Abstract
Programmed death-1 (PD-1) regulates T cell exhaustion during chronic infections. Blocking the PD-1:PD-ligand (PD-L) pathway reinvigorates exhausted CD8 T cells. Exactly how blocking PD-1:PD-L interactions improves T cell immunity, however, remains unclear. PD-1:PD-L blockade could reprogram all exhausted T cells to become antiviral effectors. Alternatively, this blockade might selectively expand a subset of exhausted T cells. We have identified two subpopulations of exhausted CD8 T cells during chronic viral infection in mice. One subset of exhausted CD8 T cells is rescued by αPD-L1 blockade, whereas the other subset appears more terminally differentiated and responds poorly to PD-1:PD-L blockade. Blocking PD-1:PD-L interactions reduces spontaneous apoptosis and enhances expansion and protective immunity of the rescuable subset, but not the more terminally differentiated subset of exhausted CD8 T cells. These results have implications for predicting clinical responses to PD-1-based therapeutic interventions and for understanding T cell dynamics during persisting infections.
Keywords: chronic infection, lymphocytic choriomeningitis virus, T cell exhaustion, PD-1, T cell memory
Recent studies have revealed an important role for the negative regulatory molecule programmed death-1 (PD-1) in T cell exhaustion during chronic viral infections (1). PD-1, a member of the CD28:CTLA-4 family of costimulatory and coinhibitory receptors, contains both immunotyrosine inhibitory motif (ITIM) and immunotyrosine switch motif (ITSM) signaling motifs, recruits the phosphatase Shp-2, and can deliver inhibitory signals (1). PD-1 interacts with two ligands, PD-ligand 1 (PD-L1), expressed by a wide variety of cells, and PD-L2, expressed mainly by macrophages and DC (1). A role for PD-1 in regulating T cell responses to chronic viral infections was identified by using lymphocytic choriomeningitis virus (LCMV) infection of mice (2). PD-1 was highly overexpressed on exhausted CD8 T cells from chronically infected animals compared with functional memory CD8 T cells from mice that had resolved acute infection. In vivo blockade of the PD-1:PD-L pathway during chronic LCMV infection led to a dramatic increase in the number and functionality of virus-specific CD8 T cells and enhanced control of infection (2). Other animal models of viral infection also supported a major role for the PD-1 pathway in regulating antiviral T cell responses (3, 4). These observations were quickly extended to primates and humans. Simian immunodeficiency virus (SIV)-, HIV-, hepatitis C virus (HCV)-, and hepatitis B virus (HBV)-specific CD8 T cells express elevated levels of PD-1 compared with CD8 T cells specific for nonpersisting pathogens such as influenza virus or vaccinia virus (5–12). In vitro blockade of PD-1:PD-L interactions reverses exhaustion of HIV-, HBV-, and HCV-specific T cells, and the proliferative capacity of these virus-specific T cell populations is dramatically improved. In light of these recent findings, PD-1 has emerged as not only a major regulator of T cell exhaustion during chronic infection, but also as an important potential therapeutic target.
At least two models have been proposed for the mechanism of reversal of exhaustion by PD-1:PD-L blockade (13). In one model, PD-1:PD-L blockade reprograms all exhausted T cells, converting them to functional antiviral effector T cells. In a second model, PD-1:PD-L blockade selectively expands and/or enhances function of a subset of exhausted CD8 T cells. In the present work, we have addressed this question directly. We have identified two subsets of exhausted CD8 T cells during chronic LCMV infection in mice that differ in expression of PD-1 and CD44. One subset that expressed intermediate levels of PD-1 and high levels of CD44 (PD-1IntCD44Hi) could be reinvigorated by blocking PD-1:PD-L interactions. In contrast, the second subset was PD-1Hi but CD44Int and responded poorly to PD-1 pathway blockade. Thus, our data suggest that the cellular mechanism of reinvigorated T cell responses during chronic viral infection is by selective expansion of one subset of exhausted CD8 T cells, whereas another subset is more terminally differentiated and unable to be rescued by PD-1 pathway blockade alone. These observations indicate that defining the populations of exhausted CD8 T cells that can and cannot respond to PD-1-based therapeutics could be relevant for future clinical interventions.
Results
Identifying Subsets of Exhausted LCMV-Specific CD8 T Cells.
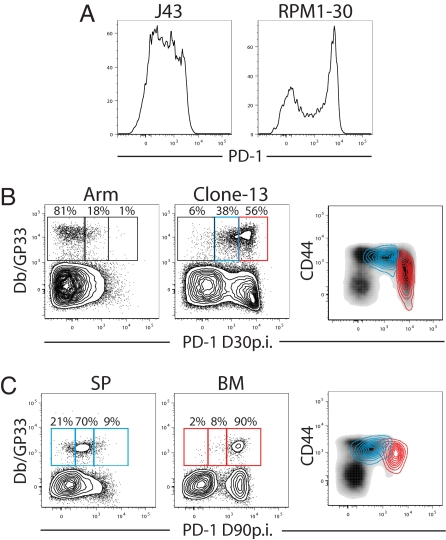
Infection of adult mice with the Armstrong strain (Arm) of LCMV causes an acute infection that is cleared by day 8–10 after infection, resulting in a highly functional population of effector and subsequently, memory CD8 T cells (14). LCMV clone 13, in contrast, causes a chronic infection with viremia for 60–80 days and exhaustion of CD8 T cells (14). All known T cell epitopes are shared between these two strains of LCMV and exhausted CD8 T cells responding to LCMV clone 13 infection can be compared directly with functional CD8 T cells of the same specificity generated after acute LCMV Arm infection (14). We evaluated PD-1 expression on LCMV-specific CD8 T cells after Arm vs. clone 13 infection by using two different clones of αPD-1 antibodies. The RPM1–30 clone of αPD-1 reveals a higher maximal expression and thus a broader range of PD-1 expression compared with the J43 clone used in many previous studies(Fig. 1A). Although DbGP33-specific CD8 T cells from Arm immune mice were predominantly PD-1Lo, two levels of higher PD-1 expression were observed on DbGP33-specific CD8 T cells from chronically infected mice (Fig. 1B). The vast majority of the virus-specific CD8 T cells found during chronic LCMV infection were PD-1Int or PD-1Hi. Thus, the RPM1–30 antibody allows virus-specific CD8 T cells to be defined as PD-1Lo, PD-1Int, or PD-1Hi rather than PD-1+ or PD-1− (Fig. 1 A and B). Interestingly, the two subsets of exhausted CD8 T cells found during chronic LCMV infection also differed in their expression of CD44 (Fig. 1B). Gating on the PD-1Int subset revealed high levels of CD44 (PD-1IntCD44Hi; blue overlay in Fig. 1B Right), whereas the PD-1Hi subset was CD44Int (red overlay in Fig. 1B Right). Decreased CD44 expression by exhausted CD8 T cells has been noted (14, 15), although the significance of this decrease has not been clear. The PD-1Hi subset also expresses slightly more cytotoxic T lymphocyte antigen-4 (CTLA-4) compared with the PD-1Int subset [supporting information (SI) Fig. S1], although previous studies suggest a minimal role for CTLA-4 on exhausted CD8 T cells (2, 16). It is also worth pointing out that the RPM1–30 clone reveals low levels of PD-1 expressed by DbGP33-specific memory CD8 T cells from LCMV Arm immune mice compared with CD44Lo CD8 T cells, consistent with ≈2–4-fold higher PD-1 mRNA in LCMV-specific memory CD8 T cells compared with naïve CD8 T cells (data not shown). Both PD-1Int and PD-1Hi subsets of GP33-specific CD8 T cells from chronically infected mice are functionally exhausted, and at day 30 after infection only 14.8 ± 1.5% and 6.4 ± 1.3% of the GP33-specific PD-1Int and PD-1Hi CD8 T cells, respectively, from the spleen (SP) coproduced IFN-γ and TNF-α in 5 h whereas >85% of GP33-specific memory CD8 T cells from LCMV Arm immune mice were able to coproduce these cytokines (data not shown). A small population of PD-1Lo virus-specific CD8 T cells is often present in chronically infected mice, but these PD-1Lo CD8 T cells still express higher levels of PD-1 than LCMV-specific memory CD8 T cells from Arm immune mice and are functionally compromised (Fig. 1 and data not shown). At later times during chronic infection (>60 days after infection) the SP often consisted of largely PD-1IntCD44Hi exhausted T cells, whereas in the same mice other tissues such as the bone marrow (BM) contained exhausted CD8 T cells that were PD-1HiCD44Int (Fig. 1C). Overlaying the SP- (Fig. 1C Right, blue overlay) and BM-derived (Fig. 1C Right, red overlay) DbGP33+ CD8 T cells with CD44 expression reveals largely distinct subsets of exhausted CD8+ T cells are present in these tissues >60 days after infection. Thus, exhausted CD8 T cells found during chronic LCMV infection could be divided into a PD-1IntCD44Hi and PD-1HiCD44Int subsets.
Fig. 1.
Identification of subsets of exhausted CD8 T cells. (A) CD8+ CD44+ T cells from Arm vs. clone 13-infected mice (day 30 after infection) were stained with either the J43 clone or the RPM1–30 clone of αPD-1. (B) Splenocytes from either LCMV Arm or clone 13-infected mice (day 30 after infection) were stained with αPD-1 clone RPM1–30 and gated on CD8+ T cells. PD-1Hi (red) and PD-1Int (blue) DbGP33+ CD8+ T cells were overlaid on total CD8 T cells (black; Right) from the same mice to assess overlap of these populations and to illustrate differences in CD44 expression. (C) Lymphocytes from SP or BM of clone 13-infected mice (day 90 after infection) were stained with αPD-1 clone RPM1–30. Plots are gated on CD8+ T cells. DbGP33+ CD8+ T cells from the SP or BM. DbGP33+ CD8+ T cells from SP (blue) or BM (red) were overlaid total CD8+ splenocytes (black; Right).
The PD-1IntCD44Hi Exhausted CD8 T Cell Subset Had Greater Proliferative Potential and Was More Responsive to PD-1 Blockade in Vivo than the PD-1HiCD44Int Subset.
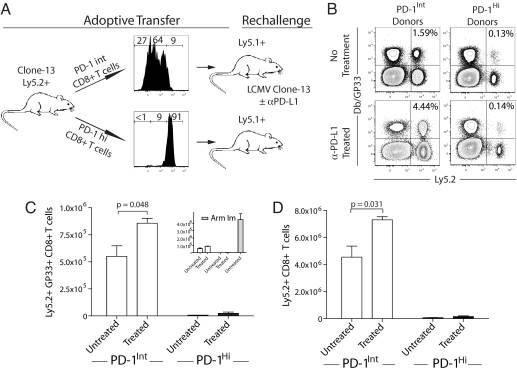
We next wanted to compare directly the properties of PD-1IntCD44Hi and PD-1HiCD44Int subsets of exhausted LCMV-specific CD8 T cells in vivo. We took advantage of the fact that the RPM1–30 antibody does not block PD-1 interactions with its ligand (17) and thus can be used to separate subsets of exhausted CD8 T cells based on the level of PD-1 expression. Exhausted LCMV-specific CD8 T cells from the SP of chronically infected mice were separated into PD-1Int and PD-1Hi subsets by using the RPM1–30 antibody (Fig. 2A). Equal numbers of GP33-specific PD-1IntCD44Hi or PD-1HiCD44Int CD8 T cells were adoptively transferred to naïve congenic recipient mice (Fig. 2A). Recipient mice were then challenged with LCMV clone 13. The PD-1Int GP33-specific CD8 T cells expanded significantly more vigorously upon challenge than the PD-1Hi GP33-specific CD8 T cells (Fig. 2 B and C). In these experiments the total donor (Ly5.2+) CD8 T cell population also expanded dramatically more in the case of the PD-1Int donor population, reflecting additional LCMV-specific CD8 T cell populations in the donor pool (Fig. 2D). It is important to point out that the responsiveness of both PD-1Hi and PD-1Int GP33-specific CD8 T cells was far less than the response of PD-1Lo GP33-specific CD8 T cells from LCMV Arm immune mice (Fig. 2C Inset), consistent with the more vigorous responsiveness of memory T cells compared with exhausted CD8 T cells (2, 18).
Fig. 2.
In vivo PD-L1 blockade enhanced expansion of splenic PD-1Int, but not the PD-1Hi subset of exhausted CD8 T cells. (A) PD-1Hi and PD-1Int splenocytes from clone 13-infected (day 30 after infection) Ly5.2+ mice were column-purified. Each fraction yielded >90% purity for PD-1Hi and PD-1Int. Note that the PD-1Int fraction contained a small percentage of PD-1Lo cells, but these cells expressed higher PD-1 levels compared with memory CD8 T cells. DbGP33+ CD8+ T cells (6 × 104) were adoptively transferred to Ly5.1 naïve recipient mice followed by rechallenge with LCMV clone 13 with or without αPD-L1 antibody treatment (200 μg i.p. on days 0, 3, and 6 after infection). As a control, LCMV-specific memory CD8 T cells from Ly5.2+ LCMV Arm immune mice were transferred to Ly5.1+ naïve recipients and rechallenged. (B–D) On day 7.5 after rechallenge, recipient mice were killed, and total donor Ly5.2+ or DbGP33+ Ly5.2+ CD8+ T cells were identified by flow cytometry (n = 3–5 mice per group, data are representative of three similar experiments).
To compare how the two exhausted CD8 T cell subsets responded to PD-L1 blockade, some recipient mice were treated with αPD-L1 for the first 7 days after viral challenge. Blocking the PD-1 pathway significantly improved the responsiveness of the PD-1Int subset, and the number of DbGP33-specific CD8 T cells increased significantly compared with untreated mice (Fig. 2C). In contrast, there was little improvement in the response of the PD-1Hi subset in the presence of PD-L1 blockade in any experiment. Similar results were observed for the total donor Ly5.2+ CD8 T cell population (Fig. 2D). (Note that many of the DbGP33 tetramer− Ly5.2+ CD8+ T cells are specific for other LCMV epitopes and will also respond in this experimental design.) This differential responsiveness of PD-1Int and PD-1Hi subsets of exhausted CD8 T cells was unlikely caused by incomplete blockade because treatment with 5-fold higher doses of blocking antibody (1 mg per injection) did not improve the responsiveness of the PD-1Hi subset, but had a similar impact on the PD-1Int subset, consistent with saturation of this pathway by the blockade (data not shown).
Differential Proliferative Potential of Tissue-Defined Exhausted CD8 T Cell Subsets.
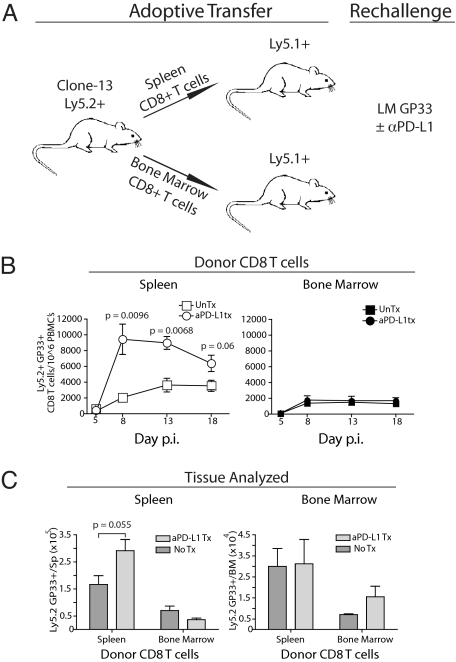
To examine the responsiveness of subsets of exhausted CD8 T cells in vivo by using an approach that did not depend on separation by using an antibody against PD-1, we took advantage of the anatomical differences of PD-1IntCD44Hi and PD-1HiCD44Int subsets described in Fig. 1. We next tested whether or not LCMV-specific CD8 T cells from the SP (PD-1Int) vs. BM (PD-1Hi) differed in their response to challenge infection in vivo and whether PD-L1 blockade had a differential impact on these tissue-defined subsets of exhausted CD8 T cells. Equal numbers of LCMV DbGP33-specific CD8 T cells (Ly5.2+) from each location were adoptively transferred to naïve congenic (Ly5.1+) mice (Fig. 3A). In these experiments, recipient mice were challenged with Listeria monocytogenes expressing the LCMV GP33 epitope (LMGP33) to assess the responsiveness of the exhausted CD8 T cell subsets to a second pathogen (Fig. 3A). Thus, recipients of PD-1IntCD44Hi CD8 T cells from the SP or PD-1HiCD44Int CD8 T cells from the BM were infected with LMGP33 in the presence or absence of αPD-L1 antibody. The response of the SP-derived (PD-1Int) GP33-specific CD8 T cells was ≈2-fold greater at 2 weeks after challenge compared with the BM-derived PD-1Hi CD8 T cells (Fig. 3B). PD-L1 blockade had virtually no impact on the proliferative expansion of the PD-1HiCD44Int exhausted CD8 T cell subset from the BM. In contrast, blocking the PD-1 pathway significantly improved the responsiveness of PD-1Int virus-specific CD8 T cells from the SP (Fig. 3B). Tissue homing or residence was unlikely to account for these differences because the absolute number of BM-derived virus-specific CD8 T cells was reduced in the SP and BM of recipient mice (Fig. 3C).
Fig. 3.
In vivo PD-L1 blockade enhanced expansion of tissue-defined PD-1Int (SP-derived), but not the PD-1Hi (BM-derived) subset of exhausted CD8 T cells. (A) PD-1Hi CD8+ T cells were derived from the BM and PD-1Int CD8+ T cells from the SP of clone 13-infected (day 90 after infection) Ly5.2+ mice. CD8 T cells from BM and SP were column-purified, CD8+ T cell populations were normalized to contain 6 × 104 DbGP33+ CD8+T cells, and cells were adoptively transferred to Ly5.1 naïve recipient mice followed by rechallenge with LMGP33 (3 × 104 cfu) with or without αPD-L1 antibody treatment (200 μg i.p. on days 0, 3, and 6 after infection). (B) Ly5.2+ DbGP33+ CD8+ T cells were monitored in the blood of recipient mice after LMGP33 rechallenge (n = 3–5 mice per group, data are representative of five experiments). (C) Total donor DbGP33+ Ly5.2+ CD8+ T cell number was assessed in recipient SP and BM on day 18 after challenge.
Protective Immunity of Subsets of Exhausted CD8 T Cells.
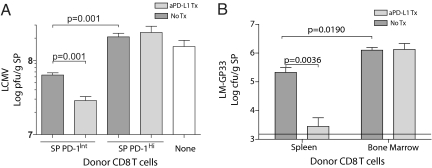
We next examined protective immunity after LCMV or LMGP33 rechallenge by using the experimental designs described above. Three days after challenge with LCMV clone 13 viral titers were determined in the SP of recipient mice that did or did not receive PD-1:PD-L blockade. Recipients of PD-1Int exhausted CD8 T cells controlled LCMV clone 13 challenge significantly better than recipients of PD-1Hi exhausted CD8 T cells (Fig. 4A). In addition, PD-1:PD-L blockade led to a significant enhancement of viral control by the PD-1Int exhausted CD8 T cell subset, but this blockade had no impact on viral control by the PD-1Hi subset (Fig. 4A). To examine protective immunity by a single defined specificity of exhausted CD8 T cells, mice receiving equal numbers of GP33-specific exhausted CD8 T cells of each subset were challenged with LMGP33. In the absence of PD-1:PD-L blockade, the PD-1IntCD44Hi subset was ≈6-fold more efficient at bacterial control compared with the PD-1HiCD44Int subset (Fig. 4B). Again, PD-L1 blockade had essentially no impact on pathogen control by the PD-1HiCD44Int subset, but dramatically improved protective immunity by the PD-1IntCD44Hi subset of exhausted GP33-specific CD8 T cells (Fig. 4B). Thus, whether defined phenotypically or anatomically, subsets of exhausted CD8 T cells responded differently to blockade of the PD-1:PD-L pathway upon antigen encounter in vivo. Blockade of the PD-1:PD-L pathway in vivo during challenge infection had little impact on the expansion of the PD-1Hi subset of exhausted CD8 T cells, but rather this blockade selectively augmented the expansion and protective immunity of the PD-1Int subset of exhausted CD8 T cells.
Fig. 4.
Protective immunity by subsets of exhausted CD8 T cells in the absence or presence PD-L1 blockade. (A) PD-1Hi and PD-1Int splenocytes were purified as in Fig. 2, and 1 × 106 LCMV-specific CD8+ T cells [normalized based on responsiveness via ICS to a pool of 20 known LCMV CD8 T cell epitopes described by Kotturi et al. (24)] were transferred to Ly5.1 naïve recipient mice followed by rechallenge with LCMV clone 13 (2 × 106pfu i.v.) with or without αPD-L1 antibody treatment as in Fig. 2. As a control, Ly5.1+ mice that received no donors cells were also infected. Mice were killed on day 3 after infection. and LCMV quantified in the SP. (B) Adoptive transfers were performed as described in Fig. 3. Recipients were then rechallenged with LMGP33 (1.5 × 104 cfu i.v.) with or without αPD-L1 antibody (200 μg i.p. on days 0 and 3 after infection). Mice were killed on day 4 after infection, and LMGP33 was quantified in the SP.
αPD-L1 Blockade Preferentially Rescues PD-1Lo/Int Exhausted CD8 T Cells from Apoptosis.
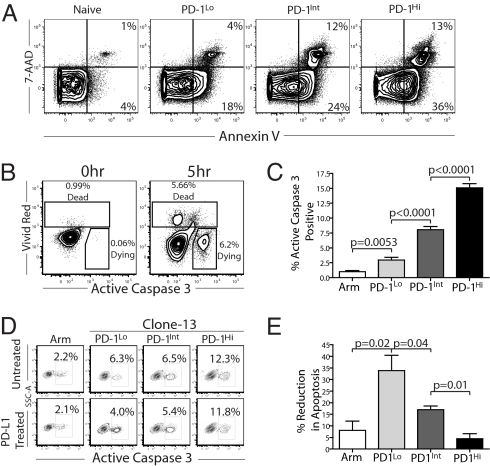
PD-L1 blockade in vivo alone or in combination with therapeutic vaccination reduces the level of apoptosis of exhausted CD8 T cells (19). In addition, higher levels of PD-1 expression correlate with an increased rate of apoptosis in HIV-specific CD8 T cells, and engaging PD-1 on SIV-specific CD8 T cells increases cell death (6, 20). Based on these observations, we examined the PD-1Lo, PD-1Int, and PD-1Hi subsets of virus-specific CD8 T cells from chronically infected mice for direct ex vivo annexin V and 7AAD staining (Fig. 5A). The percentages of annexin V+ and annexin V+ 7AAD+ CD8 T cells increased in a stepwise fashion on PD-1Lo, PD-1Int, and PD-1Hi CD8 T cells from chronically infected mice, suggesting that the levels of cell death increased in vivo as PD-1 levels increased. These results suggest that PD-1:PD-L1 blockade could rescue those CD8 T cells that express the highest levels of PD-1 from apoptosis. Thus, we developed an assay to evaluate spontaneous cell death of exhausted CD8 T cell subsets. By examining T cells that incorporated a dye indicating cell death (vivid red; VR) and staining for active caspase 3 (ACasp3) as an indicator of early stages of apoptosis, cells in late (VR+ ACasp3 +/−) vs. early (VR− ACasp3+) stages of cell death could be identified (Fig. 5B). In the following studies, the analysis is restricted to VR− ACasp3+ cells that are likely in the process of dying. By using this assay, higher levels of PD-1 expression on LCMV-specific CD8 T cells correlated directly with increased ACasp3 after culture for 5 h at 37°C (Fig. 5C). The level of spontaneous caspase 3 activation was highest in the PD-1Hi subset compared with the PD-1Int subset of LCMV-specific CD8 T cells from chronically infected mice, consistent with previous reports for HIV- and SIV-specific CD8 T cells (6, 20). The small subset of PD-1Lo GP33-specific CD8 T cells present in the chronically infected mice displayed a lower level of ACasp3 staining, but this level was still greater than that found in GP33-specific CD8 T cells from LCMV Arm immune mice (Fig. 5C). Thus, spontaneous ex vivo caspase 3 activation was increased in exhausted CD8 T cells compared with memory CD8 T cells, and the level of ACasp3 staining increased as PD-1 levels increased.
Fig. 5.
Differential inhibition of apoptosis of exhausted CD8 T cell subsets by PD-1:PD-L blockade. (A) Splenocytes from clone 13-infected mice (day 30 after infection) were isolated and stained with 7-AAD and annexin V directly ex vivo. (B) ACasp3 was used as an indicator of apoptosis in live cells. Dead cells were excluded by using an amine-reactive dye (vivid red), and ACasp3 was detected by intracellular staining. (C) Splenocytes from clone 13-infected mice were incubated at 37°C for 5 h in medium. ACasp3 was detected in live DbGP33+ CD8 T cells. (D and E) PD-1Hi, PD-1Int, and PD-1Lo exhausted CD8 T cells subsets were sorted by flow cytometry and incubated at 37°C for 5 h with or without αPD-L1 (10 μg/ml), and ACasp3 was detected in the live cell subset by flow cytometry (data are representative of three independent experiments). Note that PD-1 expression did not change in 5 h in vitro (data not shown). Similar results were observed in the presence of added peptide (data not shown).
To determine whether blocking the PD-1:PD-L pathway influenced death of exhausted CD8 T cell subsets, CD44Hi/Int CD8 T cells were sorted into PD-1Hi, PD-1Int, and PD-1Lo subsets and incubated 5 h at 37°C in medium. Increased PD-1 expression again correlated with higher levels of ACasp3 in these highly purified CD8 T cell populations. However, αPD-L1 blockade preferentially rescued the PD-1Int and PD-1Lo subsets from caspase 3 activation but had minimal effect on the PD-1Hi subset (Fig. 5 D and E). Memory CD8 T cells from LCMV Arm immune mice showed little spontaneous apoptosis, but also little change in cell death in the presence of PD-L1 blockade consistent with the low expression of PD-1 by this population. Together, these results suggest that not only are PD-1Int/Lo exhausted CD8 T cells less susceptible to induction of cell death than PD-1Hi exhausted subset, but they are also more likely to benefit from αPD-L1 blockade by greater survival.
Discussion
We identified subsets of exhausted CD8 T cells based on PD-1 and CD44 expression and tissue distribution. Our results support a model in which one subset of exhausted CD8 T cells found during chronic viral infection is rescuable by blocking PD-1 signals whereas the other subset is more terminally differentiated and is not rescued by blocking PD-1:PD-L interactions. The differential responses of the two subsets of exhausted CD8 T cells had a substantial impact on not only the expansion of these virus-specific CD8 T cells, but also on protective immunity in vivo. Thus, our data support a model in which blockade of PD-1:PD-L interactions selectively rescues one subset of exhausted antiviral CD8 T cells and suggest that restoration of antiviral immunity by PD-1:PD-L blockade does not need to induce reprogramming of all exhausted CD8 T cells to achieve a beneficial effect. In this regard it is worth noting that even in previous studies a subpopulation of exhausted CD8 T cells was observed that failed to respond and proliferate after PD-1:PD-L blockade (2). The present results also suggest that it should be possible to predict which T cell responses and which chronically infected subjects will respond most effectively to therapeutic intervention with PD-1:PD-L blockade based on the level of PD-1 expression and/or distribution of exhausted T cell subsets.
What prevents reversal of exhaustion for the PD-1HiCD44Int subset of exhausted CD8 T cells? Although incomplete blockade in vivo is one possibility, we believe that this is unlikely because similar results were observed even when 5-fold higher amounts of blocking antibody were used (data not shown). Another possibility is that CD8 T cells expressing the highest levels of PD-1 are already committed to cell death, such that PD-1:PD-L blockade at this point is insufficient to rescue their survival. A third possibility is that the PD-1HiCD44Int subset of exhausted CD8 T cells is restrained by additional regulatory pathways or cellular deficiencies. Our recent gene expression studies of exhausted CD8 T cells point to a number of additional pathways that may contribute to T cell exhaustion, including other inhibitory molecules, potential signaling changes, and metabolic defects that are likely to compromise the ability of exhausted CD8 T cells to respond to stimulation (15). During HIV infection, a second inhibitory receptor CTLA-4 can be overexpressed by exhausted CD4 T cells, and a complex pattern of PD-1 and CTLA-4 coexpression existed for these HIV-specific CD4 T cells (16). Although CTLA-4 is slightly elevated on the PD-1Hi compared with the PD-1Int subset of exhausted CD8 T cells, it is unclear how CTLA-4 or other pathways associated with T cell dysfunction mentioned above regulate the two exhausted T cell subsets defined by in the present work. CTLA-4 appears to have a minimal role on exhausted CD8 T cells (2, 16), but we would predict that additional regulatory pathways or cellular defects would be more prominent in the PD-1HiCD44Int vs. PD-1IntCD44Hi subset of exhausted CD8 T cells. Even for the less terminally differentiated exhausted CD8 T cells, additional negative regulatory pathways and cellular defects may be present. Future studies will be necessary to examine these issues. However, both increased apoptosis and additional inhibitory pathways or metabolic deficiencies should be amenable to complementary types of therapeutic intervention to enhance the effectiveness of PD-1:PD-L blockade.
Restoration of T cell responses by PD-1:PD-L blockade is a promising approach to enhance immunity to persisting infections and tumors. Our data indicate that there is heterogeneity in T cells responding to a chronic viral infection. PD-1:PD-L blockade has a dramatic effect restoring immunity and rescuing responsiveness of one subpopulation of exhausted LCMV-specific T cells, but another subset of exhausted CD8 T cells exists that is less efficiently rescued by blocking this inhibitory pathway alone. During SIV infection it appears that three levels of PD-1 expression could also be present. Velu et al. (21) demonstrated that by 12 weeks after infection there were PD-1− and PD-1+ CD8 T cells, but the SIV tetramer+ CD8 T cells expressed the highest level of PD-1 even among the PD-1+ CD8 T cells (21) similar to the PD-1Lo, PD-1Int, and PD-1Hi exhausted CD8+ T cells described here for mice. Radziewicz et al. (10) found a similar expression pattern in human HCV infection where the HCV-specific CD8 T cells in some subjects clearly expressed a higher level of PD-1 compared with the rest of the PD-1+ CD8 T cells. Thus, it is likely that populations of PD-1Lo, PD-1Int, and PD-1Hi CD8 T cells also exist in humans. However, the precise phenotypic characteristics of exhausted T cells subsets could vary in different infections or even anatomical sites. Indeed, recent studies suggest that although the circulating pool of HCV-specific CD8 T cells can express PD-1, the level of PD-1 is substantially lower in the PBMC compared with the level observed in the liver (22), and similar observations have been reported for different nonlymphoid tissues compared with the blood during SIV infection (21). Moreover, circulating PD-1Lo/Int HCV-specific CD8 T cells in humans respond more efficiently to PD-1:PD-L blockade in vitro compared with the intrahepatic PD-1Hi subset. Together, these observations from human and primate studies suggest that our findings regarding subsets of exhausted CD8+ T cells in mice could have direct relevance to human chronic viral infections. Thus, the subsets of exhausted CD8 T cells present in different settings may have substantial relevance to predicting the outcome of future clinical interventions during chronic infections.
Overall, our findings suggest that reversal of exhaustion does not need to operate through functional reprogramming of all exhausted T cells. Rather, selective expansion of a subset of exhausted T cells, although not excluding changes in per cell function, appears to be a major mechanism for improved T cell responses after PD-1:PD-L blockade during chronic viral infection. These results also suggest that additional strategies might be necessary to restore responsiveness of the PD-1Hi subset of exhausted T cells. Defining the subpopulations of exhausted CD8 T cells present before PD-1:PD-L blockade intervention and devising strategies to rescue the subset of exhausted CD8 T cells refractory to this blockade are important future goals.
Methods
Mice, Virus, Infections, and PD-L1 Blockade.
Four- to six-week-old C57BL/6 mice were purchased from The Jackson Laboratory. LCMV Arm and clone 13, recombinant L. monocytogenes (LMGP33), strains were used as described (14, 23). Mice were infected with LCMV Arm (2 × 105 pfu) i.p. or with LCMV clone 13 (2 × 106 pfu) i.v. PD-1:PD-L blockade was performed as described in ref. 2. All mice were used in accordance with IACUC guidelines.
Flow Cytometry, Intracellular Cytokine Staining (ICS), and Apoptosis Assays.
MHC class I peptide tetramers were made and used as described in ref. 14. Antibodies were purchased from ebioscience or BD Biosciences. Lymphocytes were stained and analyzed as described (14, 23). ICS was performed as described (14, 23). In apoptosis assays, cells were stained first with the amine reactive dye vivid red (Molecular Probes) and CD8, CD44, and PD-1. Cells were fixed and permeabilized with Cytofix/Cytoperm (BD Biosciences) and stained intracellularly with an anti-active caspase 3 antibody. In direct ex vivo assays, apoptosis was detected by using annexin V–FITC and 7-AAD (BD Bioscience).
Cell Purification and Adoptive Transfers.
CD8 T cells were purified to >90% purity by using magnetic beads (Miltenyi Biotec) or by FACS sorting. PD1Hi and PD1Int populations were separated by using α-PD-1 PE (RPM1–30) and anti-PE magnetic beads, resulting in PD1Hi and PD1Int populations of >90% purity (MACS beads; Miltenyi Biotec). In each individual experiment, identical numbers of DbGP33 tetramer+ CD8 T cells were adoptively transferred to each recipient mouse. For apoptosis assays, cells were separated as described above or by FACS sorting.
Supplementary Material
Acknowledgments.
We thank Dan Barber, Dave Masopust, Kyong-Mi Chang, and the members of the Wherry laboratory for helpful discussions. This work was supported by National Institutes of Health Grants AI071309 (to E.J.W.), AI56299 (to G.J.F.), and HHSN 26620050030C (to E.J.W. and G.J.F.) and by the Commonwealth Universal Research Enhancement Program, Pennsylvania Department of Health (to E.J.W.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0801497105/DCSupplemental.
References
- 1.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8:239–245. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 2.Barber DL, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 3.Iwai Y, Terawaki S, Ikegawa M, Okazaki T, Honjo T. PD-1 inhibits antiviral immunity at the effector phase in the liver. J Exp Med. 2003;198:39–50. doi: 10.1084/jem.20022235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Isogawa M, Furuichi Y, Chisari FV. Oscillating CD8(+) T cell effector functions after antigen recognition in the liver. Immunity. 2005;23:53–63. doi: 10.1016/j.immuni.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Day CL, et al. PD-1 expression on HIV-specific T cells is associated with T cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 6.Petrovas C, et al. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med. 2006;203:2281–2292. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trautmann L, et al. Up-regulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 8.Zhang JY, et al. PD-1 up-regulation is correlated with HIV-specific memory CD8+ T cell exhaustion in typical progressors, but not in long-term nonprogressors. Blood. 2007;109:4671–4678. doi: 10.1182/blood-2006-09-044826. [DOI] [PubMed] [Google Scholar]
- 9.Urbani S, et al. PD-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustion. J Virol. 2006;80:11398–11403. doi: 10.1128/JVI.01177-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Radziewicz H, et al. Liver-infiltrating lymphocytes in chronic human hepatitis C virus infection display an exhausted phenotype with high PD-1 and low CD127 expression. J Virol. 2006;81:2545–2553. doi: 10.1128/JVI.02021-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boni C, et al. Characterization of hepatitis B virus (HBV)-specific T cell dysfunction in chronic HBV infection. J Virol. 2007;81:4215–4225. doi: 10.1128/JVI.02844-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boettler T, et al. Expression of the interleukin-7 receptor α chain (CD127) on virus-specific CD8+ T cells identifies functionally and phenotypically defined memory T cells during acute resolving hepatitis B virus infection. J Virol. 2006;80:3532–3540. doi: 10.1128/JVI.80.7.3532-3540.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blattman JN, Greenberg PD. PD-1 blockade: Rescue from a near-death experience. Nat Immunol. 2006;7:227–228. doi: 10.1038/ni0306-227. [DOI] [PubMed] [Google Scholar]
- 14.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77:4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wherry EJ, et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 16.Kaufmann DE, et al. Up-regulation of CTLA-4 by HIV-specific CD4(+) T cells correlates with disease progression and defines a reversible immune dysfunction. Nat Immunol. 2007;8:1246–1254. doi: 10.1038/ni1515. [DOI] [PubMed] [Google Scholar]
- 17.Matsumoto K, et al. B7-DC regulates asthmatic response by an IFN-γ-dependent mechanism. J Immunol. 2004;172:2530–2541. doi: 10.4049/jimmunol.172.4.2530. [DOI] [PubMed] [Google Scholar]
- 18.Wherry EJ, Blattman JN, Ahmed R. Low CD8 T cell proliferative potential and high viral load limit the effectiveness of therapeutic vaccination. J Virol. 2005;79:8960–8968. doi: 10.1128/JVI.79.14.8960-8968.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ha SJ, et al. Enhancing therapeutic vaccination by blocking PD-1-mediated inhibitory signals during chronic infection. J Exp Med. 2008;205:543–555. doi: 10.1084/jem.20071949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petrovas C, et al. SIV-specific CD8+ T cells express high levels of PD1 and cytokines but have impaired proliferative capacity in acute and chronic SIVmac251 infection. Blood. 2007;110:928–936. doi: 10.1182/blood-2007-01-069112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Velu V, et al. Elevated expression levels of inhibitory receptor programmed death 1 on simian immunodeficiency virus-specific CD8 T cells during chronic infection but not after vaccination. J Virol. 2007;81:5819–5828. doi: 10.1128/JVI.00024-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakamoto N, et al. Functional restoration of HCV-specific CD8 T cells by PD-1 blockade is defined by PD-1 expression and compartmentalization. Gastroenterology. 2008;134:1927–1937. doi: 10.1053/j.gastro.2008.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wherry EJ, et al. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 24.Kotturi MF, et al. The CD8+ T cell response to lymphocytic choriomeningitis virus involves the L antigen: Uncovering new tricks for an old virus. J Virol. 2007;81:4928–4940. doi: 10.1128/JVI.02632-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.