Abstract
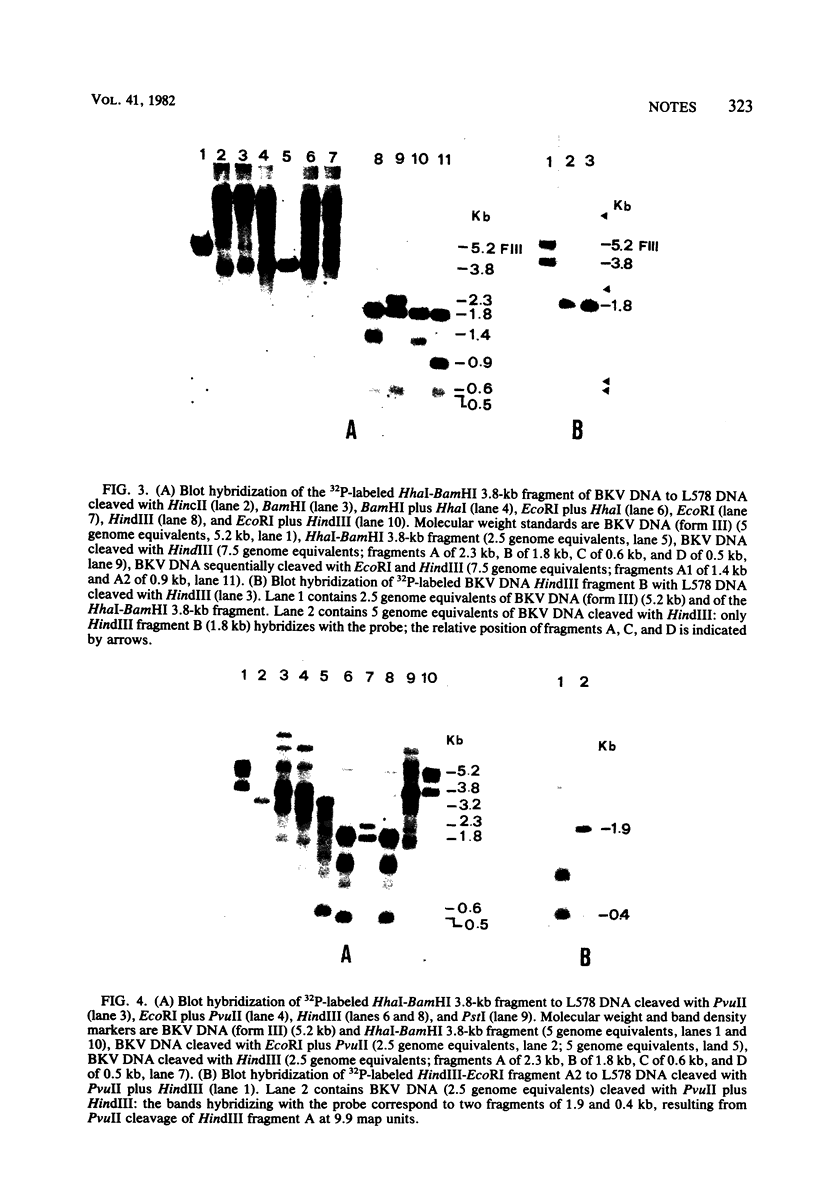
Hamster kidney cells were transformed, with comparable efficiency, by circular or linear molecules of complete BK virus (BKV) genome and by agarose gel-purified fragments of BKV DNA obtained by single or double digestions with various restriction endonucleases. Only fragments containing the complete early region of BKV DNA displayed transforming activity. Analysis by blot hybridization of the arrangement of viral DNA sequences in a cloned cell line transformed by a 3.8-kilobase fragment, obtained after sequential digestion of BKV DNA with HhaI and BamHI, showed the presence of seven viral integrations into the cellular DNA. Apparently all of the integrated viral molecules contained the entire early region of BKV DNA. Large T antigen, small t antigen, and the 56,000-dalton nonviral Tau antigen were detected in transformed cells by immunoprecipitation. The pattern of integration of viral sequences in transformed cells was constant over many generations. Likewise, large T antigen was always detected in transformed cells at various passage levels. These results may suggest that all of the sequences of the early region coding for large T antigen are required for transformation by BKV. Alternatively, subgenomic segments of the BKV DNA early region may be unable to transform because the appropriate polyadenylation site, necessary to obtain a complete functional transcriptional unit, is removed by the restriction enzyme cleavage.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahams P. J., Mulder C., Van De Voorde A., Warnaar S. O., van der Eb A. J. Transformation of primary rat kidney cells by fragments of simian virus 40 DNA. J Virol. 1975 Oct;16(4):818–823. doi: 10.1128/jvi.16.4.818-823.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouck N., Beales N., Shenk T., Berg P., di Mayorca G. New region of the simian virus 40 genome required for efficient viral transformation. Proc Natl Acad Sci U S A. 1978 May;75(5):2473–2477. doi: 10.1073/pnas.75.5.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockman W. W. Transformation of BALB/c-3T3 cells by tsA mutants of simian virus 40: temperature sensitivity of the transformed phenotype and retransofrmation by wild-type virus. J Virol. 1978 Mar;25(3):860–870. doi: 10.1128/jvi.25.3.860-870.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugge J. S., Butel J. S. Role of simian virus 40 gene A function in maintenance of transformation. J Virol. 1975 Mar;15(3):619–635. doi: 10.1128/jvi.15.3.619-635.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenciner N., Grossi M. P., Meneguzzi G., Corallini A., Manservigi R., Barbanti-Brodano G., Milanesi G. State of viral DNA in BK virus-transformed rabbit cells. Virology. 1980 May;103(1):138–148. doi: 10.1016/0042-6822(80)90132-4. [DOI] [PubMed] [Google Scholar]
- Chenciner N., Meneguzzi G., Corallini A., Grossi M. P., Grassi P., Barbanti-Brodano G., Milanesi G. Integrated and free viral DNA in hamster tumors induced by BK virus. Proc Natl Acad Sci U S A. 1980 Feb;77(2):975–979. doi: 10.1073/pnas.77.2.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury K., Light S. E., Garon C. F., Ito Y., Israel M. A. A cloned polyoma DNA fragment representing the 5' half of the early gene region is oncogenic. J Virol. 1980 Nov;36(2):566–574. doi: 10.1128/jvi.36.2.566-574.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa J., Yee C., Tralka T. S., Rabson A. S. Hamster ependymomas produced by intracerebral inoculation of a human papovavirus (MMV). J Natl Cancer Inst. 1976 Apr;56(4):863–864. doi: 10.1093/jnci/56.4.863. [DOI] [PubMed] [Google Scholar]
- Frisque R. J., Rifkin D. B., Topp W. C. Requirement for the large T and small T proteins of SV40 in the maintenance of the transformed state. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):325–331. doi: 10.1101/sqb.1980.044.01.037. [DOI] [PubMed] [Google Scholar]
- Graessmann M., Graessmann A., Mueller C. Monkey cells transformed by SV40 DNA fragments: flat revertants synthesize large and small T antigens. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):605–610. doi: 10.1101/sqb.1980.044.01.063. [DOI] [PubMed] [Google Scholar]
- Hassell J. A., Topp W. C., Rifkin D. B., Moreau P. E. Transformation of rat embryo fibroblasts by cloned polyoma virus DNA fragments containing only part of the early region. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3978–3982. doi: 10.1073/pnas.77.7.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howley P. M., Martin M. A. Uniform representation of the human papovavirus BK genome in transformed hamster cells. J Virol. 1977 Jul;23(1):205–208. doi: 10.1128/jvi.23.1.205-208.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Spurr N. Polyoma virus T antigens expressed in transformed cells: significance of middle T antigen in transformation. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):149–157. doi: 10.1101/sqb.1980.044.01.017. [DOI] [PubMed] [Google Scholar]
- Kimura G., Itagaki A. Initiation and maintenance of cell transformation by simian virus 40: a viral genetic property. Proc Natl Acad Sci U S A. 1975 Feb;72(2):673–677. doi: 10.1073/pnas.72.2.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R. G., Chou J. Y. Simian virus 40 functions required for the establishment and maintenance of malignant transformation. J Virol. 1975 Mar;15(3):599–612. doi: 10.1128/jvi.15.3.599-612.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R. G., Setlow V. P., Edwards C. A., Vembu D. The roles of the simian virus 40 tumor antigens in transformation of Chinese hamster lung cells. Cell. 1979 Jul;17(3):635–643. doi: 10.1016/0092-8674(79)90271-x. [DOI] [PubMed] [Google Scholar]
- Mason D. H., Jr, Takemoto K. K. Transformation of rabbit kidney cells by BKV(MM) human papovavirus. Int J Cancer. 1977 Mar 15;19(3):391–395. doi: 10.1002/ijc.2910190317. [DOI] [PubMed] [Google Scholar]
- McIntosh P. K., Dunker R., Mulder C., Brown N. C. DNA of Bacillus subtilis bacteriophage SPP1: physical mapping and localization of the origin of replication. J Virol. 1978 Dec;28(3):865–876. doi: 10.1128/jvi.28.3.865-876.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meneguzzi G., Chenciner N., Corallini A., Grossi M. P., Barbanti-Brodano G., Milanesi G. The arrangement of integrated viral DNA is different in BK virus-transformed mouse and hamster cells. Virology. 1981 May;111(1):139–153. doi: 10.1016/0042-6822(81)90660-7. [DOI] [PubMed] [Google Scholar]
- Novak U., Dilworth S. M., Griffin B. E. Coding capacity of a 35 percent fragment of the polyoma virus genome is sufficient to initiate and maintain cellular transformation. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3278–3282. doi: 10.1073/pnas.77.6.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M., Weber K. Simian virus 40 gene A function and maintenance of transformation. J Virol. 1975 Mar;15(3):636–644. doi: 10.1128/jvi.15.3.636-644.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif I., Khoury G., Dhar R. The genome of human papovavirus BKV. Cell. 1979 Dec;18(4):963–977. doi: 10.1016/0092-8674(79)90209-5. [DOI] [PubMed] [Google Scholar]
- Shenk T. E., Carbon J., Berg P. Construction and analysis of viable deletion mutants of simian virus 40. J Virol. 1976 May;18(2):664–671. doi: 10.1128/jvi.18.2.664-671.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D. T. Characterization of tau antigens isolated from uninfected and simian virus 40-infected monkey cells and papovavirus-transformed cells. J Virol. 1980 Nov;36(2):519–525. doi: 10.1128/jvi.36.2.519-525.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleigh M. J., Topp W. C., Hanich R., Sambrook J. F. Mutants of SV40 with an altered small t protein are reduced in their ability to transform cells. Cell. 1978 May;14(1):79–88. doi: 10.1016/0092-8674(78)90303-3. [DOI] [PubMed] [Google Scholar]
- Takemoto K. K., Rabson A. S., Mullarkey M. F., Blaese R. M., Garon C. F., Nelson D. Isolation of papovavirus from brain tumor and urine of a patient with Wiskott-Aldrich syndrome. J Natl Cancer Inst. 1974 Nov;53(5):1205–1207. doi: 10.1093/jnci/53.5.1205. [DOI] [PubMed] [Google Scholar]
- Tegtmeyer P. Function of simian virus 40 gene A in transforming infection. J Virol. 1975 Mar;15(3):613–618. doi: 10.1128/jvi.15.3.613-618.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman R., Novak U., Favaloro J., Kamen R. Transformation of rat cells by an altered polyoma virus genome expressing only the middle-T protein. Nature. 1981 Aug 13;292(5824):595–600. doi: 10.1038/292595a0. [DOI] [PubMed] [Google Scholar]
- Yang R. C., Wu R. BK virus DNA: complete nucleotide sequence of a human tumor virus. Science. 1979 Oct 26;206(4417):456–462. doi: 10.1126/science.228391. [DOI] [PubMed] [Google Scholar]
- Yogo Y., Furuno A., Nozawa A., Uchida S. Organization of viral genome in a T antigen-negative hamster tumor induced by human papovavirus BK. J Virol. 1981 May;38(2):556–563. doi: 10.1128/jvi.38.2.556-563.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]