Abstract
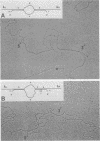
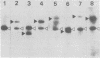
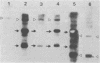
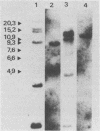
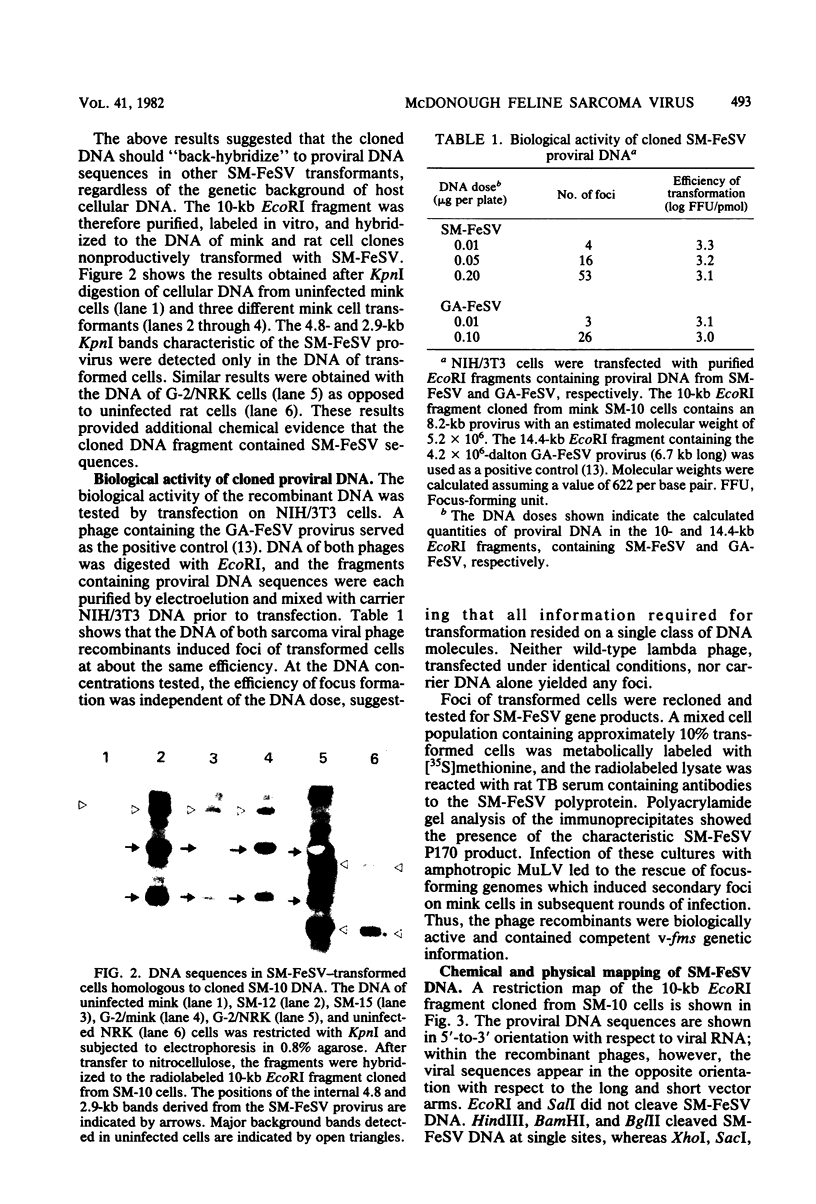
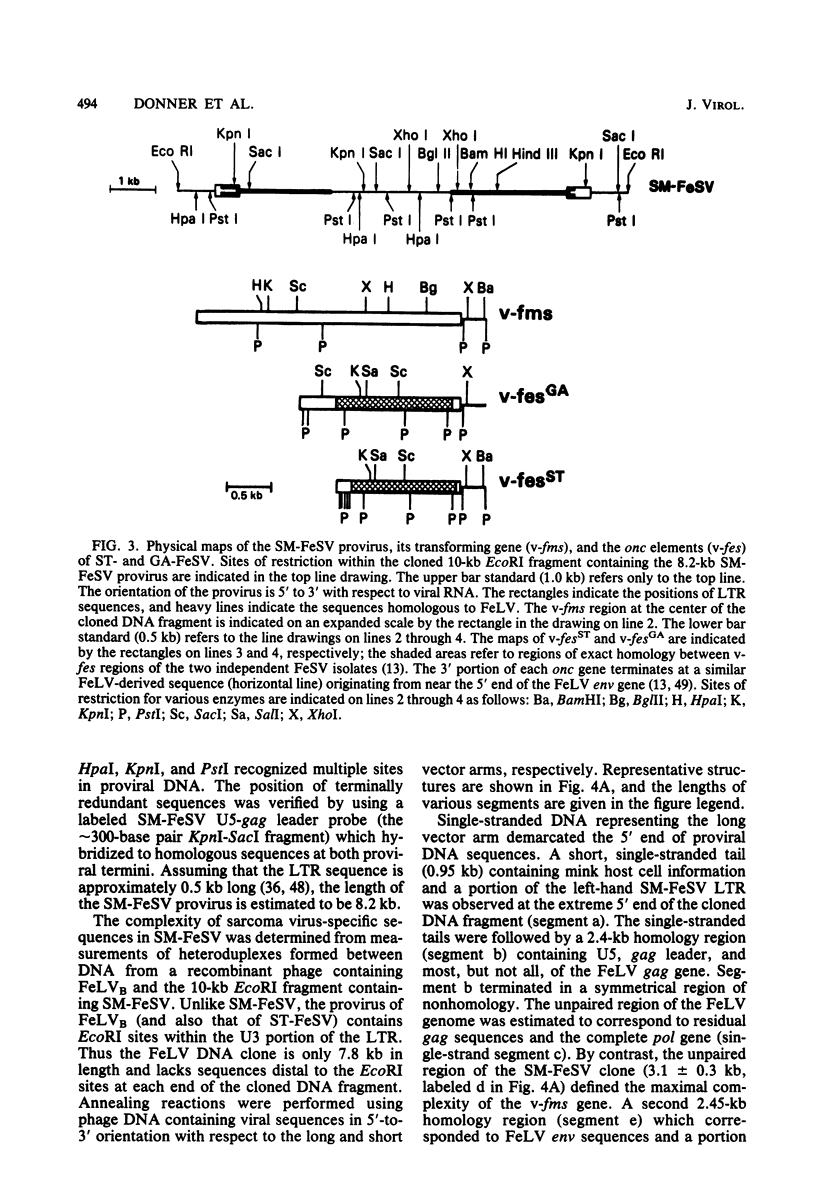
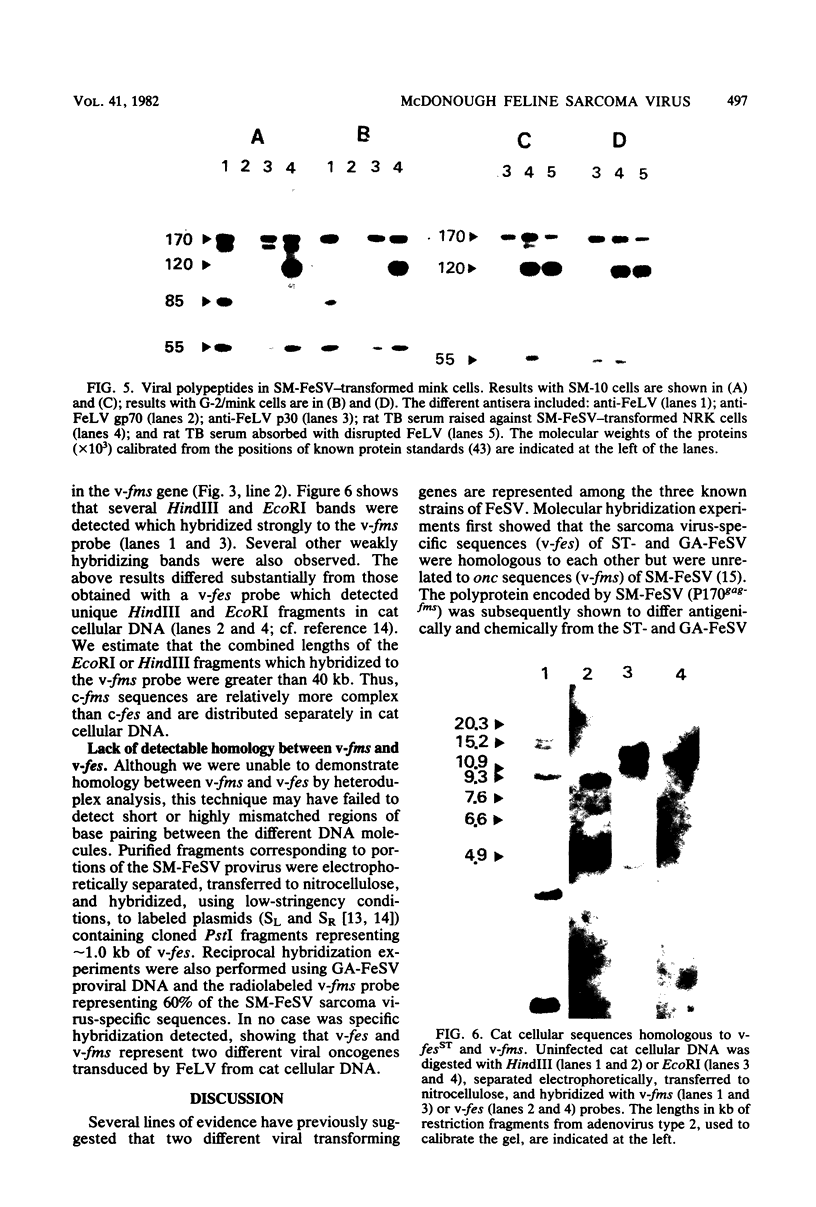
The genetic structure of the McDonough strain of feline sarcoma virus (SM-FeSV) was deduced by analysis of molecularly cloned, transforming proviral DNA. The 8.2-kilobase pair SM-FeSV provirus is longer than those of other feline sarcoma viruses and contains a transforming gene (v-fms) flanked by sequences derived from feline leukemia virus. The order of genes with respect to viral RNA is 5'-gag-fms-env-3', in which the entire feline leukemia virus env gene and an almost complete gag sequence are represented. Transfection of NIH/3T3 cells with cloned SM-FeSV proviral DNA induced foci of morphologically transformed cells which expressed SM-FeSV gene products and contained rescuable sarcoma viral genomes. Cells transformed by viral infection or after transfection with cloned proviral DNA expressed the polyprotein (P170gag-fms) characteristic of the SM-FeSV strain. Two proteolytic cleavage products (P120fms and pp55gag) were also found in immunoprecipitates from metabolically labeled, transformed cells. An additional polypeptide, detected at comparatively low levels in SM-FeSV transformants, was indistinguishable in size and antigenicity from the envelope precursor (gPr85env) of feline leukemia virus. The complexity of the v-fms gene (3.1 +/- 0.3 kilobase pairs) is approximately twofold greater than the viral oncogene sequences (v-fes) of Snyder-Theilen and Gardner-Arnstein FeSV. By heteroduplex, restriction enzyme, and nucleic acid hybridization analyses, v-fms and v-fes sequences showed no detectable homology to one another. Radiolabeled DNA fragments representing portions of the two viral oncogenes hybridized to different EcoRI and HindIII fragments of normal cat cellular DNA. Cellular sequences related to v-fms (designated c-fms) were much more complex than c-fes and were distributed segmentally over more than 40 kilobase pairs in cat DNA. Comparative structural studies of the molecularly cloned proviruses of Synder-Theilen, Gardner-Arnstein, and SM-FeSV showed that a region of the feline-leukemia virus genome derived from the pol-env junction is represented adjacent to v-onc sequences in each FeSV strain and may have provided sequences preferred for recombination with cellular genes.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baluda M. A., Roy-Burman P. Partial characterization of RD114 virus by DNA-RNA hybridization studies. Nat New Biol. 1973 Jul 11;244(132):59–62. doi: 10.1038/newbio244059a0. [DOI] [PubMed] [Google Scholar]
- Barbacid M., Beemon K., Devare S. G. Origin and functional properties of the major gene product of the Snyder-Theilen strain of feline sarcoma virus. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5158–5162. doi: 10.1073/pnas.77.9.5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbacid M., Donner L., Ruscetti S. K., Sherr C. J. Transformation-defective mutants of Snyder-Theilen feline sarcoma virus lack tyrosine-specific protein kinase activity. J Virol. 1981 Jul;39(1):246–254. doi: 10.1128/jvi.39.1.246-254.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbacid M., Lauver A. V., Devare S. G. Biochemical and immunological characterization of polyproteins coded for by the McDonough, Gardner-Arnstein, and Snyder-Theilen strains of feline sarcoma virus. J Virol. 1980 Jan;33(1):196–207. doi: 10.1128/jvi.33.1.196-207.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Sherr C. J., Todaro G. J. Evolution of type C viral genes: origin of feline leukemia virus. Science. 1975 Nov 28;190(4217):886–888. doi: 10.1126/science.52892. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Donner L., Turek L. P., Ruscetti S. K., Fedele L. A., Sherr C. J. Transformation-defective mutants of feline sarcoma virus which express a product of the viral src gene. J Virol. 1980 Jul;35(1):129–140. doi: 10.1128/jvi.35.1.129-140.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R. W., Defeo D., Shih T. Y., Gonda M. A., Young H. A., Tsuchida N., Lowy D. R., Scolnick E. M. The p21 src genes of Harvey and Kirsten sarcoma viruses originate from divergent members of a family of normal vertebrate genes. Nature. 1981 Aug 6;292(5823):506–511. doi: 10.1038/292506a0. [DOI] [PubMed] [Google Scholar]
- Enquist L., Sternberg N. In vitro packaging of lambda Dam vectors and their use in cloning DNA fragments. Methods Enzymol. 1979;68:281–298. doi: 10.1016/0076-6879(79)68020-5. [DOI] [PubMed] [Google Scholar]
- Essex M., Cotter S. M., Hardy W. D., Jr, Hess P., Jarrett W., Jarrett O., Mackey L., Laird H., Perryman L., Olsen R. G. Feline oncornavirus-associated cell membrane antigen. IV. Antibody titers in cats with naturally occurring leukemia, lymphoma, and other diseases. J Natl Cancer Inst. 1975 Aug;55(2):463–467. [PubMed] [Google Scholar]
- Fedele L. A., Even J., Garon C. F., Donner L., Sherr C. J. Recombinant bacteriophages containing the integrated transforming provirus of Gardner--Arnstein feline sarcoma virus. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4036–4040. doi: 10.1073/pnas.78.7.4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchini G., Even J., Sherr C. J., Wong-Staal F. onc sequences (v-fes) of Snyder-Theilen feline sarcoma virus are derived from noncontiguous regions of a cat cellular gene (c-fes). Nature. 1981 Mar 12;290(5802):154–157. doi: 10.1038/290154a0. [DOI] [PubMed] [Google Scholar]
- Frankel A. E., Gilbert J. H., Porzig K. J., Scolnick E. M., Aaronson S. A. Nature and distribution of feline sarcoma virus nucleotide sequences. J Virol. 1979 Jun;30(3):821–827. doi: 10.1128/jvi.30.3.821-827.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner M. B., Rongey R. W., Arnstein P., Estes J. D., Sarma P., Huebner R. J., Rickard C. G. Experimental transmission of feline fibrosarcoma to cats and dogs. Nature. 1970 May 30;226(5248):807–809. doi: 10.1038/226807a0. [DOI] [PubMed] [Google Scholar]
- Gilboa E., Mitra S. W., Goff S., Baltimore D. A detailed model of reverse transcription and tests of crucial aspects. Cell. 1979 Sep;18(1):93–100. doi: 10.1016/0092-8674(79)90357-x. [DOI] [PubMed] [Google Scholar]
- Goldfarb M. P., Weinberg R. A. Generation of novel, biologically active Harvey sarcoma viruses via apparent illegitimate recombination. J Virol. 1981 Apr;38(1):136–150. doi: 10.1128/jvi.38.1.136-150.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. Transformation of rat cells by DNA of human adenovirus 5. Virology. 1973 Aug;54(2):536–539. doi: 10.1016/0042-6822(73)90163-3. [DOI] [PubMed] [Google Scholar]
- Hardy W. D., Jr, Old L. J., Hess P. W., Essex M., Cotter S. Horizontal transmission of feline leukaemia virus. Nature. 1973 Aug 3;244(5414):266–269. doi: 10.1038/244266a0. [DOI] [PubMed] [Google Scholar]
- Hayward W. S., Neel B. G., Astrin S. M. Activation of a cellular onc gene by promoter insertion in ALV-induced lymphoid leukosis. Nature. 1981 Apr 9;290(5806):475–480. doi: 10.1038/290475a0. [DOI] [PubMed] [Google Scholar]
- Hoover E. A., Olsen R. G., Hardy W. D., Jr, Schaller J. P., Mathes L. E. Feline leukemia virus infection: age-related variation in response of cats to experimental infection. J Natl Cancer Inst. 1976 Aug;57(2):365–369. doi: 10.1093/jnci/57.2.365. [DOI] [PubMed] [Google Scholar]
- Hsu T. W., Sabran J. L., Mark G. E., Guntaka R. V., Taylor J. M. Analysis of unintegrated avian RNA tumor virus double-stranded DNA intermediates. J Virol. 1978 Dec;28(3):810–818. doi: 10.1128/jvi.28.3.810-818.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JARRETT W. F., MARTIN W. B., CRIGHTON G. W., DALTON R. G., STEWART M. F. TRANSMISSION EXPERIMENTS WITH LEUKEMIA (LYMPHOSARCOMA). Nature. 1964 May 9;202:566–567. doi: 10.1038/202566a0. [DOI] [PubMed] [Google Scholar]
- Jarrett O., Hardy W. D., Jr, Golder M. C., Hay D. The frequency of occurrence of feline leukaemia virus subgroups in cats. Int J Cancer. 1978 Mar 15;21(3):334–337. doi: 10.1002/ijc.2910210314. [DOI] [PubMed] [Google Scholar]
- Jarrett O., Laird H. M., Hay D. Determinants of the host range of feline leukaemia viruses. J Gen Virol. 1973 Aug;20(2):169–175. doi: 10.1099/0022-1317-20-2-169. [DOI] [PubMed] [Google Scholar]
- Jarrett W., Jarrett O., Mackey L., Laird H., Hardy W., Jr, Essex M. Horizontal transmission of leukemia virus and leukemia in the cat. J Natl Cancer Inst. 1973 Sep;51(3):833–841. doi: 10.1093/jnci/51.3.833. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Leder P., Tiemeier D., Enquist L. EK2 derivatives of bacteriophage lambda useful in the cloning of DNA from higher organisms: the lambdagtWES system. Science. 1977 Apr 8;196(4286):175–177. doi: 10.1126/science.322278. [DOI] [PubMed] [Google Scholar]
- Mackey L. J., Jarrett W. F., Laird H. M. An experimental study of virus leukemia in cats. J Natl Cancer Inst. 1972 Jun;48(6):1663–1670. [PubMed] [Google Scholar]
- Mackey L., Jarrett W., Jarrett O., Laird H. Anemia associated with feline leukemia virus infection in cats. J Natl Cancer Inst. 1975 Jan;54(1):209–217. doi: 10.1093/jnci/54.1.209. [DOI] [PubMed] [Google Scholar]
- McDonough S. K., Larsen S., Brodey R. S., Stock N. D., Hardy W. D., Jr A transmissible feline fibrosarcoma of viral origin. Cancer Res. 1971 Jul;31(7):953–956. [PubMed] [Google Scholar]
- Mullins J. I., Casey J. W., Nicolson M. O., Burck K. B., Davidson N. Sequence arrangement and biological activity of cloned feline leukemia virus proviruses from a virus-productive human cell line. J Virol. 1981 May;38(2):688–703. doi: 10.1128/jvi.38.2.688-703.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naso R. B., Arcement L. J., Arlinghaus R. B. Biosynthesis of Rauscher leukemia viral proteins. Cell. 1975 Jan;4(1):31–36. doi: 10.1016/0092-8674(75)90130-0. [DOI] [PubMed] [Google Scholar]
- Porzig K. J., Barbacid M., Aaronson S. A. Biological properties and translational products of three independent isolates of feline sarcoma virus. Virology. 1979 Jan 15;92(1):91–107. doi: 10.1016/0042-6822(79)90217-4. [DOI] [PubMed] [Google Scholar]
- Quintrell N., Varmus H. E., Bishop J. M., Nicholson M. O., McAllister R. M. Homologies among the nucleotide sequences of the genomes of C-type viruses. Virology. 1974 Apr;58(2):568–575. doi: 10.1016/0042-6822(74)90090-7. [DOI] [PubMed] [Google Scholar]
- Reynolds F. H., Jr, Van de Ven W. J., Blomberg J., Stephenson J. R. Involvement of a high-molecular-weight polyprotein translational product of Snyder-Theilen Feline sarcoma virus in malignant transformation. J Virol. 1981 Feb;37(2):643–653. doi: 10.1128/jvi.37.2.643-653.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickard C. G., Post J. E., Noronha F., Barr L. M. A transmissible virus-induced lymphocytic leukemia of the cat. J Natl Cancer Inst. 1969 Jun;42(6):987–1014. [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Ruscetti S. K., Turek L. P., Sherr C. J. Three independent isolates of feline sarcoma virus code for three distinct gag-x polyproteins. J Virol. 1980 Jul;35(1):259–264. doi: 10.1128/jvi.35.1.259-264.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma P. S., Log T. Subgroup classification of feline leukemia and sarcoma viruses by viral interference and neutralization tests. Virology. 1973 Jul;54(1):160–169. doi: 10.1016/0042-6822(73)90125-6. [DOI] [PubMed] [Google Scholar]
- Shank P. R., Hughes S. H., Kung H. J., Majors J. E., Quintrell N., Guntaka R. V., Bishop J. M., Varmus H. E. Mapping unintegrated avian sarcoma virus DNA: termini of linear DNA bear 300 nucleotides present once or twice in two species of circular DNA. Cell. 1978 Dec;15(4):1383–1395. doi: 10.1016/0092-8674(78)90063-6. [DOI] [PubMed] [Google Scholar]
- Sherr C. J., Fedele L. A., Donner L., Turek L. P. Restriction endonuclease mapping of unintegrated proviral DNA of Snyder-Theilen feline sarcoma virus: localization of sarcoma-specific sequences. J Virol. 1979 Dec;32(3):860–875. doi: 10.1128/jvi.32.3.860-875.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr C. J., Fedele L. A., Oskarsson M., Maizel J., Vande Woude G. Molecular cloning of Snyder-Theilen feline leukemia and sarcoma viruses: comparative studies of feline sarcoma virus with its natural helper virus and with Moloney murine sarcoma virus. J Virol. 1980 Apr;34(1):200–212. doi: 10.1128/jvi.34.1.200-212.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr C. J., Sen A., Todaro G. J., Sliski A., Essex M. Pseudotypes of feline sarcoma virus contain an 85,000-dalton protein with feline oncornavirus-associated cell membrane antigen (FOCMA) activity. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1505–1509. doi: 10.1073/pnas.75.3.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder S. P., Theilen G. H. Transmissible feline fibrosarcoma. Nature. 1969 Mar 15;221(5185):1074–1075. doi: 10.1038/2211074a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stephenson J. R., Khan A. S., Sliski A. H., Essex M. Feline oncornavirus-associated cell membrane antigen: evidence for an immunologically crossreactive feline sarcoma virus-coded protein. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5608–5612. doi: 10.1073/pnas.74.12.5608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Ven W. J., Reynolds F. H., Jr, Nalewaik R. P., Stephenson J. R. Characterization of a 170,000-dalton polyprotein encoded by the McDonough strain of feline sarcoma virus. J Virol. 1980 Jul;35(1):165–175. doi: 10.1128/jvi.35.1.165-175.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Ven W. J., Reynolds F. H., Jr, Stephenson J. R. The nonstructural components of polyproteins encoded by replication-defective mammalian transforming retroviruses are phosphorylated and have associated protein kinase activity. Virology. 1980 Feb;101(1):185–197. doi: 10.1016/0042-6822(80)90495-x. [DOI] [PubMed] [Google Scholar]