Abstract
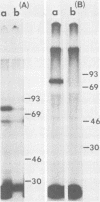
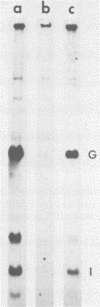
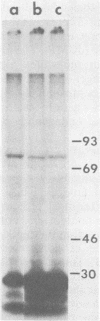
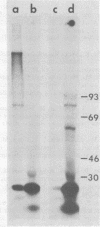
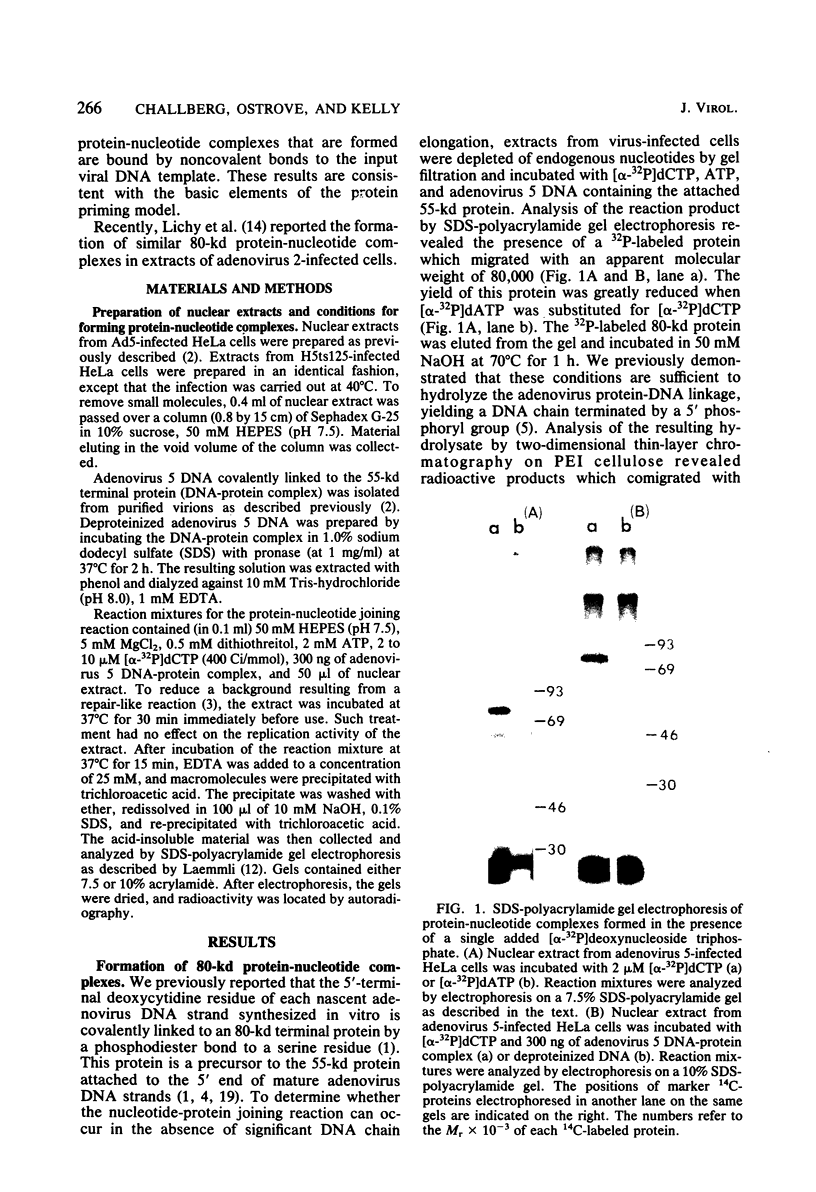
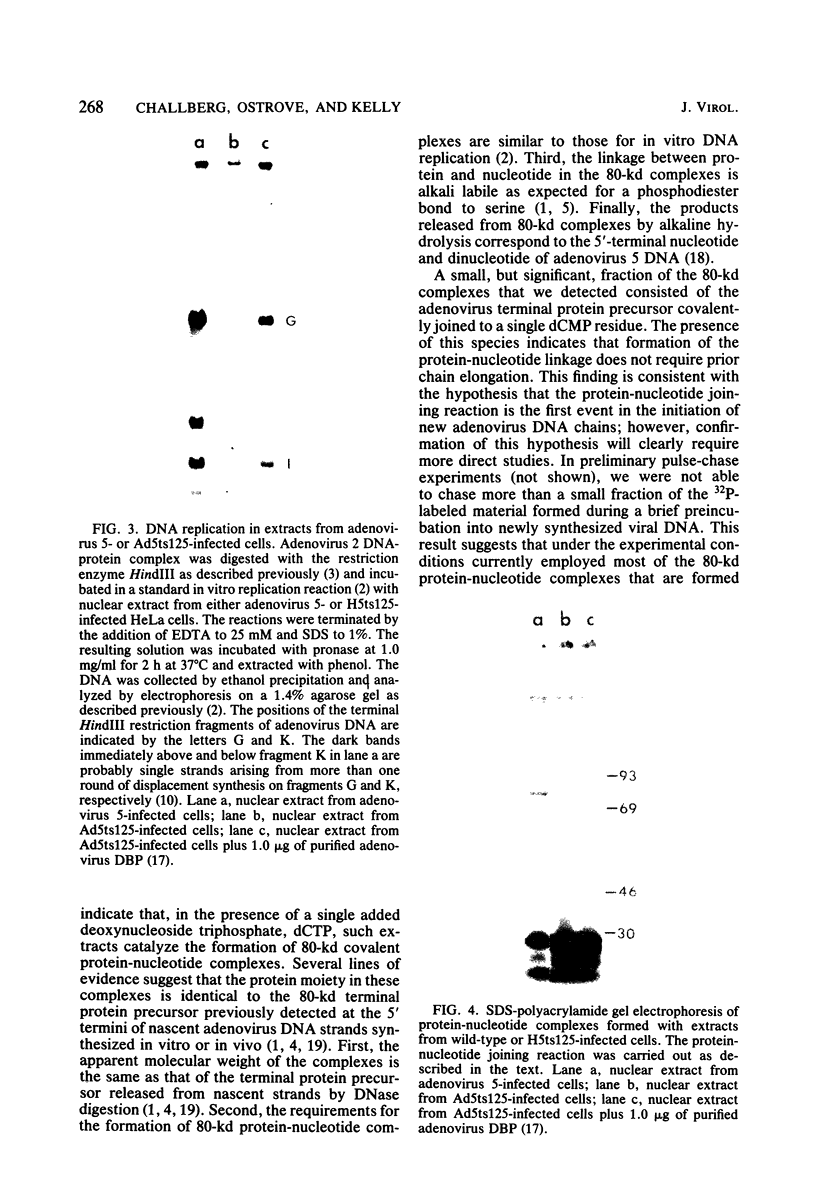
We have previously shown that the 5'-terminal deoxycytidine residue of each nascent adenovirus 5 DNA strand synthesized in vitro is covalently linked to the 80-kilodalton (kd) terminal protein precursor via a phosphodiester bond to a serine residue in the protein. When extracts prepared from adenovirus 5-infected cells are incubated with [alpha-33P]dCTP as the only added deoxynucleoside triphosphate, complexes consisting of nucleotide covalently linked to the 80-kd protein can be detected. The nucleotide moieties present in such complexes include d(pC) and d(pCpA), the 5'-terminal nucleotide and dinucleotide of adenovirus 5 DNA, respectively, as well as some longer oligonucleotides. The formation of these complexes requires the presence of adenovirus DNA containing the attached 55-kd terminal protein and ATP. Extracts from H5ts125-infected cells which are defective in DNA replication catalyze complex formation to the same extent as extracts prepared from wild-type infected cells; thus, the presence of the adenovirus-coded 72-kd DNA-binding protein is apparently not required. Most, if not all, of the 80-kd protein-nucleotide complexes that are formed are noncovalently bound to the input viral DNA. These observations are consistent with the protein-priming model for the initiation of adenovirus DNA replication.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Challberg M. D., Kelly T. J., Jr Adenovirus DNA replication in vitro. Proc Natl Acad Sci U S A. 1979 Feb;76(2):655–659. doi: 10.1073/pnas.76.2.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challberg M. D., Kelly T. J., Jr Adenovirus DNA replication in vitro: origin and direction of daughter strand synthesis. J Mol Biol. 1979 Dec 25;135(4):999–1012. doi: 10.1016/0022-2836(79)90524-2. [DOI] [PubMed] [Google Scholar]
- Challberg M. D., Kelly T. J., Jr Processing of the adenovirus terminal protein. J Virol. 1981 Apr;38(1):272–277. doi: 10.1128/jvi.38.1.272-277.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desiderio S. V., Kelly T. J., Jr Structure of the linkage between adenovirus DNA and the 55,000 molecular weight terminal protein. J Mol Biol. 1981 Jan 15;145(2):319–337. doi: 10.1016/0022-2836(81)90208-4. [DOI] [PubMed] [Google Scholar]
- Ensinger M. J., Ginsberg H. S. Selection and preliminary characterization of temperature-sensitive mutants of type 5 adenovirus. J Virol. 1972 Sep;10(3):328–339. doi: 10.1128/jvi.10.3.328-339.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg H. S., Lundholm U., Linné T. Adenovirus DNA-binding protein in cells infected with wild-type 5 adenovirus and two DNA-minus, temperature-sensitive mutants, H5ts125 and H5ts149. J Virol. 1977 Jul;23(1):142–151. doi: 10.1128/jvi.23.1.142-151.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M., Piña M., Kimes R., Wensink P. C., MacHattie L. A., Thomas C. A., Jr Adenovirus DNA. I. Molecular weight and conformation. Proc Natl Acad Sci U S A. 1967 May;57(5):1302–1309. doi: 10.1073/pnas.57.5.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. S., Ariga H. Multiple rounds of adenovirus DNA synthesis in vitro. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1476–1480. doi: 10.1073/pnas.78.3.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. S. Temperature-sensitive replication of H5ts125 adenovirus DNA in vitro. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4291–4295. doi: 10.1073/pnas.75.9.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan L. M., Ariga H., Hurwitz J., Horwitz M. S. Complementation of the temperature-sensitive defect in H5ts125 adenovirus DNA replication in vitro. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5534–5538. doi: 10.1073/pnas.76.11.5534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lechner R. L., Kelly T. J., Jr The structure of replicating adenovirus 2 DNA molecules. Cell. 1977 Dec;12(4):1007–1020. doi: 10.1016/0092-8674(77)90165-9. [DOI] [PubMed] [Google Scholar]
- Lichy J. H., Horwitz M. S., Hurwitz J. Formation of a covalent complex between the 80,000-dalton adenovirus terminal protein and 5'-dCMP in vitro. Proc Natl Acad Sci U S A. 1981 May;78(5):2678–2682. doi: 10.1073/pnas.78.5.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekosh D. M., Russell W. C., Bellet A. J., Robinson A. J. Identification of a protein linked to the ends of adenovirus DNA. Cell. 1977 Jun;11(2):283–295. doi: 10.1016/0092-8674(77)90045-9. [DOI] [PubMed] [Google Scholar]
- Schechter N. M., Davies W., Anderson C. W. Adenovirus coded deoxyribonucleic acid binding protein. Isolation, physical properties, and effects of proteolytic digestion. Biochemistry. 1980 Jun 10;19(12):2802–2810. doi: 10.1021/bi00553a041. [DOI] [PubMed] [Google Scholar]
- Steenbergh P. H., Maat J., van Ormondt H., Sussenbach J. S. The nucleotide sequence at the termini of adenovirus type 5 DNA. Nucleic Acids Res. 1977 Dec;4(12):4371–4389. doi: 10.1093/nar/4.12.4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillman B. W., Lewis J. B., Chow L. T., Mathews M. B., Smart J. E. Identification of the gene and mRNA for the adenovirus terminal protein precursor. Cell. 1981 Feb;23(2):497–508. doi: 10.1016/0092-8674(81)90145-8. [DOI] [PubMed] [Google Scholar]
- Van Der Vliet P. C., Levine A. J., Ensinger M. J., Ginsberg H. S. Thermolabile DNA binding proteins from cells infected with a temperature-sensitive mutant of adenovrius defective in viral DNA synthesis. J Virol. 1975 Feb;15(2):348–354. doi: 10.1128/jvi.15.2.348-354.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Vliet P. C., Zandberg J., Jansz H. S. Evidence for a function of the adenovirus DNA-binding protein in initiation in DNA synthesis as well as in elongation of nascent DNA chains. Virology. 1977 Jul 1;80(1):98–110. doi: 10.1016/0042-6822(77)90383-x. [DOI] [PubMed] [Google Scholar]
- Vliet P. C., Sussenbach J. S. An adenovirus type 5 gene function required for initiation of viral DNA replication. Virology. 1975 Oct;67(2):415–426. doi: 10.1016/0042-6822(75)90443-2. [DOI] [PubMed] [Google Scholar]
- Winnacker E. L. Adenovirus DNA: structure and function of a novel replicon. Cell. 1978 Aug;14(4):761–773. doi: 10.1016/0092-8674(78)90332-x. [DOI] [PubMed] [Google Scholar]
- van der Vliet P. C., Keegstra W., Jansz H. S. Complex formation between the adenovirus type 5 DNA-binding protein and single-stranded DNA. Eur J Biochem. 1978 May 16;86(2):389–398. doi: 10.1111/j.1432-1033.1978.tb12321.x. [DOI] [PubMed] [Google Scholar]
- van der Vliet P. C., Levine A. J. DNA-binding proteins specific for cells infected by adenovirus. Nat New Biol. 1973 Dec 12;246(154):170–174. doi: 10.1038/newbio246170a0. [DOI] [PubMed] [Google Scholar]