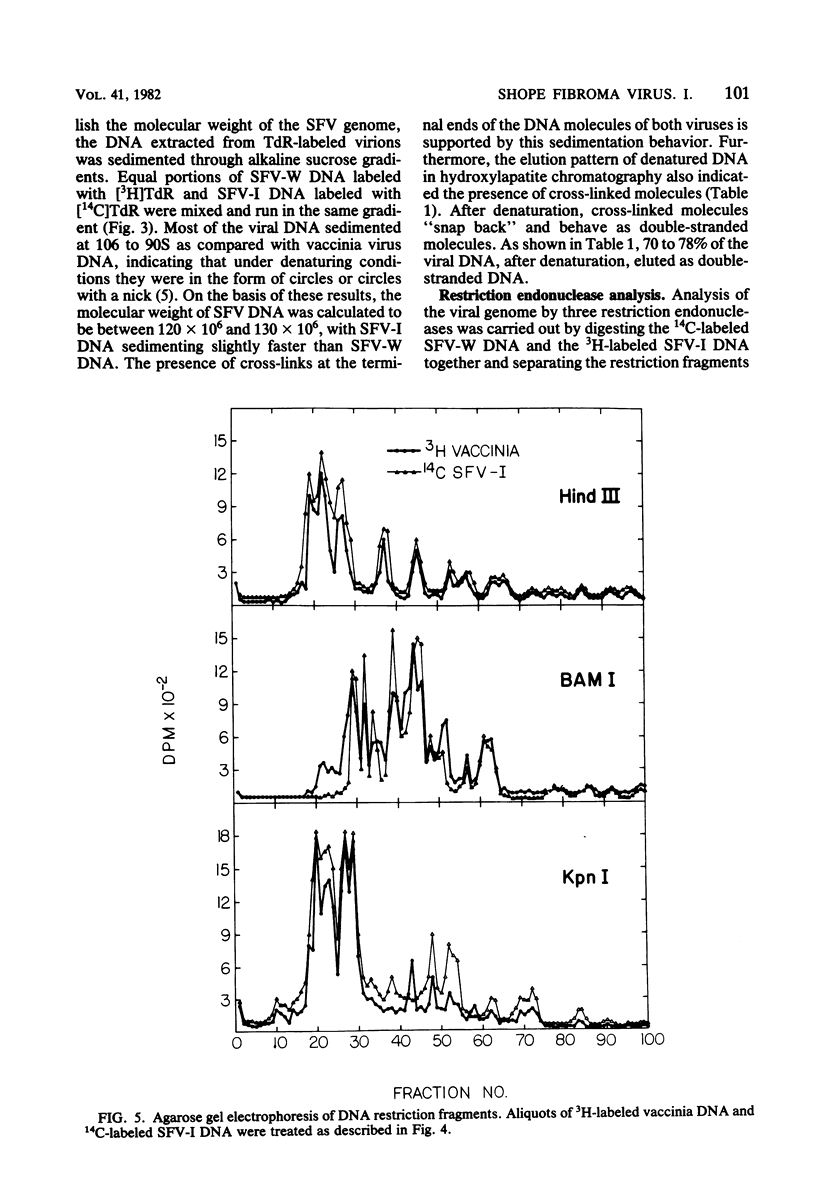
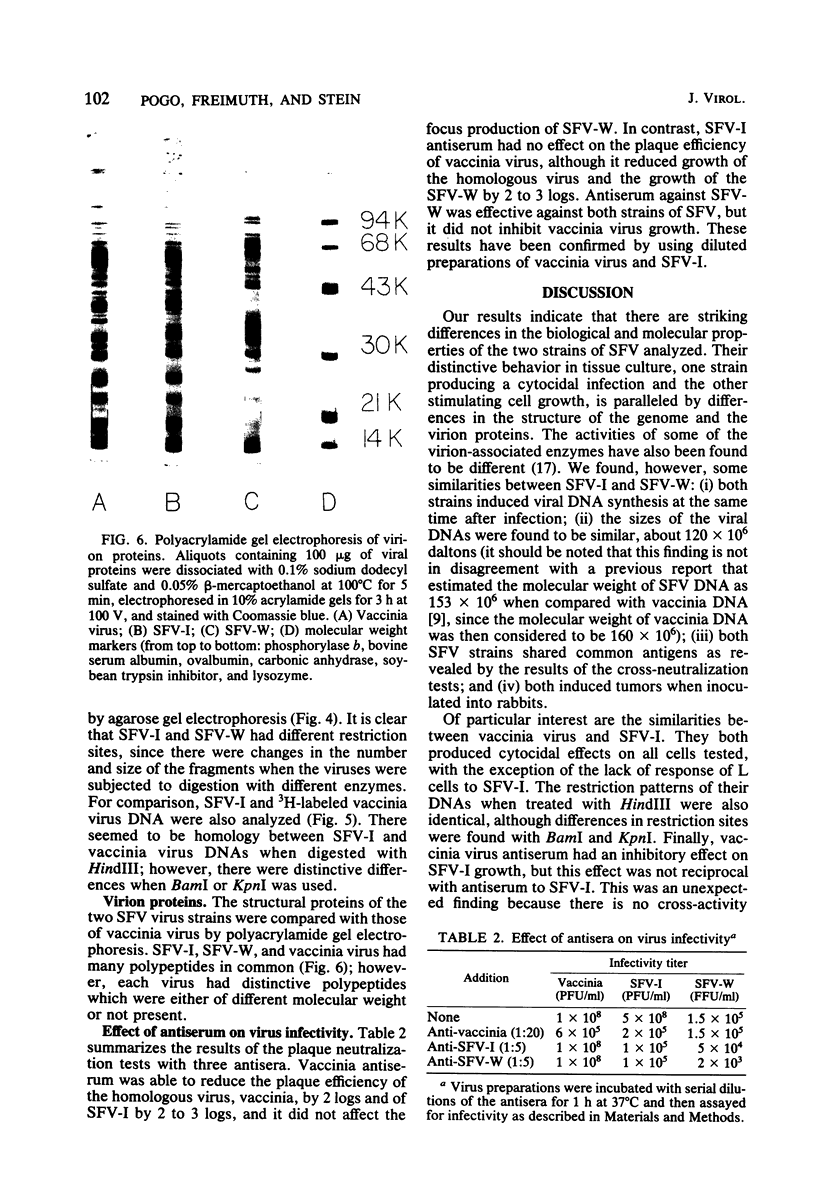

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DALES S. The uptake and development of vaccinia virus in strain L cells followed with labeled viral deoxyribonucleic acid. J Cell Biol. 1963 Jul;18:51–72. doi: 10.1083/jcb.18.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewton D., Hodes M. E. Nucleic acid synthesis in HeLa cells infected with Shope fibroma virus. Virology. 1967 Sep;33(1):77–83. doi: 10.1016/0042-6822(67)90095-5. [DOI] [PubMed] [Google Scholar]
- HINZE H. C., WALKER D. L. RESPONSE OF CULTURED RABBIT CELLS TO INFECTION WITH THE SHOPE FIBROMA VIRUS. I. PROLIFERATION AND MORPHOLOGICAL ALTERATION OF THE INFECTED CELLS. J Bacteriol. 1964 Oct;88:1185–1194. doi: 10.1128/jb.88.4.1185-1194.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinze H. C., Walker D. L. Comparison of cytocidal and noncytocidal strains of Shope rabbit fibroma virus. J Virol. 1971 May;7(5):577–581. doi: 10.1128/jvi.7.5.577-581.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemont B., Gazzolo L. Existence d'une synth'ese de DNA cellulaire liée à l'infection virale dans les cellules infectées par le virus du fibrome de Shope. C R Acad Sci Hebd Seances Acad Sci D. 1971 Jul 12;273(2):253–256. [PubMed] [Google Scholar]
- OVERMAN J. R., TAMM I. Multiplication of vaccinia virus in the chorioallantoic membrane in vitro. Virology. 1957 Feb;3(1):173–184. doi: 10.1016/0042-6822(57)90031-4. [DOI] [PubMed] [Google Scholar]
- Padgett B. L., Walker D. L. Effect of persistent fibroma virus infection on susceptibility of cells to other viruses. J Virol. 1970 Feb;5(2):199–204. doi: 10.1128/jvi.5.2.199-204.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogo B. G. Changes in parental vaccinia virus DNA after viral penetration into cells. Virology. 1980 Mar;101(2):520–524. doi: 10.1016/0042-6822(80)90466-3. [DOI] [PubMed] [Google Scholar]
- Pogo B. G., Dales S. Biogenesis of poxviruses: further evidence for inhibition of host and virus DNA synthesis by a component of the invading inoculum particle. Virology. 1974 Apr;58(2):377–386. doi: 10.1016/0042-6822(74)90073-7. [DOI] [PubMed] [Google Scholar]
- Pogo B. G. Elimination of naturally occurring crosslinks in vaccinia virus DNA after viral penetration into cells. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1739–1742. doi: 10.1073/pnas.74.4.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogo B. G., O'Shea M. T. The mode of replication of vaccinia virus DNA. Virology. 1978 Jan;84(1):1–8. doi: 10.1016/0042-6822(78)90213-1. [DOI] [PubMed] [Google Scholar]
- Pogo B. G., O'Shea M., Freimuth P. Initiation and termination of vaccinia virus DNA replication. Virology. 1981 Jan 15;108(1):241–248. doi: 10.1016/0042-6822(81)90543-2. [DOI] [PubMed] [Google Scholar]
- Pogo B. G., Stein A., Freimuth P. Shope fibroma virus. II. Role of the virion-associated nucleases. J Virol. 1982 Jan;41(1):104–109. doi: 10.1128/jvi.41.1.104-109.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompkins W. A., Walker D. L., Hinze H. C. Cellular deoxyribonucleic acid synthesis and loss of contact inhibition in irradiated and contact-inhibited cell cultures infected with fibroma virus. J Virol. 1969 Nov;4(5):603–609. doi: 10.1128/jvi.4.5.603-609.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- des Gouttes Olgiati D., Pogo B. G., Dales S. Biogenesis of vaccinia: specific inhibition of rapidly labeled host DNA in vaccinia inoculated cells. Virology. 1976 May;71(1):325–335. doi: 10.1016/0042-6822(76)90116-1. [DOI] [PubMed] [Google Scholar]