Abstract
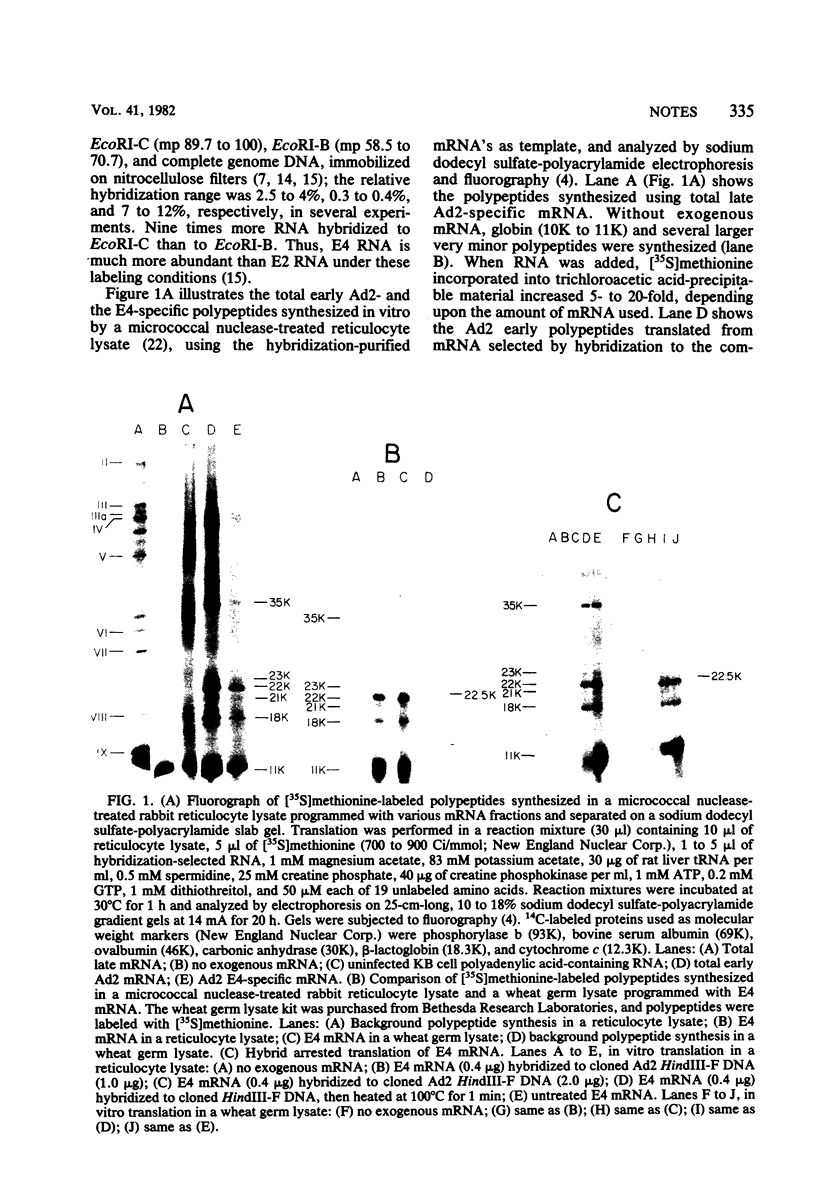
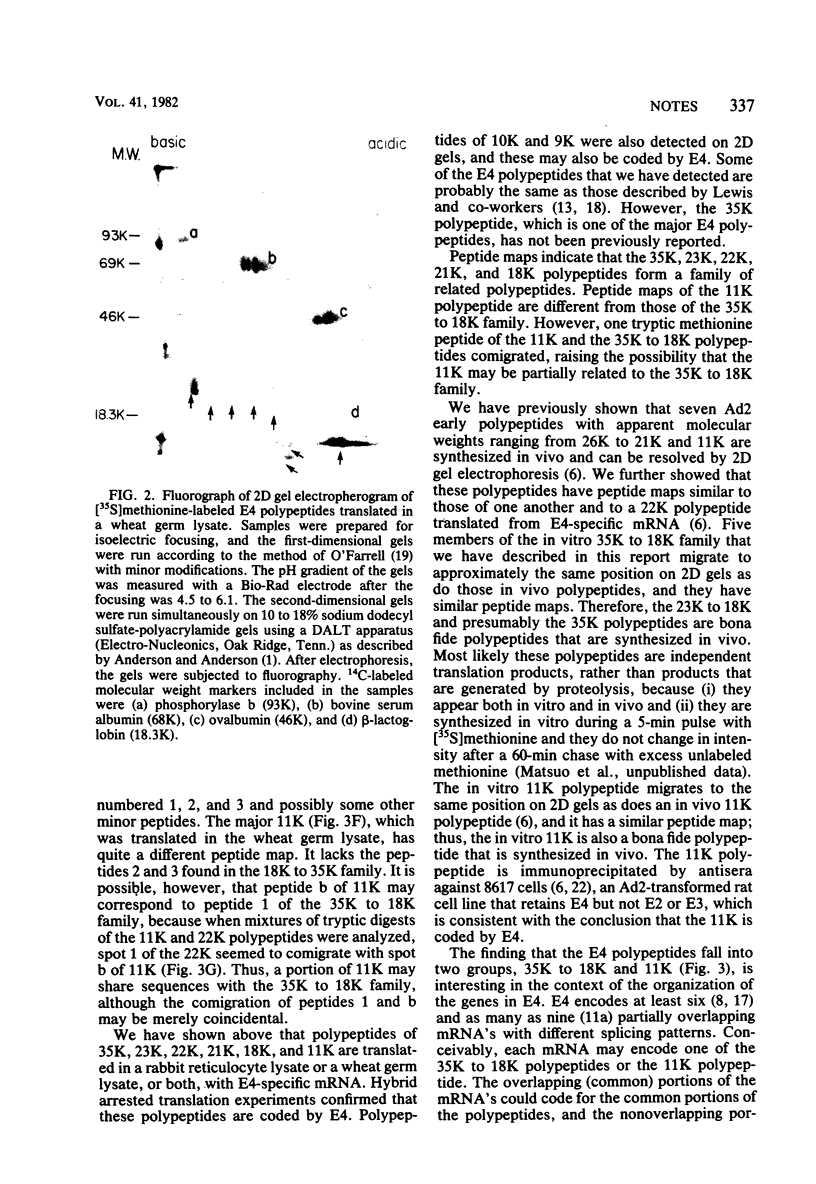
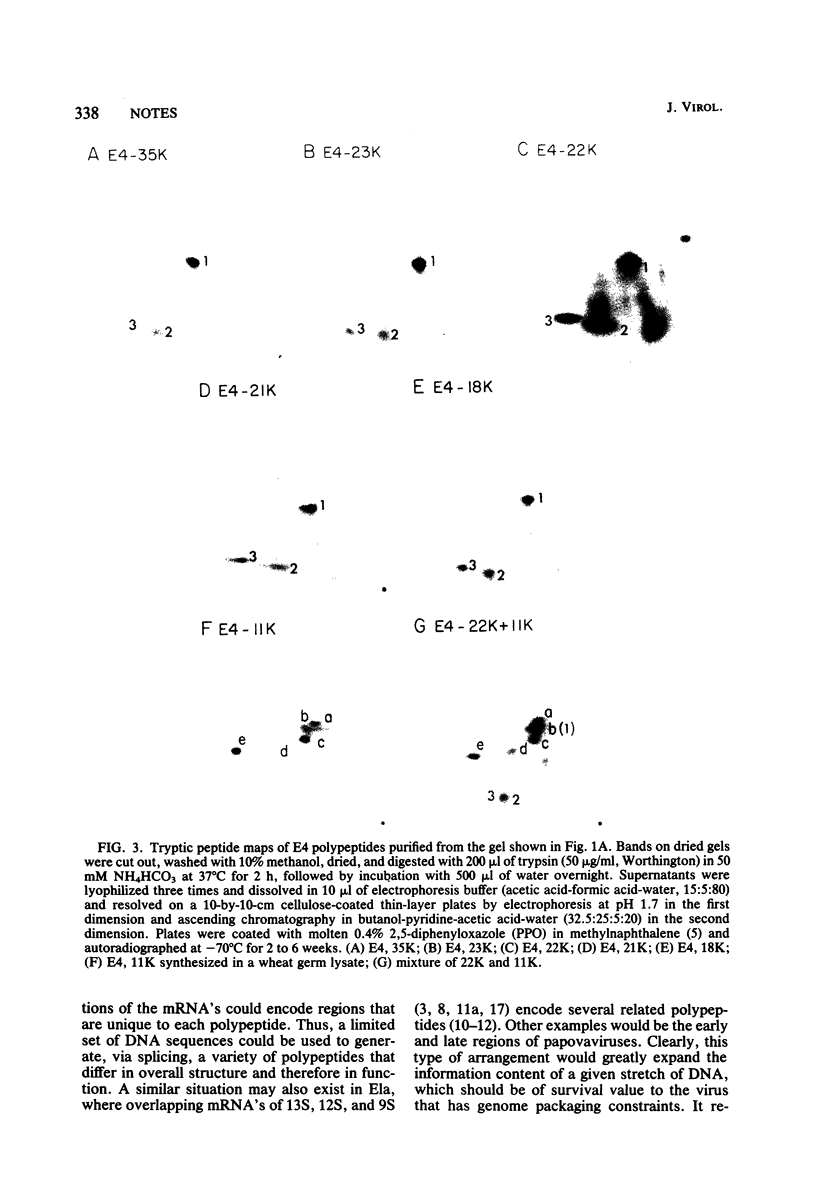
The mRNA species encoded by early region 4 (E4) (map position [mp] 91.5 to 99.3) of adenovirus 2 were isolated from the polysomes of infected KB cells and were purified by hybridization to the cloned HindIII-F fragment (mp 89.5 to 97.3) or to EcoRI-C fragment (mp 89.7 to 100). The mRNA's were translated in vitro using [35S]methionine as a labeled precursor in rabbit reticulocyte lysates treated with micrococcal nuclease as well as in wheat germ lysates. Five major (35,000-molecular-weight [35K], 23K, 22K, 21K, 18K) polypeptides were observed when the reticulocyte lysate was used. The 23K, 22K, 21K, and 18K polypeptides were also observed with the wheat germ lysate, as well as a very prominent 11K polypeptide; the 35K polypeptide was not observed. Assignment of these polypeptides to E4 was further established by hybrid arrested translation. Two-dimensional gel electrophoresis of a wheat germ translate resolved five polypeptides ranging from 18K to 23K, the major 11K polypeptide, and polypeptides of 10K and 9K. The in vitro 23K to 18K and 11K polypeptides migrated to approximately the same positions on two-dimensional gels as did seven 26K to 21K polypeptides and an 11K polypeptide synthesized in vivo (Brackmann et al., J. Biol. Chem, 255:6772--6779, 1980). Two-dimensional tryptic peptide maps demonstrated that the 35K, 23K, 22K, 21K, and 18K polypeptides are related. The peptide map of 11K is different from those of the above polypeptides, although 11K may share one tryptic methionine polypeptide with them. These results indicate that E4 encodes a major 11K polypeptide, as well as major 35K, 23K, 22K, 21K, and 18K polypeptides.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson N. L., Anderson N. G. Analytical techniques for cell fractions. XXII. Two-dimensional analysis of serum and tissue proteins: multiple gradient-slab gel electrophoresis. Anal Biochem. 1978 Apr;85(2):341–354. doi: 10.1016/0003-2697(78)90230-0. [DOI] [PubMed] [Google Scholar]
- Baker C. C., Ziff E. B. Biogenesis, structures, and sites of encoding of the 5' termini of adenovirus-2 mRNAs. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):415–428. doi: 10.1101/sqb.1980.044.01.045. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Structure of the adenovirus 2 early mRNAs. Cell. 1978 Jul;14(3):695–711. doi: 10.1016/0092-8674(78)90252-0. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Stedman J. D. Efficient fluorography of 3H and 14C on thin layers. Anal Biochem. 1978 Aug 15;89(1):247–256. doi: 10.1016/0003-2697(78)90747-9. [DOI] [PubMed] [Google Scholar]
- Brackmann K. H., Green M., Wold W. S., Cartas M., Matsuo T., Hashimoto S. Identification and peptide mapping of human adenovirus type 2-induced early polypeptides isolated by two-dimensional gel electrophoresis and immunoprecipitation. J Biol Chem. 1980 Jul 25;255(14):6772–6779. [PubMed] [Google Scholar]
- Büttner W., Veres-Molnár Z., Green M. Preparative isolation and mapping of adenovirus 2 early messenger RNA species. J Mol Biol. 1976 Oct 25;107(2):93–114. doi: 10.1016/s0022-2836(76)80020-4. [DOI] [PubMed] [Google Scholar]
- Chow L. T., Broker T. R., Lewis J. B. Complex splicing patterns of RNAs from the early regions of adenovirus-2. J Mol Biol. 1979 Oct 25;134(2):265–303. doi: 10.1016/0022-2836(79)90036-6. [DOI] [PubMed] [Google Scholar]
- Elder J. H., Pickett R. A., 2nd, Hampton J., Lerner R. A. Radioiodination of proteins in single polyacrylamide gel slices. Tryptic peptide analysis of all the major members of complex multicomponent systems using microgram quantities of total protein. J Biol Chem. 1977 Sep 25;252(18):6510–6515. [PubMed] [Google Scholar]
- Esche H., Mathews M. B., Lewis J. B. Proteins and messenger RNAs of the transforming region of wild-type and mutant adenoviruses. J Mol Biol. 1980 Sep 25;142(3):399–417. doi: 10.1016/0022-2836(80)90279-x. [DOI] [PubMed] [Google Scholar]
- Green M., Wold W. S., Brackmann K. H., Cartas M. A. Identification of families of overlapping polypeptides coded by early "transforming" gene region 1 of human adenovirus type 2. Virology. 1979 Sep;97(2):275–286. doi: 10.1016/0042-6822(79)90339-8. [DOI] [PubMed] [Google Scholar]
- Green M., Wold W. S., Büttner W. Integration and transcription of group C human adenovirus sequences in the DNA of five lines of transformed rat cells. J Mol Biol. 1981 Sep 25;151(3):337–366. doi: 10.1016/0022-2836(81)90001-2. [DOI] [PubMed] [Google Scholar]
- Halbert D. N., Spector D. J., Raskas H. J. In vitro translation products specified by the transforming region of adenovirus type 2. J Virol. 1979 Sep;31(3):621–629. doi: 10.1128/jvi.31.3.621-629.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harter M. L., Lewis J. B. Adenovirus type 2 early proteins synthesized in vitro and in vivo: identification in infected cells of the 38,000- to 50,000- molecular-weight protein encoded by the left end of the adenovirus type 2 genome. J Virol. 1978 Jun;26(3):736–749. doi: 10.1128/jvi.26.3.736-749.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto S., Green M. Adenovirus 2 early messenger RNA-genome mapping of 5'-terminal RNase T1 oligonucleotides and heterogeneity of 5'-termini. J Biol Chem. 1980 Jul 25;255(14):6780–6788. [PubMed] [Google Scholar]
- Hashimoto S., Green M. Methylated 5'-terminal caps of adenovirus type 2 early mRNA: evidence for at least six 5'-termini. Virology. 1979 Apr 30;94(2):254–272. doi: 10.1016/0042-6822(79)90460-4. [DOI] [PubMed] [Google Scholar]
- Hashimoto S., Pursley M. H., Green M. Nucleotide sequences and mapping of novel heterogenous 5'-termini of adenovirus 2 early region 4 mRNA. Nucleic Acids Res. 1981 Apr 10;9(7):1675–1689. doi: 10.1093/nar/9.7.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitchingman G. R., Westphal H. The structure of adenovirus 2 early nuclear and cytoplasmic RNAs. J Mol Biol. 1980 Feb 15;137(1):23–48. doi: 10.1016/0022-2836(80)90155-2. [DOI] [PubMed] [Google Scholar]
- Lewis J. B., Atkins J. F., Baum P. R., Solem R., Gesteland R. F., Anderson C. W. Location and identification of the genes for adenovirus type 2 early polypeptides. Cell. 1976 Jan;7(1):141–151. doi: 10.1016/0092-8674(76)90264-6. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Parsons J. T., Green M. Biochemical studies on adenovirus multiplication. 18. Resolution of early virus-specific RNA species in Ad 2 infected and transformed cells. Virology. 1971 Jul;45(1):154–162. doi: 10.1016/0042-6822(71)90122-x. [DOI] [PubMed] [Google Scholar]
- Paterson B. M., Roberts B. E., Kuff E. L. Structural gene identification and mapping by DNA-mRNA hybrid-arrested cell-free translation. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4370–4374. doi: 10.1073/pnas.74.10.4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Wold W. S., Green M. Adenovirus type 2 early polypeptides immunoprecipitated by antisera to five lines of adenovirus-transformed rat cells. J Virol. 1979 Apr;30(1):297–310. doi: 10.1128/jvi.30.1.297-310.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]