Abstract
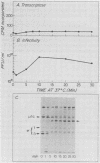
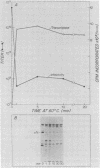
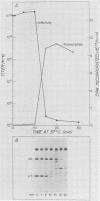
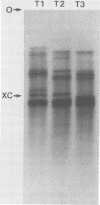
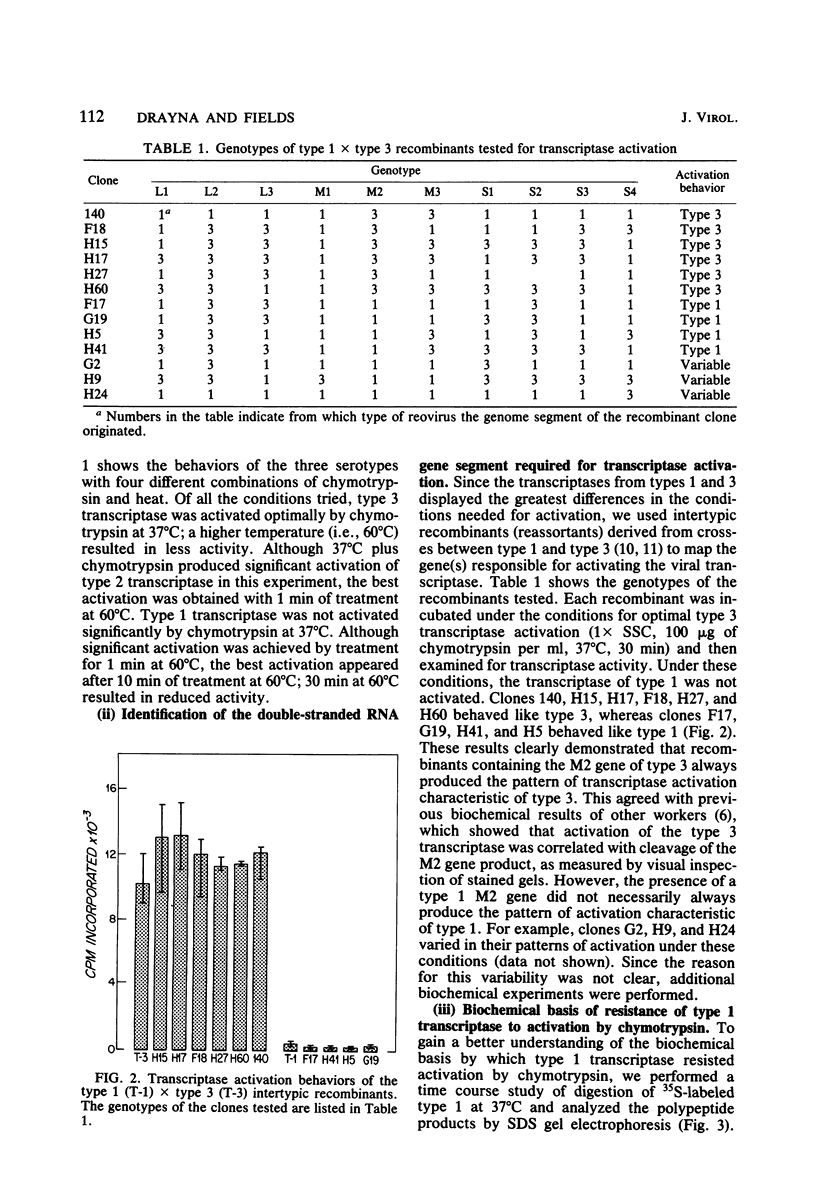
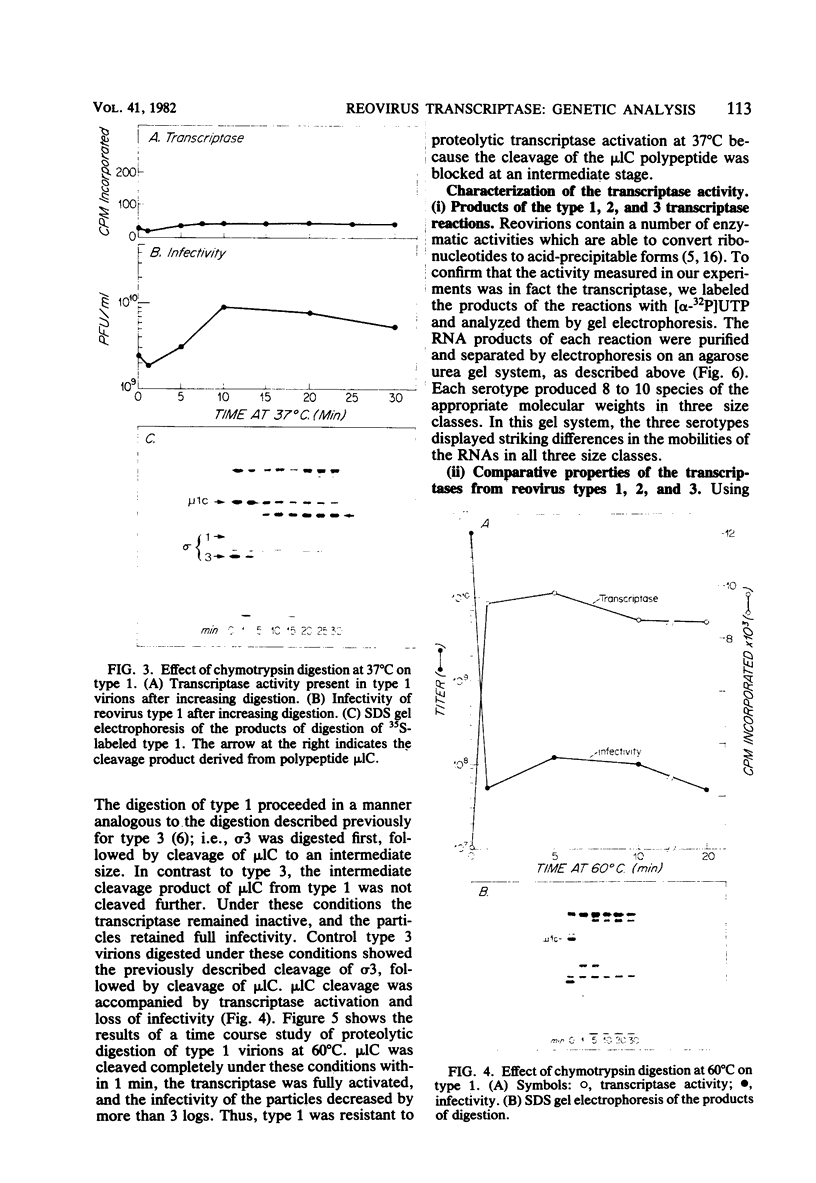
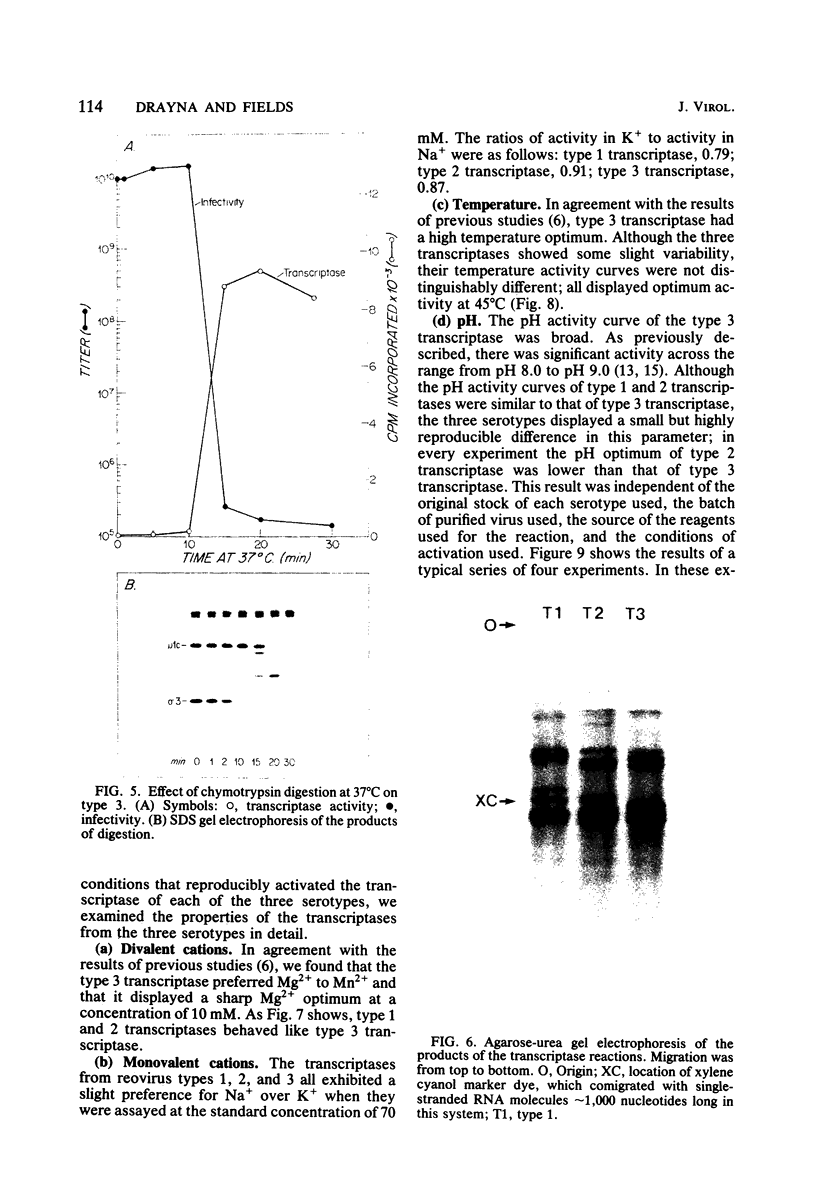
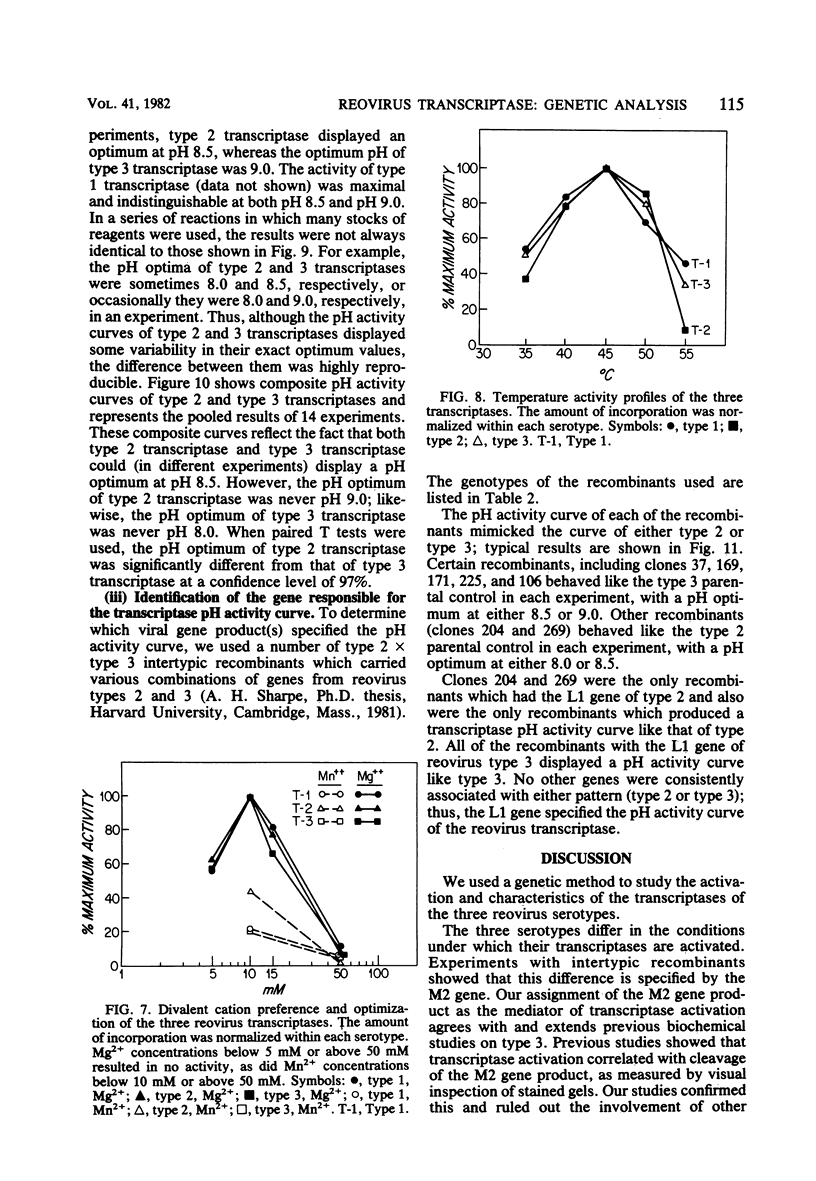
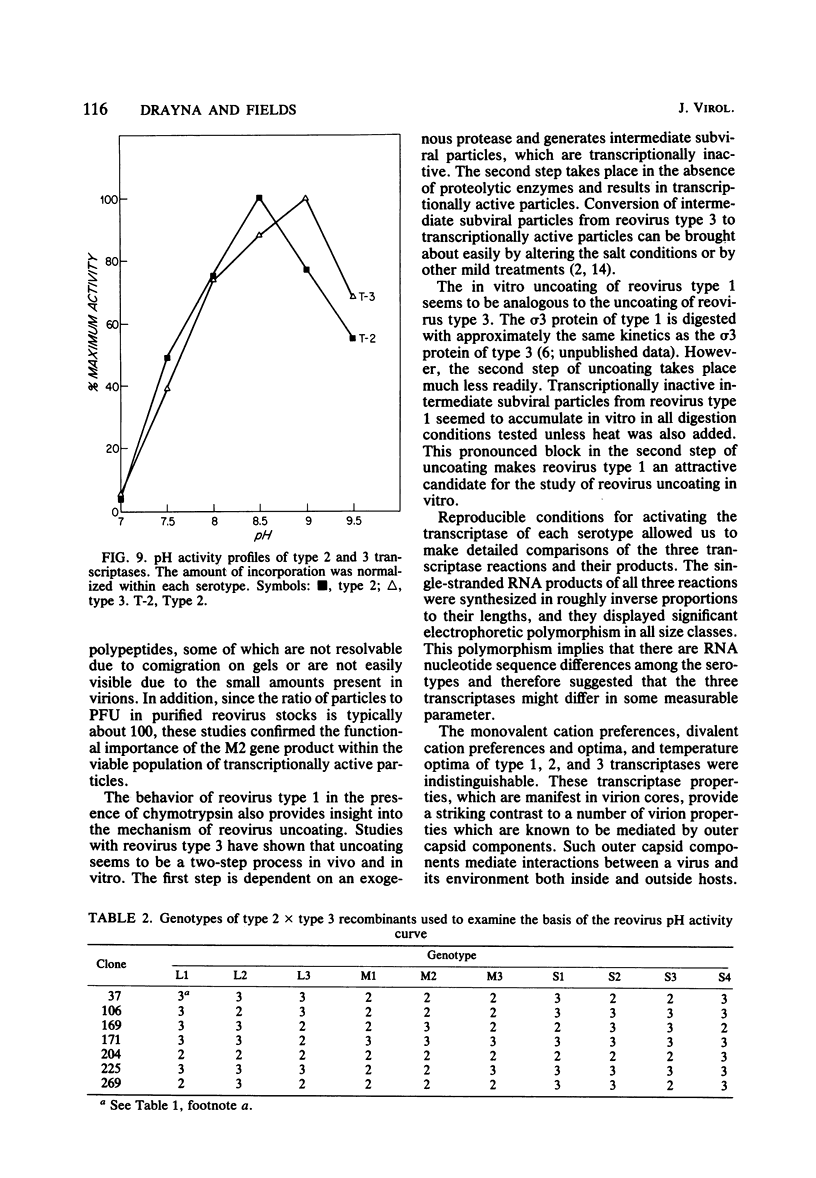
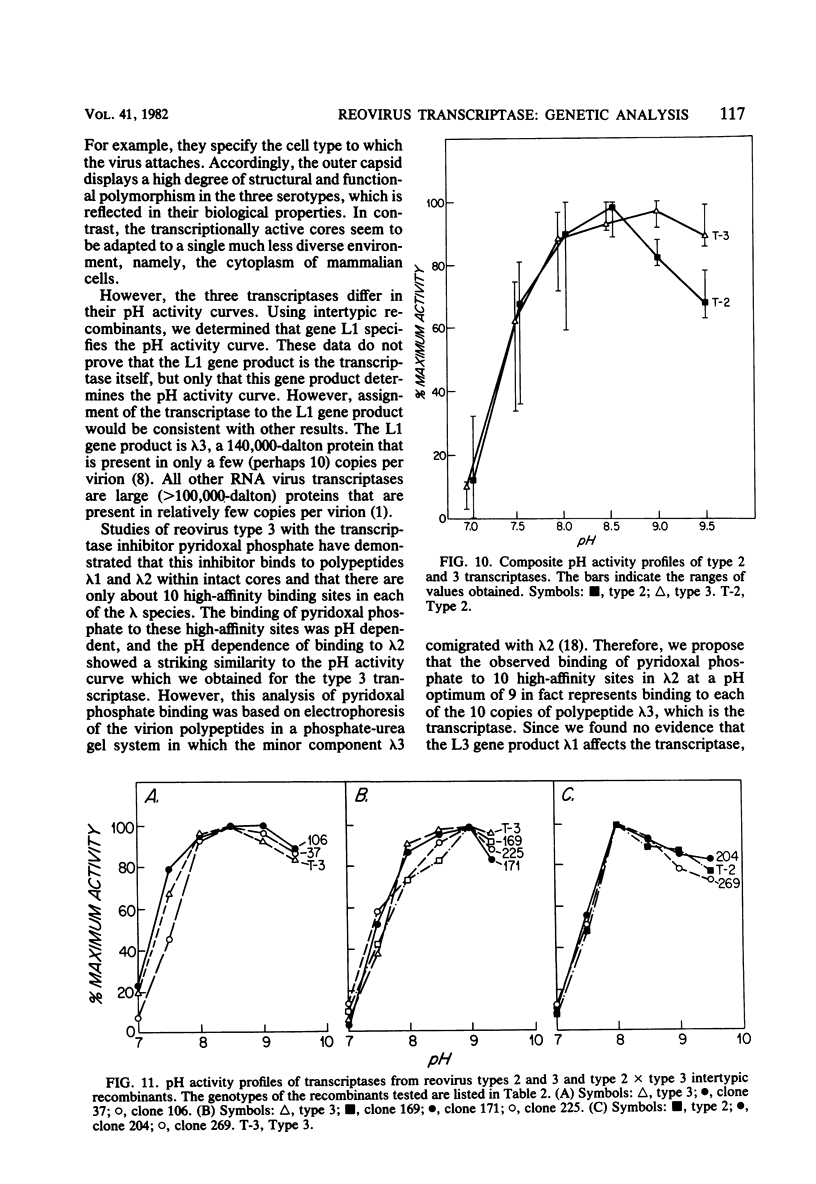
We studied the ability of chymotrypsin to activate the transcriptases of the three serotypes of reovirus. When we used conditions that reproducibly caused the activation of type 3 transcriptase by chymotrypsin alone, type 2 transcriptase was sometimes activated, and type 1 transcriptase was never activated. Using intertypic recombinants containing various combinations of genome segments from reovirus types 3 and 1, we showed that the M2 segment determined this difference. Biochemical experiments indicated that the digestion of reovirus type 1 by chromotrypsin was blocked at an intermediate stage in uncoating. We found conditions which reproducibly activated the transcriptases of all three serotypes. This allowed us to compare the biochemical properties of the three transcriptases. Although the monovalent cation preferences, divalent cation preferences and optima, and temperature optima of type 1, 2, and 3 transcriptases were indistinguishable, the pH activity curves were reproducibly different. The largest difference was between type 2 and 3 transcriptases; the pH optimum of type 2 transcriptase was lower than the pH optimum of type 3 transcriptase. Using intertypic recombinants containing various combinations of genome segments from reovirus types 2 and 3, we demonstrated that the L1 segment specified this difference.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borsa J., Graham A. F. Reovirus: RNA polymerase activity in purified virions. Biochem Biophys Res Commun. 1968 Dec 30;33(6):895–901. doi: 10.1016/0006-291x(68)90396-3. [DOI] [PubMed] [Google Scholar]
- Borsa J., Sargent M. D., Lievaart P. A., Copps T. P. Reovirus: evidence for a second step in the intracellular uncoating and transcriptase activation process. Virology. 1981 May;111(1):191–200. doi: 10.1016/0042-6822(81)90664-4. [DOI] [PubMed] [Google Scholar]
- Cross R. K., Fields B. N. Temperature-sensitive mutants of reovirus type 3: studies on the synthesis of viral RNA. Virology. 1972 Dec;50(3):799–809. doi: 10.1016/0042-6822(72)90434-5. [DOI] [PubMed] [Google Scholar]
- Furuichi Y., Morgan M., Muthukrishnan S., Shatkin A. J. Reovirus messenger RNA contains a methylated, blocked 5'-terminal structure: m-7G(5')ppp(5')G-MpCp-. Proc Natl Acad Sci U S A. 1975 Jan;72(1):362–366. doi: 10.1073/pnas.72.1.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joklik W. K. Studies on the effect of chymotrypsin on reovirions. Virology. 1972 Sep;49(3):700–715. doi: 10.1016/0042-6822(72)90527-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McCrae M. A., Joklik W. K. The nature of the polypeptide encoded by each of the 10 double-stranded RNA segments of reovirus type 3. Virology. 1978 Sep;89(2):578–593. doi: 10.1016/0042-6822(78)90199-x. [DOI] [PubMed] [Google Scholar]
- Morgan E. M., Kingsbury D. W. Pyridoxal phosphate as a probe of reovirus transcriptase. Biochemistry. 1980 Feb 5;19(3):484–489. doi: 10.1021/bi00544a014. [DOI] [PubMed] [Google Scholar]
- Mustoe T. A., Ramig R. F., Sharpe A. H., Fields B. N. A genetic map of reovirus. III. Assignment of the double-stranded RNA-positive mutant groups A, B, and G to genome segments. Virology. 1978 Apr;85(2):545–556. doi: 10.1016/0042-6822(78)90460-9. [DOI] [PubMed] [Google Scholar]
- Ramig R. F., Cross R. K., Fields B. N. Genome RNAs and polypeptides of reovirus serotypes 1, 2, and 3. J Virol. 1977 Jun;22(3):726–733. doi: 10.1128/jvi.22.3.726-733.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramig R. F., Mustoe T. A., Sharpe A. H., Fields B. N. A genetic map of reovirus. II. Assignment of the double-stranded RNA-negative mutant groups C, D, and E to genome segments. Virology. 1978 Apr;85(2):531–534. doi: 10.1016/0042-6822(78)90459-2. [DOI] [PubMed] [Google Scholar]
- Shatkin A. J., Sipe J. D. RNA polymerase activity in purified reoviruses. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1462–1469. doi: 10.1073/pnas.61.4.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein S. C., Astell C., Levin D. H., Schonberg M., Acs G. The mechanisms of reovirus uncoating and gene activation in vivo. Virology. 1972 Mar;47(3):797–806. doi: 10.1016/0042-6822(72)90571-5. [DOI] [PubMed] [Google Scholar]
- Skehel J. J., Joklik W. K. Studies on the in vitro transcription of reovirus RNA catalyzed by reovirus cores. Virology. 1969 Dec;39(4):822–831. doi: 10.1016/0042-6822(69)90019-1. [DOI] [PubMed] [Google Scholar]
- Stoltzfus C. M., Morgan M., Banerjee A. K., Shatkin A. J. Poly(A) polymerase activity in reovirus. J Virol. 1974 Jun;13(6):1338–1345. doi: 10.1128/jvi.13.6.1338-1345.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner H. L., Drayna D., Averill D. R., Jr, Fields B. N. Molecular basis of reovirus virulence: role of the S1 gene. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5744–5748. doi: 10.1073/pnas.74.12.5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White C. K., Zweerink H. J. Studies on the structure of reovirus cores: selective removal of polypeptide lambda 2. Virology. 1976 Mar;70(1):171–180. doi: 10.1016/0042-6822(76)90247-6. [DOI] [PubMed] [Google Scholar]