Abstract
We normally live in symbiosis with ∼1013 bacteria present in the colon. Among the several mechanisms maintaining the bacteria/host balance, there is limited understanding of the structure, function, and properties of intestinal mucus. We now demonstrate that the mouse colonic mucus consists of two layers extending 150 μm above the epithelial cells. Proteomics revealed that both of these layers have similar protein composition, with the large gel-forming mucin Muc2 as the major structural component. The inner layer is densely packed, firmly attached to the epithelium, and devoid of bacteria. In contrast, the outer layer is movable, has an expanded volume due to proteolytic cleavages of the Muc2 mucin, and is colonized by bacteria. Muc2−/− mice have bacteria in direct contact with the epithelial cells and far down in the crypts, explaining the inflammation and cancer development observed in these animals. These findings show that the Muc2 mucin can build a mucus barrier that separates bacteria from the colon epithelia and suggest that defects in this mucus can cause colon inflammation.
Keywords: commensal bacteria, proteomics, ulcerative colitis, large intestine, colon cancer
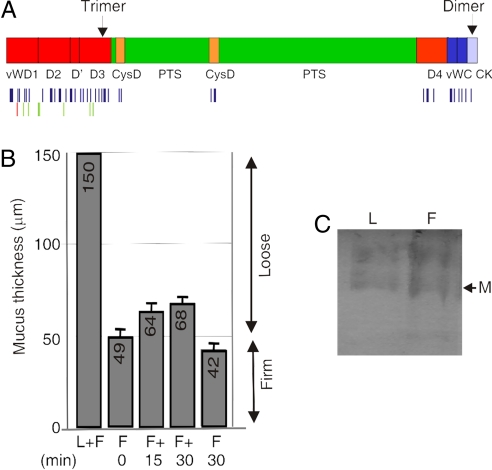
The digestive tract is home to 10 times as many bacteria as human cells in an ecosystem that has evolved to ensure homeostasis. How we can manage this enormous bacterial load without overt immune responses from the adaptive and innate systems is not well understood. When the equilibrium is altered, as in the disease ulcerative colitis, inflammatory responses are initiated against the commensal bacteria. An important component, often neglected due to lack of understanding, is the mucus layer that covers the epithelial cells. The major components of mucus, the gel-forming mucins, are part of the innate immunity and are well preserved in evolution (1). The secreted gel-forming mucin, MUC2, is a major mucin of the colon mucus (2). This is a large glycoprotein characterized by abundant and variable O-linked glycans attached to hydroxy amino acids clustered in PTS or mucin domains, Fig. 1A (1). The assembly process of MUC2 is well documented (2–4): MUC2 dimerizes in the endoplasmic reticulum via its C terminus, becomes heavily O-glycosylated in the Golgi and polymerizes via the N terminus by disulfide-bonded trimerization (4). The glycosylated primary translation product of MUC2 has a mass ≈2.5 MDa, and the secreted polymer has a huge, net-like structure.
Fig. 1.
The loose and the firm mucus layers of the colon consist mainly of the Muc2 mucin. (A) Schematic view of the domain organization of the MUC2 mucin and indication of where the sites for polymerization are localized. The human apoprotein is 5,174 aa long (2); vW identifies von Willebrand C (dark blue) or D (red) domain; PTS, the mucin domain (green); CysD (yellow), and CK, the cysteine knot domain (light blue) (1). The bands in C were digested with trypsin, and the peptides identified with LC-MS/MS and searched against the mucin database. Identified Muc2 peptides are schematically marked with one line per peptide under the Muc2 protein sequence. Peptides identified in both fractions are marked blue, those found only in the firm fraction are marked green, and those only found in the loose fraction are marked red. A full peptide list is presented in Table S1. (B) In vivo measurements of the mucus thickness in the distal colon reveal the presence of a firm and a loose layer. The thickness of the total mucus (F+L) and of the remaining firm (F) mucus after removal of the loose (L) layer is presented. The regeneration of the loose mucus is determined 15 and 30 min after its removal. C57BL/6 mice (n = 5) were analyzed, values are mean ± SEM. (C) Total loose (L) and firm (F) mucus from the measured area in B was separated on composite AgPAGE and visualized by staining the gel with Alcian blue. The fast migrating band corresponding to the smallest identified form of Muc2, most likely the monomer, is indicated by M.
Previous studies in rat colon have shown that there are two mucus layers. An inner adherent mucus layer that is possible to remove only by gentle scraping and an outer loosely adherent mucus layer that is easy to remove by gentle suctioning (5). To understand how these mucus layers are formed and their function, we have analyzed the composition and properties of these two mucus layers. The results show that both these layers are formed largely by the Muc2 mucin, that the two layers have different properties, and that the inner layer excludes the bacteria.
Results
The organization of the large-intestinal mucus, where most of the intestinal bacteria are localized, is not well understood. To address this, we first measured the thickness of the mucus in colon in vivo in C57BL/6 mice by a micropipette that can penetrate the mucus layer down to the epithelial cells (5). In the mouse colon, the mucus extended ≈150 μm above the epithelial cells and was composed of two layers with distinct physical properties (Fig. 1B, F+L). Most of the mucus could be aspirated off and represented the loose layer (≈100 μm thick); the remaining fraction was adhering firmly to the epithelia and was identified as firm (≈50 μm thick) (Fig. 1B, F 0 min). This firmly adherent layer could, however, be removed by gentle scraping. Similar measurements in the rat colon have shown firm and loose mucus layers of ≈100- and 700-μm thickness, respectively (5). After aspiration, the loose layer was slowly replenished, as shown by the increasing mucus thickness when measurements were taken 15 and 30 min after the initial aspiration (Fig. 1B, F + 15 and F + 30 min). To prove that the newly formed layer was indeed loosely adherent mucus, we performed a second aspiration that decreased the thickness to the firm level (Fig. 1B, F 30 min).
Composition of the Two Mucus Layers.
We next investigated the components of the two colonic mucus layers by separating reduced and alkylated samples of mucus by composite agarose-PAGE (AgPAGE). Upon gel staining with Alcian blue to visualize mucin bands, we detected two major bands for Muc2, corresponding to Muc2 monomer (the smallest detectable component of Muc2, marked M) and a likely dimer (3) that were similarly represented in the loose- and firm-mucus layer preparations (Fig. 1C). Mass spectrometry of the tryptic peptides showed Muc2 as the only gel-forming mucin detected in these bands. The identified peptides encompassed 30% of the protein (excluding the PTS domains) and included most of the peptides expected not to be glycosylated [Fig. 1A and supporting information (SI) Table S1]. Only minor peptide differences, localized to the N-terminal part, were observed between Muc2 from the firm and loose mucus. The intensity of the bands and peptide representation suggest that the Muc2 mucin is a major constituent of both the firm and loose mucus layers. Upon analysis of the small-sized protein components of the loose and firm mucus by PAGE and Coomassie blue staining, identical patterns for the two mucus layers were observed (Fig. S1). A detailed comparison identified proteins that were intracellular components, serum proteins, and likely mucus constituents. Out of these, the secreted proteins and proteins with large extracellular domains as well as their association to the loose and/or firm layers is presented in Table 1 and Tables S2 and S3, revealing that the proteins were present in both the firm and loose mucus layers (some proteins were only identified under less-stringent conditions). The expression of some of these proteins was further verified by immunostaining (Fig. S2) showing Clca3 expression in the granules of the goblet cells as shown before (6), a localization also shown for Fcgbp (7). That the composition of the loose and firm mucus layers is almost identical suggest that the loose mucus layer is generated from the firm mucus layer.
Table 1.
The proteins of the loose (L) and firm (F) mucus were separated by PAGE (Fig. S1) and the proteins identified as tryptic peptides by LC-MS/MS
| Protein name | Gene | Loc | L* | F* |
|---|---|---|---|---|
| Anterior gradient 2 | Agr2 | sec | x | x |
| Ca-activated Cl channel 3 | Clca3 | sec | x | x |
| Ca-activated Cl channel 6 | Clca6 | sec | x | x |
| IgG Fc-binding protein | Fcgbp | sec | x | x |
| γ-Glutamyl hydrolase | Ggh | sec | x | x |
| Immunoglobulin | Ig | sec | x | x |
| Lumican | Lum | sec | x | x |
| Mucin 2 | Muc2 | sec | x | x |
| Major urinary proteins 11 and 8 | Mup | sec | x | x |
| Oncoprotein-induced transcript 1 | Oit1 | sec | x | x |
| Mucosal pentraxin | Ptx | sec | x | x |
| Arginyl aminopeptidase | Rnpep | sec | x | x |
| Seminal vesicle secretory protein IV | Svs4 | sec | x | x |
| Zymogen granule membrane protein 16 | Zg16 | sec | x | x |
| Mucin 3(17), orthologue hMUC17 | Muc3(17) | sec-tm1 | x | (x) |
| Vomeroglandin | Dmbt1 | sec-tm1 | x | (x) |
| Polymeric immunoglobulin receptor | Pigr | sec-tm1 | x | (x) |
| Quiescinsulfhydryl oxidase 1 | Qsox1 | sec-tm1 | x | (x) |
| Mucin13 | Muc13 | tm1 | x | x |
| Basigin | Bsg | tm1 | x | x |
| Uvomorulin | Cdh1 | tm1 | x | x |
| Cadherin 17 | Cdh17 | tm1 | x | x |
| CEACAM 1 | Ceacam1 | tm1 | x | x |
| Hephaestin | Heph | tm1 | x | x |
| Mucin-like protocadherin | Mupcdh | tm1 | x | x |
| Nicastrin | Ncstn | tm1 | x | x |
| Protocadherin LKC | Pcdh | tm1 | x | x |
| Tumor-associated Ca signal transducer 1 | Tacstd1 | tm1 | x | x |
| Maltase-glucoamylase | Mgam | tm2 | x | (x) |
Only secreted proteins or transmembrane proteins with a large extracellular domain are listed. A detailed list is presented in Table S1. Loc, localization; sec, secreted protein; tm1, type 1 transmembrane protein; tm2, type 2 transmembrane protein.
*Identification: One peptide at 99% significance level (individual peptide score cutoff = 47) and one supporting peptide at 95% significance level (individual peptide score cutoff = 40) are marked with an x. Proteins only identified by peptides with ion score cutoff = 25 are marked by (x). Multiple entries for the same protein were combined, and identification in two of three analyses of at least one sample was required for inclusion.
When the amount of the Muc2 mucin recovered from an identical sealed surface area was compared, the firm layer was estimated to contain at least the double amount of Muc2 as compared with the loose layer when measured by the band intensity of Alcian blue stained gels (data not shown). Considering that the loose mucus layer is approximately twice as thick as the firm (Fig. 1B), this suggests a higher concentration of Muc2 by a factor of four in the firm relative to the loose mucus layer.
Properties of the Two Mucus Layers.
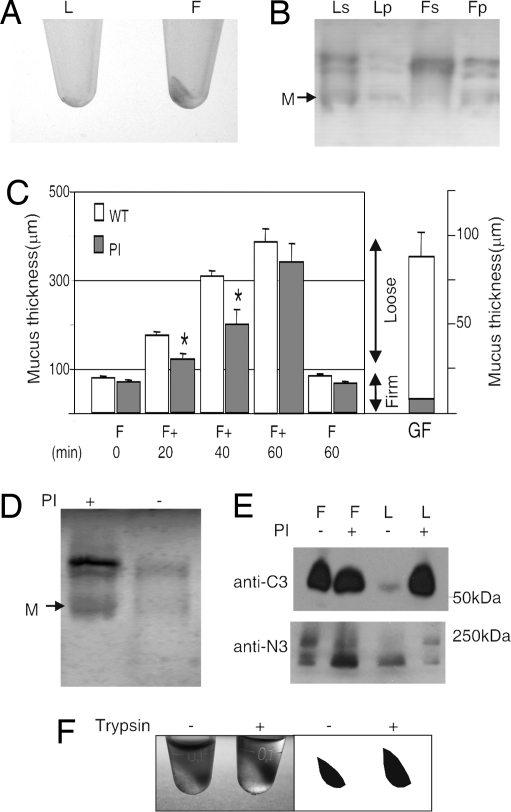
The Muc2 mucin from the intestine has been observed to be partly insoluble in chaotropic salts like guanidinium chloride (8, 9). To study whether the loose and firm mucus layers had different properties in this respect, both layers extracted with guanidinium chloride were separated by centrifugation into a soluble supernatant and an insoluble pellet. A clearly visible pellet could be detected only in the firm (F) layer, but not in the loose (L) (Fig. 2A). After reduction, alkylation, and dialysis, the samples were analyzed for mucins by AgPAGE and Alcian blue staining (Fig. 2B). The pellet of the guanidinium-insoluble firm mucus layer (Fp) contained the Muc2 mucin but only small amounts in the loose pellet.
Fig. 2.
The firm and loose mucus layers have different properties. (A) Loose (L) and firm (F) mucus were extracted by 6 M guanidinium chloride into soluble and insoluble pellet fractions. The insoluble pellet was photographed and is clearly visible in the firm mucus. (B) The soluble (s) and insoluble (p) fractions of loose (L) and firm (F) mucus after reduction of disulfide bonds were analyzed for mucins by AgPAGE and the gel stained with Alcian blue. The firm mucus pellet (Fp) contained Muc2 mucin, whereas the loose pellet (Lp) was almost devoid of Muc2. (C) The mucus thickness measured in rat distal colon in vivo overlaid with protease inhibitors (n = 4) or control (n = 16). The protease inhibitors (PI) were added after the first removal of the loose mucus. The firm (F) mucus is presented together with the regeneration of the loose (F+) after 20, 40, and 60 min and a final measurement of the firm (F) mucus after removal of the loose mucus. Values are mean ± SEM. *, P < 0.05 vs. untreated. The germ-free (GF) mice had a loose (white) and firm (gray) mucus layer as shown to the right (n = 5). (D) Mucus from the same measured surface area of the loose material was removed at 60 min after protease inhibitor (PI) treatment, reduced and analyzed on AgPAGE, and stained with Alcian blue. M, Muc2 monomer. (E) Western blot analysis of reduced loose and firm mucus after 60 min with or without protease inhibitor treatment. The bands were detected by the anti-MUC2N3 (anti-N3) or anti-MUC2C3 (anti-C3) antisera. (F) Equal amounts by volume of guanidinium chloride-insoluble mucus pellets from mouse colon (A) were treated or nontreated with trypsin for 3 h, resulting in volume expansion of the pellet in the trypsin-treated sample as shown by the photo and a graphic representation of the pellets.
Because the loose mucus layer had different properties than the firm layer, yet the Muc2 mucin was a major component, we hypothesized that proteolytic cleavages could be responsible for the transformation from firm to loose mucus layer. After removal of the loose mucus in rat colon, a protease inhibitor (PI) mixture inhibiting serine and cysteine proteases was added to the firm mucus layer in vivo (Fig. 2C). This caused a decreased replenishing rate of the loose mucus (F + 20 and 40 min), but the thickness of the firm layer was not affected (F 60 min). To our surprise, the newly formed loose mucus as analyzed by reduced AgPAGE revealed an increased amount of intact full-length Muc2 mucin in the PI-treated sample by a factor of three (Fig. 2D). The loose mucus was also analyzed for proteolytic fragments of the Muc2 mucin by reduced PAGE and Western blot using anti-Muc2 N- and C-terminal specific antisera (Fig. 2E). The band patterns were similar for the PI-treated loose layer and firm layer that showed identical patterns irrespectively of PI addition. However, the nontreated loose mucus was different, suggesting that the Muc2 mucin of the loose mucus layer normally undergoes additional proteolytic cleavages, deleting fragments especially from the C terminus. The smaller volume and higher Muc2 concentration in the PI-treated loose mucus imply a protease-dependent volume expansion in this layer. The presence of a loose mucus layer also in germ-free animals suggests that the formation of a loose mucus is due to endogenous processes/proteases (Fig. 2C Right). The identities of these activities are still not known, but all proteases identified by proteomics are presented in Table S4. As the mucus gel is still intact, some of the proteolytic cleavages must maintain an intact Muc2 polymer that allow volume expansion. Such phenomena are most likely due to cleavages in the disulfide bond-stabilized, compact N- and C-terminal domains (4, 10). This interpretation is substantiated by the observation that treatment of the guanidinium chloride-insoluble Muc2 pellet with trypsin does not dissolve the pellet, but allows a volume expansion (Fig. 2F).
Bacteria Are Excluded from the Inner Stratified Mucus Layer.
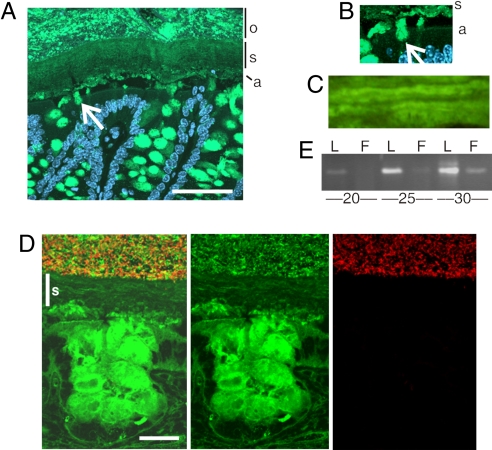
Immunostaining of Carnoy-fixed mouse distal colon with an anti-Muc2 antiserum (green) identified two major mucus layers in addition to Muc2-positive goblet cells (Fig. 3A and control Fig. S3). The staining of the inner layer was characterized by a well organized stratified lamellar appearance, suggesting that it was formed by sheets of polymerized Muc2 (s in Fig. 3 A and C). This is consistent with the notion that the N-terminal trimers in the Muc2 polymers (4) should favor such an organization. This layer was after fixation ≈25-μm thick and probably represented the firm mucus layer observed in vivo (Fig. 1B). Under this, a thin and more intensely stained layer was observed that could correspond to a not yet fully organized mucus layer (a in Fig. 3 A and B). This is directly linked to the goblet cells with Muc2-positive streaks (white arrow). As the firm mucus layer is attached to the epithelia in colon, it is possible that this anchoring is to the goblet cells and mediated by these Muc2-containing streaks. The outer Muc2 mucus layer (o in Fig. 3A) is characterized by a differently stained Muc2 and probably corresponds to the loose mucus layer. The border was relatively sharp, suggesting a controlled transition from the inner stratified to the outer layer.
Fig. 3.
The firm mucus layer is devoid of bacteria. (A) Muc2-positive goblet cells and overlaying mucus layers in a section of the mouse distal colon were detected by using the anti-MUC2C3 antiserum (green). The section is counterstained with DAPI to visualize nuclei. An inner stratified mucus layer (s) is linked via Muc2-stained threads (white arrow) to the goblet cells of the surface epithelia. The lowest part of the inner mucus layer is stained differently (a), suggesting a different organized form compared to the stratified (s) layer. The outer mucus layer (o) is mixed with the luminal content. (Scale bar: 50 μm.) (B) Magnification of the interface between the epithelial surface and the mucus layer, marked as in A. (C) Magnification of the inner mucus layer displayed a stratified pattern suggesting a lamellar organization. (D) Combined Muc2 immunostaining (green) as in A and FISH analysis using the general bacterial probe EUB338-Alexa Fluor 555 (red) of distal colon shows Muc2-positive goblet cells and an inner stratified (s) mucus layer on the epithelium. This layer is devoid of bacteria, which can only be detected in the outer mucus layer. The inner mucus generates a spatial separation between the cells and the microflora. (Scale bar: 20 μm.) (E) Semiquantitative PCR of bacterial 16S gene using DNA isolated from equal surface areas of the loose (L) and firm (F) mucus demonstrated more bacteria in the loose mucus layer. Amplification was done for 20, 25, and 30 cycles as indicated.
Next, we analyzed the tissue sections for bacterial presence by in situ hybridization using a general 16S rRNA probe (Fig. 3D, negative control in Fig. S4). Bacteria were detected (red) in the outer mucus layer and were excluded from the inner stratified layer (s). This observation was supported by semiquantitative PCR using primers specific for conserved regions of the bacterial 16S rRNA genes performed on DNA isolated from the loose and firm mucus layers. Considerably less bacterial DNA was detected by PCR in the firm mucus layer (Fig. 3E) where the smaller amounts of bacteria in the firm layer could be due to difficulties in fully removing the loose layer. Thus the inner firm mucus layer, devoid of bacteria, could act as a physical barrier impenetrable to bacteria, whereas the expanded Muc2 in the loose mucus layer is a major habitat for commensal bacteria.
Mice Lacking the Muc2 Mucin Have Bacteria in Direct Contact with the Epithelial Cells.
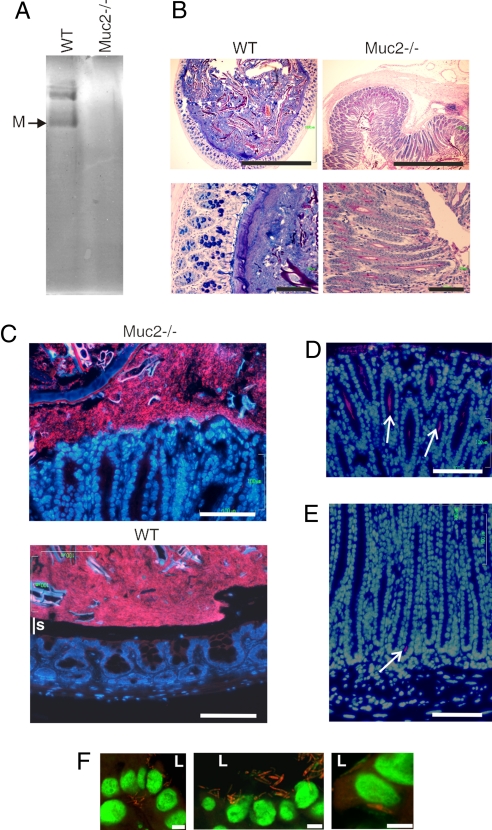
Because Muc2 is a major component of the colonic mucus, we first asked whether mice lacking the Muc2 mucin (11) expressed compensatory mucins. AgPAGE did not reveal any major mucin bands (Fig. 4A), as was also confirmed by proteomics studies of Muc2−/− mice that failed to identify peptides corresponding to other gel-forming mucins. Furthermore, there was a loss of Alcian blue-positive goblet cells (Fig. 4B), cells still identified by Clca3 (Fig. S2). A dramatic elongation of the crypts was observed (11, 12). We next analyzed Muc2−/− colonic sections for bacteria by using FISH analysis. Contrary to wild-type mice, the colonic epithelium of Muc2−/− mice was not covered by a mucus layer devoid of bacteria (Fig. 4C). Instead, bacteria were in direct contact with the epithelial cells (nuclei stained with DAPI). The bacteria not only reached the epithelium but were also detected deep down into the crypts (Figs. 4 D and E). In some instances, bacteria were also observed close to the nuclei or below the nuclei, indicating intracellular bacteria in some epithelial cells (Fig. 4F). These data are in agreement with the fact that Muc2−/− mice were characterized by a slower growth and developed colitis by 7 weeks of age (Fig. S5). A similar phenotype was reported previously as well as the development of colon cancer after 6–12 months (11, 12).
Fig. 4.
In Muc2−/− mice the bacteria are in close contact with the epithelia and enter into the crypts and cells. (A) Total mucus scraped from C57BL/6 (WT) and Muc2−/− colon were separated by reduced AgPAGE and the gel stained with Alcian blue. No major compensating mucins were observed in the sample from Muc2−/− mice as also revealed by proteomics. (B) Fixed sections of the distal colon from WT or Muc2−/− mice were stained with Alcian blue/PAS. The samples are shown in the same scale relative to each other. Muc2−/− animals have an enlarged colon diameter with elongated crypts. (Scale bars: 1 mm (Upper) and 100 μm (Lower). (C) FISH using the EUB338-Alexa Fluor 555 probe staining bacteria and DAPI DNA staining in colon show a clear separation of the bacterial DNA and epithelial surface in WT mice, but not in Muc2−/−. This separation corresponds to the stratified mucus layer (s). (Scale bar: 100 μm.) (D and E) Alcian blue/PAS and FISH staining of Muc2−/− colon show bacteria far down into the crypts as indicated by white arrows. This was never observed in WT mice. (Scale bar: 100 μm.) (F) Bacteria were detected close to the nuclei (1–5 μm) or basal of the nuclei in some epithelial and in detached cells. Confocal microscopy of a 1.5-μm optical slice with bacteria (EUB338) red and DNA green. L, lumen. (Scale bars: 5 μm.)
Discussion
The firm mucus layer of colon is dense with a high concentration of Muc2 and with special properties as reflected by the Muc2 mucin guanidinium chloride insolubility. This inner layer is devoid of bacteria, suggesting small pore sizes that physically block bacteria from entering. Specific antimicrobial properties in the firm layer could be an alternative explanation, but the similar protein composition shown for these two layers makes this less likely. Such a physical separation of bacteria from the epithelial cells has previously been suggested (13). The firm layer is attached to the underlying epithelia. This is in contrast to the small intestine where the same Muc2 mucin is not attached and directly forms a soluble mucus gel (5). The mechanism for attaching the inner firm mucus layer is not known, but the Muc2-containing streaks suggest an attachment to the goblet cells, maybe via the Muc2 mucin.
Because the mucin and general protein components of both the firm and loose layers are identical, the most likely explanation is that the loose mucus layer is formed from the firm. The mechanisms for this transition from firm to a loose layer and detachment from the epithelium is currently not understood. The thickness of the firm layer is remarkably constant with a small variation from animal to animal also after a second removal of the loose mucus (see Fig. 1 B and C). This suggests a well controlled process. That the thickness of the firm layer was not affected by the PI treatment excludes the involvement of serine and cysteine proteases but not other proteases. In contrast to this, the volume expansion of the formed outer loose mucus that results in a less dense and guanidinium chloride-soluble gel, involve proteolytic enzymes that target the Muc2 mucin. The proteases responsible for this process have not been identified, and could be of cellular and bacterial origin. However, because germ-free mice still had a loose mucus layer, cellular proteases are more likely. Proteolytic cleavages within the large Cys-rich Muc2 N- and C-termini allows a volume expansion of the gel without breaking the covalent polymer structure, as exemplified by the effect of the natural digestive enzyme, trypsin. Interestingly, the colon pathogen Entamoeba histolytica, which is able to penetrate the colonic mucus layer, secretes a very specific cysteine protease that cleaves the guanidinium-insoluble Muc2 mucin of the inner firm layer at one specific site (14). This dissolves the gel and allows the parasite to penetrate. This cleavage disrupts the gel because the target sequence is localized outside of the Cys-rich C terminus of the human MUC2 mucin. Trypsin, on the other hand, only cleaves within the Cys-rich N- and C-terminal domains that remain held together by intramolecular disulfide bonds.
The outer loose mucus layer is thus less dense and contains a high number of bacteria where it seems to provide an ideal habitat for the commensal flora. The numerous O-glycans of the Muc2 mucin can provide attachment sites that could be used by the bacterial adhesins but, more importantly, act as an important energy sources for the commensal bacteria (15). The inner firmly attached mucus layer forms a specialized physical barrier that excludes the resident bacteria from a direct contact with the underlining epithelium. This organization of the colon mucus, as based on the properties of the Muc2 mucin, should be ideal for excluding bacteria from contacting the epithelial cells and thus also the immune system. Alterations or the absence of these protective layers, as in the Muc2−/− mouse colon, allow bacteria to have a direct contact with epithelial cells, to penetrate lower into the crypts and also translocate into epithelial cells. That such a close contact between bacteria and epithelia can trigger an inflammatory response is easy to understand and chronic inflammation is known to be an important ingredient in colon cancer development. The importance of the Muc2 mucin in organizing the colon mucus protection is further strengthen by the recent observation that two mouse strains with diarrhea and colon inflammation were shown to have two separate spontaneous mutations in the Muc2 mucin (16). Taken together, the MUC2 mucin can be suggested to have a role in the pathogenesis of the inflammatory colon disease ulcerative colitis. However, further studies on the properties of the MUC2 mucin and other mucus components in relation to mucus formation, commensal flora, and ulcerative colitis are necessary to further advance our understanding of the normal and diseased colon.
Materials and Methods
Animals.
All mice including the germ-free were C57BL/6, and the rats were Sprague–Dawley. The Muc2−/− mice have been described before (11). Animal experimental procedures were approved by the Animal Ethical Committee in Gothenburg and Uppsala.
Measurements of the Mucus Thickness in Vivo.
The animals were anesthetized and prepared and the mucus measured with a micropipette connected to a micromanipulator in a 30° angle as described before for rat (5) and adapted to mouse (17). The mucus thickness, the vertical distance between the cell surface and the luminal mucus surface, was calculated (18). The results are expressed as mean ± SEM. To compare single values, Student's t test for paired or unpaired data was used. The differences were regarded as significant at P < 0.05. After removal of the loose layer during mucus measurements in rat, the firm mucus was covered with 2× complete EDTA-free protease inhibitor (Roche) in PBS. Mucus measurements were performed at specified times, followed by a second removal. Untreated rats were used as controls by following the same protocol.
SDS–Agarose Composite Gel Electrophoresis for Separation of Mucins.
Mucus from the colon was removed from an identical, measured epithelial surface by suction (loosely adherent) or scraped (firmly adherent), and protease inhibitors and complete EDTA-free protease inhibitor (Roche) were added. The samples, equalized to identical surface area were reduced in sample buffer with 100 mM dithiotreitol DTT at 95°C and alkylated by iodoacetamide or 4-vinyl pyridine (2.5 molar excess of DTT). A composite gel (AgPAGE) containing agarose (0.5–1% gradient), acrylamide (0–6%), and glycerol (0–10%) was used for analysis (19). The ImageJ software (National Institutes of Health) was used for relative quantification of Alcian blue stained bands.
Polyacrylamide Gel Separation and Western Blot Analysis.
Loose and firm mucus was sampled from the measured area or from the distal half of a dissected colon, with fecal pellets removed, and reduced by DTT in sample buffer and analyzed by 4–12% SDS/PAGE. The gels were stained by Coomassie with Imperial stain (Pierce) or blotted by semidry Western blot to Immobilone P membranes (Millipore). Muc2 was detected with a polyclonal serum against the human MUC2 C-terminal peptide PHYVTFDGLYYSYQGNC (anti-MUC2C3) reacting with the mouse Muc2 or with a serum against the N-terminal D3 domain (20). The HRP-conjugated secondary antibody was detected by Supersignal West Pico (Pierce).
Trypsin Digestion of Proteins, LC-MS/MS Analysis, and Protein/Mucin Identification.
Alcian blue-stained bands from AgPAGE or whole lane (total protein content in 22 bands) from 4–12% SDS/PAGE stained with Imperial stain (Pierce) were selected and excised. The proteins were digested in-gel by trypsin (Promega) and the peptides eluted and analyzed by nano-LC coupled to a hybrid linear ion trap-FT-ICR MS equipped with a 7T ICR magnet (LTQ-ICR(FT); Thermo) (21). Searches were performed by using MASCOT (Matrix Science). The search parameters were set to MS accuracy 5 ppm, MS/MS accuracy 0.5 Da, one missed cleavage allowed, fixed carbamidomethyl, S-pyridylethyl or propionamid modification of cysteine, and variable modification of oxidized methionine. The mass spectra from the LC-ICR MS/MS experiments were searched against an in-house mucin database containing the assembled mouse mucin sequences (www.medkem.gu.se/mucinbiology/) or the nonredundant protein sequence database downloaded from the National Center for Biotechnology Information (NCBI) October 11, 2007. The mucin bands in each lane from AgPAGE were merged, and the results from three analyses were assembled. The total protein content from one SDS/PAGE lane were merged (22 excised bands), and three separate analysis of loose and firm were compared.
Histology and Immunostaining.
Segments of the distal colon without washing from 10- to 14-week-old animals were fixed in Methanol–Carnoy's fixative. Paraffin-embedded sections were dewaxed and hydrated. Antigens were retrieved by Retrievagen A (BD). Sections were stained with Alcian blue/PAS or by the anti-MUC2C3 antiserum with FITC-conjugated goat anti-rabbit immunoglobulins (DAKO) and DAPI. Pictures were obtained by using a Nikon Eclipse E1000 fluorescence microscope, and a LSM 510 (Zeiss) or a Radiance 2000 (Bio-Rad) confocal microscope.
Isolation and Preparation of Soluble and Insoluble Mucins.
Loose and firm mucus samples were extracted in 6 M guanidinium chloride (22) supplemented with protease inhibitors 2× Complete protease inhibitor mix (Roche), stirred overnight at +4°C and centrifuged for 20 min at 16,000 × g. Extractions were repeated three times. Pellets were photographed, and samples were reduced in guanidinium chloride with DTT at 37°C for 3 h and alkylated with iodoacetamide overnight. The samples were dialyzed against water and solubilized in sample buffer.
Trypsin Treatment of Insoluble Mucus Pellet.
Guanidinium chloride extracted mucus was washed three times in 100 mM Tris·HCl (pH 8.5) and mixed with 10 μg of trypsin (Lonza) or buffer as control. The mucus was pelleted by centrifugation at 16,000 × g for 20 min and incubated at 37°C for 3h. The volumes of the pellets were photographed with bromophenol blue in the supernatant.
PCR of Bacteria 16S rRNA Genes in Loose and Firm Mucus.
The loose mucus was sampled from the distal part of a dissected colon, and the colon was washed once with PBS before sampling the firm mucus, both loose and firm were from an identical surface area. DNA was prepared with the Nuclospin tissue kit (Macharey Nagel) with a preceding digestion with 2 mg of Lysozyme. Samples were analyzed by PCR with universal primers directed against a region of the 16S rRNA gene common to most bacteria: set1 (forward 5′-CCATGAAGTCGGAATCGCTAG-3′and reverse, 5′-ACTCCCATGGTGTGACGG-3′) (bp 1302–1394 in bacteria EU622773) and set2 (forward, 5′-TCCTACGGGAGGCAGCAGT-3′; reverse, 5′-GGACTACCAGGGTATCTAATCCTGTT-3) (bp 339–780 in bacteria EU622773) (23). The PCR (95°C 30 s, 58°C 30 s, 72°C 45 s) was performed for 20, 25, or 30 cycles, with an initial step of 5 min at 95°C and final extension of 5 min at 72°C by using TaqDNA polymerase (NEB). Products were analyzed by agarose gel electrophoresis. Both PCR sets show similar results represented by set 2 in Fig. 3E.
Fluorescence in Situ Hybridization.
Paraffin sections were dewaxed and washed in 95% ethanol. The tissue sections were incubated with 250 μg Alexa Fluor 555-conjugated EUB (5′-GCTGCCTCCCGTAGGAGT-3′) (bp 337–354 in bacteria EU622773) (24) in 50 μl of hybridization buffer [20 mM Tris·HCl (pH 7.4), 0.9 M NaCl, 0.1% SDS] at 50°C overnight. The sections were rinsed in wash buffer [20 mM Tris·HCl (pH 7.4), 0.9 M NaCl], washed at 50°C for 20 min and counterstaining with DAPI or Sytox Green DNA stain (Invitrogen). Coimmunostaining ith anti-MUC2C3 was performed at 4°C without antigen retrieval and mounted in prolong Gold antifade (Invitrogen).
Supplementary Material
Acknowledgments.
We acknowledge the Proteomics Core Facility and Centre for Cellular Imaging at University of Gothenburg. This work was supported by Swedish Research Council Grants 7461, 8646, and 20680 and the Swedish Research Council provided equipment. Support was also provided by the Swedish Cancer Foundation, the Swedish Foundation for Strategic Research–Mucosal Immunobiology and Vaccine Center, and the IngaBritt and Arne Lundberg Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0803124105/DCSupplemental.
References
- 1.Lang T, Hansson GC, Samuelsson T. Gel-forming mucins appeared early in metazoan evolution. Proc Natl Acad Sci USA. 2007;104:16209–16214. doi: 10.1073/pnas.0705984104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gum JR, Hicks JW, Toribara NW, Siddiki B, Kim YS. Molecular cloning of human intestinal mucin (MUC2) cDNA. Identification of the amino terminus and overall sequence similarity to prepro-von Willebrand factor. J Biol Chem. 1994;269:2440–2446. [PubMed] [Google Scholar]
- 3.Axelsson MAB, Asker N, Hansson GC. O-glycosylated MUC2 monomer and dimer from LS 174T cells are water-soluble, whereas larger MUC2 species formed early during biosynthesis are insoluble and contain nonreducible intermolecular bonds. J Biol Chem. 1998;273:18864–18870. doi: 10.1074/jbc.273.30.18864. [DOI] [PubMed] [Google Scholar]
- 4.Godl K, et al. The N-termini of the MUC2 mucin form trimers that are held together within a trypsin-resistant core fragment. J Biol Chem. 2002;277:47248–47256. doi: 10.1074/jbc.M208483200. [DOI] [PubMed] [Google Scholar]
- 5.Atuma C, Strugula V, Allen A, Holm L. The adherent gastrointestinal mucus gel layer: thickness and physical state in vivo. Am J Physiol. 2001;280:G922–G929. doi: 10.1152/ajpgi.2001.280.5.G922. [DOI] [PubMed] [Google Scholar]
- 6.Komiya T, Tanigawa Y, Hirohashi S. Cloning and identification of the gene Gob-5, which is expressed in intestinal goblet cells in mice. Biochem Biophys Res Commun. 1999;255:347–351. doi: 10.1006/bbrc.1999.0168. [DOI] [PubMed] [Google Scholar]
- 7.Kobayashi K, et al. Distribution and partial characterisation of IgG Fc binding protein in various mucin producing cells and body fluids. Gut. 2002;51:169–176. doi: 10.1136/gut.51.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carlstedt I, et al. Characterization of two different glycosylated domains from the insoluble mucin complex of rat small intestine. J Biol Chem. 1993;268:18771–18781. [PubMed] [Google Scholar]
- 9.Herrmann A, et al. Studies on the “insoluble” glycoprotein complex from human colon. J Biol Chem. 1999;274:15828–15836. doi: 10.1074/jbc.274.22.15828. [DOI] [PubMed] [Google Scholar]
- 10.Lidell ME, et al. The recombinant C-terminus of the human MUC2 mucin forms dimers in CHO cells and heterodimers with full-length MUC2 in LS 174T cells. Biochem J. 2003;372:335–345. doi: 10.1042/BJ20030003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Velcich A, et al. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science. 2002;295:1726–1729. doi: 10.1126/science.1069094. [DOI] [PubMed] [Google Scholar]
- 12.Van der Sluis M, et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology. 2006;131:117–129. doi: 10.1053/j.gastro.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 13.Swidsinski A, Weber J, Loening-Baucke V, Hale LP, Lochs H. Spatial organization and composition of the mucosal flora in patients with inflammatory bowel disease. J Clin Microbiol. 2005;43:3380–3389. doi: 10.1128/JCM.43.7.3380-3389.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lidell ME, Moncada DM, Chadee K, Hansson GC. Entamoeba histolytica cysteine proteases cleave the MUC2 mucin in its C-terminal part and dissolves the protective colonic mucus gel. Proc Natl Acad Sci USA. 2006;103:9298–9393. doi: 10.1073/pnas.0600623103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host–bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 16.Heazlewood CK, et al. Aberrant mucin assembly in mice causes endoplasmic reticulum stress and spontaneous inflammation resembling ulcerative colitis. PLoS Med. 2008;5:e54. doi: 10.1371/journal.pmed.0050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henriksnas J, et al. Impaired mucus–bicarbonate barrier in Helicobacter pylori-infected mice. Am J Physiol. 2006;291:G396–G403. doi: 10.1152/ajpgi.00017.2006. [DOI] [PubMed] [Google Scholar]
- 18.Malmberg EK, et al. Increased levels of mucins in the cystic fibrosis mouse small intestine and modulator effects of the Muc1 mucin expression. Am J Physiol. 2006;291:G203–G210. doi: 10.1152/ajpgi.00491.2005. [DOI] [PubMed] [Google Scholar]
- 19.Schultz BJ, Oxley D, Packer NH, Karlsson NG. Identification of two highly sialylated human tear-fluid DMBT1 isoforms: The major high-molecular-mass glycoproteins in human tears. Biochem J. 2002;366:511–520. doi: 10.1042/BJ20011876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asker N, Axelsson MAB, Olofsson SO, Hansson GC. Dimerization of the human MUC2 mucin in the endoplasmic reticulum is followed by a N-glycosylation-dependent transfer of the mono- and dimers to the Golgi apparatus. J Biol Chem. 1998;273:18857–18863. doi: 10.1074/jbc.273.30.18857. [DOI] [PubMed] [Google Scholar]
- 21.Andersch-Björkman Y, Thomsson KA, Holmén Larsson JM, Ekerhovd E, Hansson GC. Large-scale identification of proteins, mucins and their O-glycosylation in the endocervical mucus during the menstrual cycle. Mol Cell Proteomics. 2007;6:708–716. doi: 10.1074/mcp.M600439-MCP200. [DOI] [PubMed] [Google Scholar]
- 22.Carlstedt I, Lindgren H, Sheehan JK. The macromolecular structure of human cervical-mucus glycoproteins. Biochem J. 1983;213:427–435. doi: 10.1042/bj2130427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.An G, et al. Increased susceptibility to colitis and colorectal tumors in mice lacking core 3-derived O-glycans. J Exp Med. 2007;204:1417–1429. doi: 10.1084/jem.20061929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amann RI, et al. Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl Environ Microbiol. 1990;56:1919–1925. doi: 10.1128/aem.56.6.1919-1925.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.