Abstract
Plant growth depends on the integration of environmental cues and phytohormone-signaling pathways. During seedling emergence, elongation of the embryonic stem (hypocotyl) serves as a readout for light and hormone-dependent responses. We screened 10,000 chemicals provided exogenously to light-grown seedlings and identified 100 compounds that promote hypocotyl elongation. Notably, one subset of these chemicals shares structural characteristics with the synthetic auxins, 2,4-dichlorophenoxyacetic acid (2,4-D), and 1-naphthaleneacetic acid (1-NAA); however, traditional auxins (e.g., indole-3-acetic acid [IAA], 2,4-D, 1-NAA) have no effect on hypocotyl elongation. We show that the new compounds act as “proauxins” akin to prodrugs. Our data suggest that these compounds diffuse efficiently to the hypocotyls, where they undergo cleavage at varying rates, releasing functional auxins. To investigate this principle, we applied a masking strategy and designed a pro-2,4-D. Unlike 2,4-D alone, this pro-2,4-D enhanced hypocotyl elongation. We further demonstrated the utility of the proauxins by characterizing auxin responses in light-grown hypocotyls of several auxin receptor mutants. These new compounds thus provide experimental access to a tissue previously inaccessible to exogenous application of auxins. Our studies exemplify the combined power of chemical genetics and biochemical analyses for discovering and refining prohormone analogs with selective activity in specific plant tissues. In addition to the utility of these compounds for addressing questions related to auxin and light-signaling interactions, one can envision using these simple principles to study other plant hormone and small molecule responses in temporally and spatially controlled ways.
Keywords: chemical genetics, light
Plant growth and development are regulated by environmental and endogenous cues. Under limited light, dicotyledonous seedlings, such as those of Arabidopsis, have long hypocotyls and underdeveloped leaves. In contrast, seedlings grown under high irradiance have short hypocotyls and expanded leaves. Although genetic and physiological studies have revealed the tractability of these differential growth responses, the molecular details of how the light signal is translated into the cellular mechanics of expansion and division remain poorly understood.
Environmental signals that regulate plant growth are mediated by small-molecule phytohormones. Hypocotyl elongation is positively or negatively controlled by signals initiated by distinct plant hormones (1). Among these, brassinosteroids (BRs), auxins, and gibberellins (GAs) positively modify hypocotyl length in both light and dark conditions, as implicated by the short hypocotyl phenotype of their corresponding insensitive or deficient mutants. In accordance with this property, exogenous application of GAs and BRs to wild-type light-grown seedlings stimulate growth (2, 3). The promotion of shoot growth by auxin has been more difficult to study. Although plants that overproduce auxin have long hyocotyls (4–7), this elongation effect cannot be mimicked by exogenous application of auxin (either indole-3-acetic acid [IAA] or one of its commonly used synthetic analogs, 2,4-dichlorophenoxyacetic [2,4-D] or 1-naphthaleneacetic acid [1-NAA]) to light-grown seedlings (8). This is in contrast to the observed growth-promoting effects of auxin in excised stems and hypocotyls, which may provide a more direct route to auxin internalization (9). Although a few exceptions have been reported (5, 10–14), exogenous application of auxins usually produces an inhibitory effect (and rarely a stimulatory effect) on the hypocotyl growth of intact seedlings germinated and grown under standard conditions.
Application of small bioactive molecules (<500Da) in forward genetics screens has successfully uncovered signaling mechanisms (15–17). However, only a few attempts have been made to systematically screen for novel modifiers of a biological phenomenon of interest, an approach called chemical genetics or chemical genomics (18–22). Among the various advantages of chemical genetics is potential access to new agonists or antagonists that affecting pathways of interest in a spatially or temporally controlled fashion. These “pharmacologic” approaches can be used to confer an acute response in a controlled manner and to serve as a basis for novel forward genetic screens.
We conducted a high-throughput chemical genetic screen on Arabidopsis thaliana seedlings with the goal of identifying novel modulators of plant growth. The screen yielded 100 compounds with structural components reminiscent of auxin analogs but with what appeared to be bipartite structures composed of a synthetic auxin mimic and a chemical masking agent. Analysis of specific compounds shows that they act as “proauxins,” which undergo in situ hydrolysis in plants to liberate an auxin mimic. Thus, the “prodrug” nature of the compounds is required for efficient induction of hypocotyl length, most likely by facilitating tissue-specific localization, cellular uptake, and varying hydrolysis rates. These new bipartite auxin-like compounds allow examination of auxin signaling responses using hypocotyl elongation as a readout and provide a new platform for spatially and temporally controlled genetic screens and physiological assays.
In addition, we used the novel auxin-like compounds to characterize auxin responsiveness in hypocotyls of auxin receptor mutants in single and double mutant combinations. We found that different combinations exerted distinct degrees of auxin insensitivity, suggesting that auxin receptors play discrete roles in shoot growth. Finally, we found that the compounds can specifically rescue the hypocotyl elongation defects in an auxin-deficient mutant, sav3, but cannot rescue a BR-deficient mutant (23). The sav3 mutant is involved in auxin-mediated cell elongation under shade conditions, where the ratio between red and far-red light is low (23). Thus, our new small molecules are bona fide masked auxins that can be applied to questions in auxin signaling, as well as in interactions of auxin with different signaling pathways in the Arabidopsis shoot.
Results and Discussion
Bipartite Synthetic Auxin Small-Molecule Conjugates with Growth-Stimulating Activity in Intact Plants.
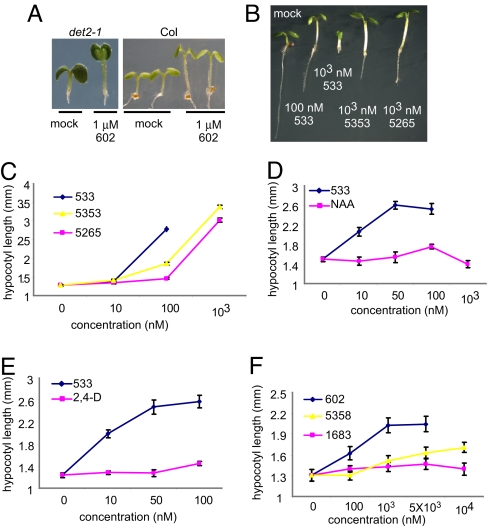
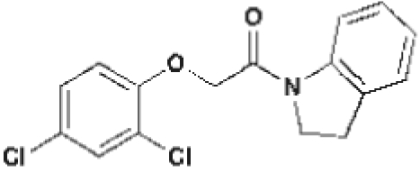
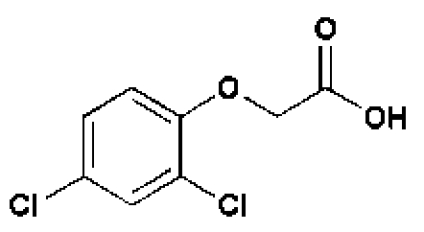
In an attempt to identify new growth-promoting compounds, we compiled a diverse chemical library and scored individual compounds for their ability to promote hypocotyl elongation in the BR-deficient dwarf mutant, det2–1.The det2–1 mutant is a steroid 5α-reductase mutant with only ≈10% of the wild-type BR levels (24).The det2–1 seedlings (ca. 10/well) were grown in 96-well plates, each well containing a different chemical, and scored for long hypocotyls. Among the 10,000 compounds screened, 100 small molecules were recorded as positive; see supporting information (SI) Table S1. These compounds also were active in stimulating the hypocotyl growth of wild-type seedlings. The effect of one compound, designated 602, is shown in Table 1 and Fig. 1 A and F). The identified compounds share a structural feature in an otherwise bipartite chemical reminiscent of the commonly used auxin analogs, NAA and 2,4-D (Fig. 1B, Table, 1, and Tables S2–S4); however, NAA and 2,4-D were unable to efficiently stimulate growth under these conditions or at other tested concentrations (Fig. 1 D and E). In addition, the conjugated moiety 2-amino-4-picoline had no effect on roots and hypocotyl length (Fig. S1A).
Table 1.
ClogD analysis at various pH of representative synthetic auxin and bipartite proauxins used in this work
| Short Name | 602 | 533 | 5265 | 5353 | 602-UC | 2,4-D | NAA |
|---|---|---|---|---|---|---|---|
| Structure | 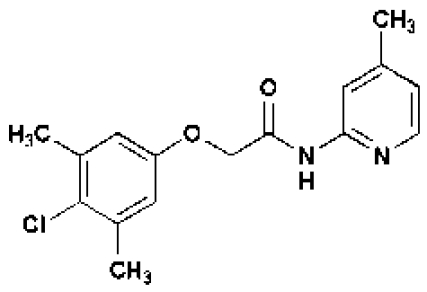 |
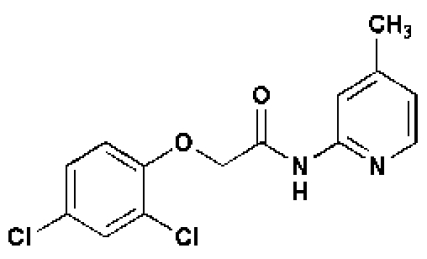 |
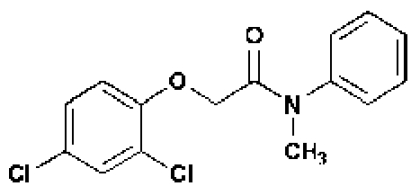 |
 |
 |
 |
|
| clog D | 3.98 (pH 5.0) | 3.56 (pH 5.0) | 3.68 (pH 5.0) | 3.66 (pH 5.0) | 1.33 (pH 5.0) | 0.19 (pH 5.0) | 2.24 (pH 5.0) |
| 4.11 (pH 5.5) | 3.69 (pH 5.5) | 3.68 (pH 5.5) | 3.66 (pH 5.5) | 0.85 (pH 5.5) | −0.28 (pH 5.5) | 1.86 (pH 5.5) | |
| 4.16 (pH 6.0) | 3.74 (pH 6.0) | 3.68 (pH 6.0) | 3.66 (pH 6.0) | 0.37 (pH 6.0) | −0.70 (pH 6.0) | 1.41 (pH 6.0) | |
| 4.17 (pH 6.5) | 3.76 (pH 6.5) | 3.68 (pH 6.5) | 3.66 (pH 6.5) | −0.08 (pH 6.5) | −1.01 (pH 6.5) | 0.93 (pH 6.5) | |
| 4.18 (pH 7.0) | 3.76 (pH 7.0) | 3.68 (pH 7.0) | 3.66 (pH 7.0) | −0.46 (pH 7.0) | −1.19 (pH 7.0) | 0.44 (pH 7.0) | |
| 4.18 (pH 7.5) | 3.76 (pH 7.5) | 3.68 (pH 7.5) | 3.66 (pH 7.5) | −0.70 (pH 7.5) | −1.27 (pH 7.5) | −0.02 (pH 7.5) |
Fig. 1.
Chemical genetic screening identified novel auxin-like bipartite compounds that induce hypocotyl growth in light-grown seedlings. (A) Compound 602 induced hypocotyl growth of light grown det2–1 (Left) and wild-type (Right) seedlings. (B and C) Comparison of different 2,4-D conjugates. Wild-type seedlings (6 days old) were grown in the presence or absence of different small molecules at varying concentrations. Note that 533, which was predicted to be hydrolyzed more efficiently, induced a hypocotyl length increase at lower concentrations as compared with 5353 and 5265. (D and E) Hypocotyl length of 6-day-old seedlings grown in the presence of 533 and NAA or 2,4-D, respectively. (F) Comparison of 602 with 5358 and 1638 bipartite compounds. The 5358 and 1683 compounds are predicted to be hydrolyzed at a slower rate compared with 602. Error bars indicate SE.
Like IAA, 2,4-D and NAA are relatively hydrophilic given their charged nature and depend to some extent on influx and efflux transporters, respectively, to move between cells (25–28; also see below and Table 1 and Tables S3 and S4). One hypothesis to explain their inability to promote hypocotyl elongation is that their relevant transporters are not active in this tissue. As such, we suspected that the identified bipartite synthetic conjugates, being largely uncharged, might diffuse more efficiently between cells before undergoing hydrolysis of their amide linkages to locally liberate active auxins. This is the case for sirtinol, a more potent auxin than either IAA or its downstream-metabolized auxin-like product (29). To test this idea, we searched a chemical database for commercially available bipartite compounds in which 2,4-D is conjugated to the same N-(4-methyl-2-pyridinyl)acetamide group as in compound 602. As predicted, one such compound, designated 5336619 (533) (www.emolecules.com), increased the height of light-grown seedlings, whereas 2,4-D and NAA did not (Table 1 and Fig. 1 B–E). In addition, we observed that 533 increased hypocotyl length when the seedlings were grown on vertically oriented plates, with the hypocotyl and cotyledons kept in direct contact with the agar medium (Fig. S1B). We conclude that cell elongation in hypocotyls depends on direct contact with the compound in question and/or its transport from the leaves rather than from the roots. Moreover, the promotion of hypocotyl elongation by exogenous application of the proauxin compounds occurs in low to moderate light intensity ranges (≈55 μmol m−2 s−1) and was almost diminished at an intensity of 90 μmol m−2 s−1.
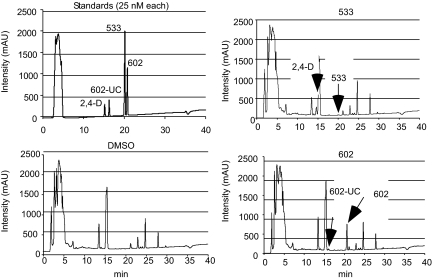
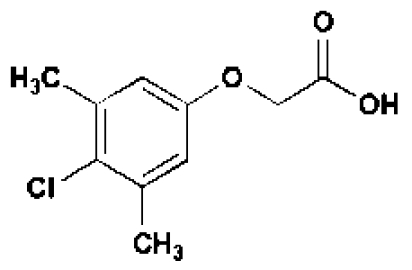
Liquid chromatography- mass spectrometry (LC-MS) analysis of seedlings incubated with 602 and 533 confirmed that the compounds underwent hydrolysis of the amide linkages to liberate 2-(4-chloro-3,5-dimethylphenoxy)acetic acid (hereafter called 602-UC) and 2,4-D, respectively (Fig. 2; Table 1, and Tables S3 and S4). This hydrolysis did not occur in extreme pH conditions, indicating that it depends on inherent enzymatic activity (Fig. S2). To further test the prediction that the bipartite structure of these masked auxin analogs was important for their effects on hypocotyl elongation, we tested the effect of conjugated 2,4-D with a methylated amide bond that was predicted to undergo much slower hydrolysis (5353 and 5265; Table 1, Table S3, and Fig. 1C). Indeed, compounds 5353 and 5265 induced hypocotyl growth but at a 10-fold higher concentration compared with 533. Similar results were obtained for other compounds that are less prone to amidase-mediated hydrolysis and that also contain within their bipartite structure 602-UC and 602-UC-like structures as the active auxin component (compounds 1683 and 5351; Fig. 1F and Table S2).
Fig. 2.
UV absorbance spectra of LC-MS analysis. Extracts from 5-day-old seedlings preincubated with the indicated compounds were analyzed by LC-MS and compared with a DMSO control and standards, as described in SI Materials and Methods. The mass and retention time of the extracted compounds correspond to the following standards: 602, (m/z) = 304.8 ([M-H]+), with a retention time of 21 min; 533, (m/z) = 311 ([M-H]+), with a retention time of 20 min; 2,4-D, (m/z) = 219 ([M-H]-), with a retention time of 14.8 min; and 602-UC, (m/z) = 213.5 ([M-H]-), with a retention time of 16.2 min.
We next analyzed the effect of a commercially available, nonconjugated 602-UC (Table 1). As opposed to 2,4-D, which did not exhibit an effect in a range of tested concentrations, 602-UC promoted hypocotyl elongation, albeit to a lesser degree and at a 10-fold higher concentration compared with the intact 602 molecule (Fig. S3). We conclude that an amide bond linking the synthetic auxin to a hydrophobic and/or heterocyclic moiety results in bipartite “prohormones” capable of more efficient cellular uptake either through transporters or directly across the membrane.
We reasoned that the differences in activity between the compounds might be due to their diffusiveness between cells. As such, we calculated the physiochemical properties of the bipartite masked auxins using principles of pharmacokinetics embodied in the Lipinski rule of five; for more information, see Table S2 (30). Although the application of this pharmacokinetic model to plants has not yet been established, the results described below fit generally with what would be expected of small-molecule descriptors based on the LipinskirRule of five. Therefore, these heuristic rules serve as a useful starting point from which to examine the physiochemical properties of small molecules in plants. Compounds that adopt a calculated logD (clogD) value less than −0.40 are very hydrophilic and diffuse poorly through membranes (31). We also calculated the pharmacokinetic properties of picloram. Exogenous application of picloram has been reported to phenocopy the auxin overproducer sur2, which has longer hypocotyls than the wild type. Indeed, in our assay, picloram promoted hypocotyl elongation at micromolar concentrations (Table S4 and Fig. S4) (5, 32).
Applying these criteria, 602, 533, 5353, and 5265 satisfy the Lipinski rule of five (Table 1 and Tables S3 and S4). Furthermore, the primary and secondary amide groups impart additional lipophilicity over the relevant physiological pH range of 5.0–7.5. All of these compounds have a high probability of readily diffusing across cell membranes. In contrast, the unconjugated forms, including IAA, NAA, and picloram, have lower calculated logP (clog P) and clogD values, because of the free carboxylic acid functional groups. This highly ionized group at physiological pH, especially in the pH range of 5.0–7.5, makes these compounds significantly less lipophilic, and with the exception of picloram (Tables S2 and S4), correlates with their weak stimulation of hypocotyl elongation. Using this set of rules, we anticipated that masked auxins with an ester linkage instead of an amide linkage, which have been reported to better penetrate the leaves then the free acid form, also will stimulate hypocotyl elongation (33, 34). Indeed, similar to 533, methyl-2,4-D exhibited a stimulatory effect on hypocotyl growth compared with 2,4-D (Fig. S5). In summary, hypocotyl elongation in the intact plant is promoted when auxins are supplied exogenously in the form of membrane-permeable proauxins.
Different Bipartite Pro-Auxin Analogs Produce Distinct Physiological Effects.
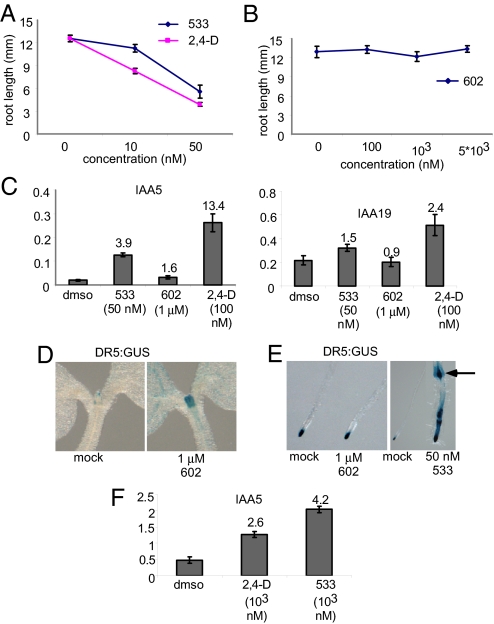
To further characterize the auxin activities, we performed a root inhibition assay. Like 2,4-D, and at similar concentrations, 533 inhibited root growth and was highly effective at 50 nM, also the concentration for maximum induction of hypocotyl elongation (Figs. 1 B–E and 3A). Similarly, less efficiently metabolized conjugates of 2,4-D (5353 and 5265) also inhibited root growth at concentrations that significantly induced hypocotyl elongation (Tables S2 and S3, Fig. 1B, and Fig. S6). In contrast, 602 had no obvious effect on root length at 1 μM, a concentration that effectively induced hypocotyl elongation, and the effect of higher concentrations (10 μM) of 602 could not be tested because of solubility problems. But at 10 μM, 602-UC inhibited root length and caused a more dramatic effect on root hair elongation compared with 533 and 2,4-D (Fig. S3). This suggests that the shoot may be more sensitive than the root to 602, or that 602 may undergo more efficient hydrolysis in the shoot.
Fig. 3.
Hypocotyl elongation and root inhibition were differentially affected by distinct auxin analogs. (A and B) Root inhibition assay. Total root length of 6-day-old seedlings grown in the presence of 533 and 2,4-D (A) and 602 (B). (C) qPCR analysis of 6-day-old seedlings treated with different auxin analogues for 1 h. Fold change compared with mock treatment is indicated on top of the columns. (D and E) Histochemical assay of the DR5:GUS reporter line. (D) Five-day-old DR5:GUS seedlings treated for 16 h with DMSO (Left) or 1 μM 602 (Right). (E) DR5:GUS seedlings grown for 5 days in the presence of the indicated small molecules. Note that whereas 1 μM 602 induced DR:GUS in the shoot, it had no apparent effect in the root. Lateral root initiation is marked by an arrow. (F) qPCR analysis of 2-day-old seedlings treated with different auxin analogues for 1 h. Fold change compared with mock treatment is indicated on top of the columns. Error bars indicate SE.
To test whether the physiological effects of the analogs were correlated with a molecular auxin response, we examined a widely used system in which the synthetic auxin response element DR5 is fused to the β-glucuronidase (GUS) reporter (35). Histochemical staining of DR5:GUS seedlings grown for 16 h in the presence of the compounds showed induction of GUS expression in the shoots (Fig. 3D; the effect of 602 is shown). Under these conditions, no clear induction of GUS expression was seen in the roots. Continuous growth of the reporter line in the presence of the compounds resulted in GUS induction in the root for 533 but not for 602 (Fig. 3E). In addition, continuous growth of seedlings in the presence of 50 nM 533, but not in the presence of 1 μM 602, led to increased lateral root formation, as implicated in the GUS reporter gene expression pattern (Fig. 3E). Next, we tested the ability of 602 and 533 to induce auxin-responsive genes at concentrations known to be highly effective in promoting hypocotyl growth (1 μM and 50 nM, respectively) in 5-day-old seedlings. The expression of 2 genes, IAA5 and IAA19, were analyzed in response to a 1-h treatment with these compounds. As expected, based on the effect of 533 on root inhibition, 533 had a stronger effect than 602, which showed no response or only a weak response, depending on the readout (Fig. 3C). The fact that 602 efficiently promoted hypocotyl elongation at this concentration supports the idea that hypocotyl elongation is sensitive to low levels of auxin. As a positive control for gene induction, 2,4-D was applied at a high concentration, which led to an elevated level of gene induction. In summary, 602 was found to act as a weak auxin analog after the unmasking of the auxin end of the bipartite synthetic conjugate. This bipartite chemical structure thus allows for straightforward and specific examination of elongation growth responses in the hypocotyl without the background responses often seen in other studies.
It was recently proposed that germination of seedlings in the presence of IAA can inhibit or promote hypocotyl elongation if applied before or ≈4 days after germination, respectively (12). To test whether the compounds discovered in the course of this study were active only at late developmental stages, we submerged germinating seedlings (up to 2 days after the end of stratification) in our newly discovered bipartite auxin synthetic conjugates and analyzed their effect on gene expression. As shown in Fig. 3F, 533 efficiently induced the expression of auxin-responsive genes. This finding supports our conclusion that the new analogs induce hypocotyl elongation due to better delivery compared with IAA, rather than being metabolized at specific developmental stages.
Bipartite Masked Auxin Analogs Can Rescue Growth Defects in an Auxin Biosynthetic Mutant, and Their Activity Depends on Members of the Auxin Receptor Family.
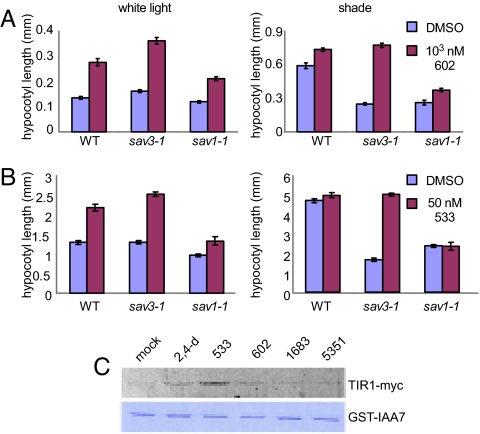
We examined whether the analogs can rescue hypocotyl elongation defects that result from auxin deficiency. The growth-promoting activity of auxin and BRs is interdependent (36). Indeed, the effect of 602 was completely abolished when the BR inhibitor brassinazole was added to the growth media (Fig. S7). Therefore, to confirm the analogs' specificity, we performed hypocotyl elongation assays using 602 and 533 in two recently isolated shade-avoidance mutants, sav3–1 and sav1–1 (23). The former is an auxin biosynthesis mutant that contains 60% of the wild-type auxin levels in white light; the latter is a weak allele of the BR biosynthesis enzyme, DWF4. When grown in white light, the hypocotyl length of sav3–1 and sav1–1 was slightly shorter than that of wild type (Fig. 4 A and B, Left). In contrast, simulated shade light conditions stimulated hypocotyl elongation in wild-type plants but not in sav3–1 or sav1–1 (Fig. 4 A and B, Right). When grown in the presence of 602 and 533, sav3–1 was rescued in shade, but sav1–1 was not (Fig. 4 A and B, Right). Thus, 602 and 533 have a bona fide auxin activity and do not simply cause elongation. These small molecules thus provide a useful, robust, and specific alternative for examining auxin signaling under different light conditions and in combination with other exogenously applied hormone-signaling agonists and antagonists.
Fig. 4.
The analogues specifically rescue auxin-deficient mutants. (A and B) Hypocotyl length of wild-type (Col-0), sav3–1, and sav1–1 grown in white light (Left) and shade (low red:far red ratio, Right) in the presence of 602 (A) and 533 (B). sav1–1 (a BR-deficient mutant) responded only slightly to the chemicals, whereas sav3–1 hypocotyl length was fully rescued in shade. (C) Pull-down assay. TIR1-myc recovery by GST-AUX/IAA7 in the presence of selected auxin analogs (Upper) and Coomassie staining of GST-AUX/IAA7 (Lower).
Auxin is perceived by direct binding to the transport inhibitor response 1 protein (TIR1), a member of a small family of F-box proteins (37, 38). This interaction accelerates the Skp1, Cdc53/Cullin1, F-box protein ubiquitin ligase-catalyzed degradation of Aux/IAA repressor proteins, allowing derepression of auxin-regulated genes by auxin response factor transcription factors (39). To further explore their utility in biological assays, we examined whether 533 and 602 could interact with the auxin receptor, TIR1, which should enhance its interaction with AUX/IAAs in a pull-down assay (37, 38). As shown in Fig. 4C, both compounds enhanced the amount of TIR1-myc recovered by GST-AUX/IAA7, with 602 being less efficient than 533, in agreement with 602's ability to stimulate hypocotyl elongation at higher concentrations. The activity of the compounds was likely a result of their hydrolysis (see SI Materials and Methods). In accordance with the predicted low hydrolysis capacity and low activity in plants, compounds 1683 and 5351 did not enhance the interaction between TIR1-myc and GST-AUX/IAA7 above background (Figs. 4 C and 1F).
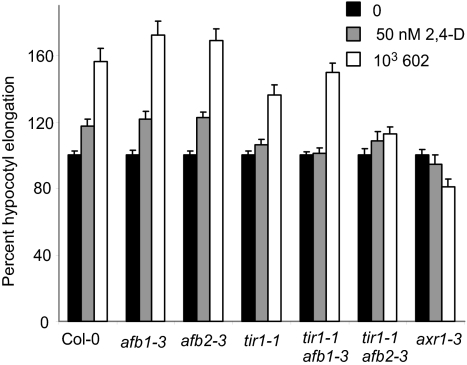
We next used 1 μM 602 (in which wild-type seedlings undergo maximal hypocotyl elongation; Figs. 1 C and D) to analyze elongation growth responsiveness in tir1–1 and newly isolated alleles of TIR1 family members in the Col-0 background: afb1 and afb2 in single and double mutant combinations (Fig. 5 and SI Materials and Methods). afb1 and afb2 retained almost wild-type sensitivity to auxin, but in combination with tir1 had minor effect or significant auxin resistance respectively. As expected, 50 nM of 2,4-D had only a limited effect on the induction of hypocotyl elongation in wild-type plants compared with 602. The auxin resistant mutant axr1 was used as a control. Taken together, these data demonstrate that TIR1 and AFB2 mediate auxin elongation responses in Arabidopsis hypocotyls; however, the extent to which AFB1 mediates elongation is not clear. AFB1 may work together with another auxin receptor, or perhaps it is not significantly involved.
Fig. 5.
TIR1, AFB1, and AFB2 contributed differently to auxin-mediated elongation response in shoots. Mutants were grown in the presence or absence of the compounds for 5 days.
Several attempts have been made in the past to study elongation growth by exogenously applying auxin. Small et al. (11) found that IAA can stimulate hypocotyl growth of light-grown seedlings if a low nutrient medium is substituted for the standard MS medium. Collet et al. (10) showed that very mild stimulation of growth occurs with added IAA in an auxin-deficient mutant grown at 26°C but not in wild-type plants grown under the same conditions. In addition, it was reported recently that IAA can promote hypocotyl elongation if it is applied daily, at high concentrations, in red light conditions and after the seedlings have reached a certain developmental stage (12). On the other hand, a different study demonstrated that the long hypocotyl and short root phenotype of the auxin overproducing mutant sur2 can be mimicked by germinating wild-type seedlings in the presence of the auxin analog picloram (5). Interestingly, the activity of picloram differs from that of 2,4-D with respect to the auxin receptor family member AFB5 (40). Finally, induction of hypocotyl elongation can be achieved when plants are grown at high temperatures, conditions that lead to an increase in endogenous auxin levels (13).
The identification of the bipartite compounds presented here allows for the efficient induction of auxin-mediated elongation response in shoots. The new compounds are robust; they can be supplied in standard growth media at normal growth temperatures under various light conditions and appear to function through interactions with multiple auxin receptors. Thus, these prohormones provide a useful new approach to address unanswered questions in auxin-signaling interactions with light and other hormone pathways. Ultimately, structure–activity relationship studies centered around these and other bipartite prohormones, which are based on the amide bond and aimed at developing a set of heuristic rules governing metabolic fate in particular tissues, should allow investigators to precisely dissect plant hormone-signaling pathways with a level of spatial and temporal specificity previously unattainable.
Materials and Methods
Plant Materials and Growth Conditions.
det2–1 seeds were used for the chemical genetic screen (41). tir1–1, axr1–3, afb1–3 and afb2–3 were as described in SI Materials and Methods. Arabidopsis (Arabidopsis thaliana) ecotype Columbia (Col-0) was the wild type. Sav1–1 and sav3–1 are as described previously (23). Surface-sterilized seeds were spotted on 0.5X Linsmaier-Skoog medium (LS; Caisson Laboratories) supplemented with 0.8% (wt/vol) phytagar and stratified in the dark at 4°C for 4 days. For white light experiments, plants were grown in a 16-h light/8-h dark cycle, at 22°C in ≈50 μmol m−2 s−1 placed in a vertical position. The 96-well plates used for the chemical genetic screens were placed horizontally. For shade avoidance experiments, the LED lights were as follows: red, 13 μmol m−2 s−1; blue, 1.23 μmol m−2 s−1; far light, 20.2 μmol m−2 s−1 (R:FR ratio: 0.7).
Chemical Genetic Screen.
The 96-well format DIVERSet library (ChemBridge) contained 10,000 small molecules in a final concentration of 2–4 mM in DMSO. For screening, agar medium supplemented with individual compounds was prepared in 96-well plates using a 3-step method. First, 50 μl of 0.5X Murashige and Skoog Salt Media (MS; Gibco BRL) was dispersed using a multichannel repetitive pipette. Next, 1 μl from the 96-well stock was dispersed to each well using a Hydra 96 microdispenser (Robbins Scientific). Finally, an additional 50 μl of 0.5X MS media supplemented with 1.6% (wt/vol) phytagar was dispersed, thereby obtaining media with 0.8% (wt/vol) phytagar and a 20–40 μM final concentration of each compound. About 10 surface-sterilized seeds were spotted in each well, stratified, and scored 6–7 days after germination for long hypocotyls.
Small Molecules, Binding Assay, and LC-MS.
Compounds used in this work and that are summarized in Table S2 were obtained from ChemBridge. 2,4-D (D6679), picloram (P5575), methyl-2,4-D, and 2-amino-4-picoline were obtained from Sigma. All compounds were dissolved in DMSO. LC-MS analysis and pull-down experiments are described in the SI Materials and Methods.
RNA Isolation and Quantitative Reverese-Transcription Polymerase Chain Reaction.
Seedlings were harvested either 2 or 7 days after the end of stratification and incubated for 1 h in 0.5X LS media supplemented with compounds or with an equivalent volume of DMSO. Total RNA was then isolated with RNeasy Plant Mini Kit (Qiagen). First-strand cDNA was synthesized from 1–3 μg of total RNA using the SuperScript III First-Strand Synthesis System kit with the oligo(dT) as a primer (Invitrogen). For quantitative polymerase chain reaction (qPCR), cDNAs were diluted 20-fold. qPCR reactions were run in BioRAD myIQ system using SYBRgreen (PE Biosystems). Primers are described in the SI Materials and Methods.
Histochemical Analysis.
For histochemical analysis of GUS activity, Arabidopsis seedlings were prefixed in 90% acetone for 10 min on ice. The seedlings were then washed three times with GUS reaction buffer (50 mM sodium phosphate buffer [pH 7.0], 0.2% Triton-X-100, 5 mM potassium ferrocyanide, 5 mM potassium ferricyanide) and incubated in GUS reaction buffer supplemented with 1 mM X-gluc at 37°C for ≈6 h. The samples were then cleared in 70% ethanol.
Hypocotyl Measurements.
Plates were scanned on a flatbed scanner. Hypocotyl length was then measured using the National Institutes of Health's Image 1.62. At least 12 seedlings were measured for each treatment.
Supplementary Material
Acknowledgments.
We thank Dr. Ana Cano-Delgado for providing the det2–1 seeds, Dr. Emmanuel Theodorakis for synthesizing an auxin analog in the initial stages of this study and Dr. Sean Cutler for the LC-MS protocol. This work was supported by grants from the National Institutes of Health (GM52413 to J.C. and GM43644 to M.E.), National Science Foundation (IOS-0649389 to J.C.; MCB-023602 to J.P.N.), and U.S. Department of Energy (DE-FG02-02ER15312 to M.E.). S.S.-G. was supported by fellowships from the United States-Israel Bi-National Agricultural Research and Development Fund (BARD) and the Salk Institute. F.P. was a Deutsche Forschungsgemeinschaft postdoctoral fellow. J.P.N. and J.C. are investigators at the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0806324105/DCSupplemental.
References
- 1.Vandenbussche F, Verbelen JP, Van Der Straeten D. Of light and length: Regulation of hypocotyl growth in Arabidopsis. Bioessays. 2005;27:275–284. doi: 10.1002/bies.20199. [DOI] [PubMed] [Google Scholar]
- 2.Cowling RJ, Harberd NP. Gibberellins control Arabidopsis hypocotyl growth via regulation of cellular elongation. J Exp Bot. 1999;50:1351–1357. [Google Scholar]
- 3.Neff MM, et al. BAS1: A gene regulating brassinosteroid levels and light responsiveness in Arabidopsis. Proc Natl Acad Sci USA. 1999;96:15316–15323. doi: 10.1073/pnas.96.26.15316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boerjan W, et al. Superroot, a recessive mutation in Arabidopsis, confers auxin overproduction. Plant Cell. 1995;7:1405–1419. doi: 10.1105/tpc.7.9.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delarue M, Prinsen E, Onckelen HV, Caboche M, Bellini C. Sur2 mutations of Arabidopsis thaliana define a new locus involved in the control of auxin homeostasis. Plant J. 1998;14:603–611. doi: 10.1046/j.1365-313x.1998.00163.x. [DOI] [PubMed] [Google Scholar]
- 6.Romano CP, Robson PR, Smith H, Estelle M, Klee H. Transgene-mediated auxin overproduction in Arabidopsis: Hypocotyl elongation phenotype and interactions with the hy6–1 hypocotyl elongation and axr1 auxin-resistant mutants. Plant Mol Biol. 1995;27:1071–1083. doi: 10.1007/BF00020881. [DOI] [PubMed] [Google Scholar]
- 7.Zhao Y, et al. A role for flavin monooxygenase-like enzymes in auxin biosynthesis. Science. 2001;291:306–309. doi: 10.1126/science.291.5502.306. [DOI] [PubMed] [Google Scholar]
- 8.Hanson JB, Trewavas AJ. Regulation of plant cell growth: The changing perspective. New Phytol. 1982;90:1–18. [Google Scholar]
- 9.Cleland RE. In: Plant Hormones: Biosynthesis, Signal Transduction, Action. Davies PJ, editor. Dordrecht: Kluwer Academic Publishers; 2004. pp. 282–303. [Google Scholar]
- 10.Collett CE, Harberd NP, Leyser O. Hormonal interactions in the control of Arabidopsis hypocotyl elongation. Plant Physiol. 2000;124:553–562. doi: 10.1104/pp.124.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smalle J, Haegman M, Kurepa J, Van Montagu M, Straeten DV. Ethylene can stimulate Arabidopsis hypocotyl elongation in the light. Proc Natl Acad Sci USA. 1997;94:2756–2761. doi: 10.1073/pnas.94.6.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Covington MF, Harmer SL. The circadian clock regulates auxin signaling and responses in Arabidopsis. PLoS Biol. 2007;5:e222. doi: 10.1371/journal.pbio.0050222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gray WM, Ostin A, Sandberg G, Romano CP, Estelle M. High temperature promotes auxin-mediated hypocotyl elongation in Arabidopsis. Proc Natl Acad Sci USA. 1998;95:7197–7202. doi: 10.1073/pnas.95.12.7197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang T, Davies PJ, Reid JB. Genetic dissection of the relative roles of auxin and gibberellin in the regulation of stem elongation in intact light-grown peas. Plant Physiol. 1996;110:1029–1034. doi: 10.1104/pp.110.3.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Estelle MA, Somerville C. Auxin-resistant mutants of Arabidopsis thaliana with an altered morphology. Mol Genl Genomics. 1987;206:200–206. [Google Scholar]
- 16.Guzman P, Ecker JR. Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. Plant Cell. 1990;2:513–523. doi: 10.1105/tpc.2.6.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang ZY, et al. Nuclear-localized BZR1 mediates brassinosteroid-induced growth and feedback suppression of brassinosteroid biosynthesis. Dev Cell. 2002;2:505–513. doi: 10.1016/s1534-5807(02)00153-3. [DOI] [PubMed] [Google Scholar]
- 18.Armstrong JI, Yuan S, Dale JM, Tanner VN, Theologis A. Identification of inhibitors of auxin transcriptional activation by means of chemical genetics in Arabidopsis. Proc Natl Acad Sci USA. 2004;101:14978–14983. doi: 10.1073/pnas.0404312101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Surpin M, et al. The power of chemical genomics to study the link between endomembrane system components and the gravitropic response. Proc Natl Acad Sci USA. 2005;102:4902–4907. doi: 10.1073/pnas.0500222102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zouhar J, Hicks GR, Raikhel NV. Sorting inhibitors (sortins): Chemical compounds to study vacuolar sorting in Arabidopsis. Proc Natl Acad Sci USA. 2004;101:9497–9501. doi: 10.1073/pnas.0402121101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao Y, et al. Chemical genetic interrogation of natural variation uncovers a molecule that is glycoactivated. Nat Chem Biol. 2007;3:716–721. doi: 10.1038/nchembio.2007.32. [DOI] [PubMed] [Google Scholar]
- 22.Norambuena L, Zouhar J, Hicks GR, Raikhel NV. Identification of cellular pathways affected by Sortin2, a synthetic compound that affects protein targeting to the vacuole in Saccharomyces cerevisiae. BMC Chem Biol. 2008;8:1–8. doi: 10.1186/1472-6769-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tao Y, et al. Rapid synthesis of auxin via a new tryptophan-dependent pathway is required for shade avoidance in plants. Cell. 2008;133:164–176. doi: 10.1016/j.cell.2008.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fujioka S, et al. The Arabidopsis de-etiolated2 mutant is blocked early in brassinosteroid biosynthesis. Plant Cell. 1997;9:1951–1962. doi: 10.1105/tpc.9.11.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raven JA. Transport of indoleacetic acid in plant cells in relation to pH and electrical potential gradients, and its significance for polar IAA transport. New Phytol. 1975;74:163–172. [Google Scholar]
- 26.Ashton FM. Absorption and translocation of radioactive 2,4-D in sugarcane and bean plants. Weeds. 1958;6:257–262. [Google Scholar]
- 27.Day BE. The absorption and translocation of 2,4-dichlorophenoxyacetic acid by bean plants. Plant Physiol. 1952;27:143–152. doi: 10.1104/pp.27.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Delbarre A, Muller P, Imhoff V, Guern J. Comparison of mechanisms controlling uptake and accumulation of 2,4-dichlorophenoxy acetic acid, naphthalene-1-acetic acid, and indole-3-acetic acid in suspension-cultured tobacco cells. Planta. 1996;198:532–541. doi: 10.1007/BF00262639. [DOI] [PubMed] [Google Scholar]
- 29.Dai X, Hayashi K, Nozaki H, Cheng Y, Zhao Y. Genetic and chemical analyses of the action mechanisms of sirtinol in Arabidopsis. Proc Natl Acad Sci USA. 2005;102:3129–3134. doi: 10.1073/pnas.0500185102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2001;46:3–26. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 31.Ghose AK, Viswanadhan VN, Wendoloski JJ. A knowledge-based approach in designing combinatorial or med chem libraries for drug discovery. J Comb Chem. 1999;1:55–68. doi: 10.1021/cc9800071. [DOI] [PubMed] [Google Scholar]
- 32.Barlier I, et al. The SUR2 gene of Arabidopsis thaliana encodes the cytochrome P450 CYP83B1, a modulator of auxin homeostasis. Proc Natl Acad Sci U S A. 2000;97:14819–14824. doi: 10.1073/pnas.260502697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crafts AS. Evidence for hydrolysis of esters of 2,4-D during absorption by plants. Weeds. 1960;8:19–25. [Google Scholar]
- 34.Gershater M, Sharples K, Edwards R. Carboxylesterase activities toward pesticide esters in crops and weeds. Phytochemistry. 2006;67:2561–2567. doi: 10.1016/j.phytochem.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 35.Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ. Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell. 1997;9:1963–1971. doi: 10.1105/tpc.9.11.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hardtke CS. Transcriptional auxin-brassinosteroid crosstalk: Who's talking? Bioessays. 2007;29:1115–1123. doi: 10.1002/bies.20653. [DOI] [PubMed] [Google Scholar]
- 37.Dharmasiri N, Dharmasiri S, Estelle M. The F-box protein TIR1 is an auxin receptor. Nature. 2005;435:441–445. doi: 10.1038/nature03543. [DOI] [PubMed] [Google Scholar]
- 38.Kepinski S, Leyser O. The Arabidopsis F-box protein TIR1 is an auxin receptor. Nature. 2005;435:446–451. doi: 10.1038/nature03542. [DOI] [PubMed] [Google Scholar]
- 39.Gray WM, Kepinski S, Rouse D, Leyser O, Estelle M. Auxin regulates SCF(TIR1)-dependent degradation of AUX/IAA proteins. Nature. 2001;414:271–276. doi: 10.1038/35104500. [DOI] [PubMed] [Google Scholar]
- 40.Walsh TA, et al. Mutations in an auxin receptor homolog AFB5 and in SGT1b confer resistance to synthetic picolinate auxins and not to 2,4-dichlorophenoxyacetic acid or indole-3-acetic acid in Arabidopsis. Plant Physiol. 2006;142:542–552. doi: 10.1104/pp.106.085969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li J, Nagpal P, Vitart V, McMorris TC, Chory J. A role for brassinosteroids in light-dependent development of Arabidopsis. Science. 1996;272:398–401. doi: 10.1126/science.272.5260.398. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.