Abstract
The highly polarized architecture of neurons is important for their function. Experimental data based on dominant-negative approaches suggest that the tumor suppressor adenomatous polyposis coli (APC), a regulator of Wnt signaling and the cytoskeleton, regulates polarity of neuroectodermal precursors and neurons, helping specify one neurite as the axon, promoting its outgrowth, and guiding axon pathfinding. However, such dominant-negative approaches might affect processes in which APC is not essential. We completely removed both APCs from Drosophila melanogaster larval neural precursors and neurons, testing whether APCs play universal roles in neuronal polarity. Surprisingly, APCs are not essential for asymmetric cell division or the stereotyped division axis of central brain (CB) neuroblasts, although they do affect cell cycle progression and spindle architecture. Likewise, CB, lobular plug, and mushroom body neurons do not require APCs for polarization, axon outgrowth, or, in the latter two cases, axon targeting. These data suggest that proposed cytoskeletal roles for APCs in mammals should be reassessed using loss of function tools.
Introduction
Neurons are exquisitely polarized with axons and dendrites specialized for sending or receiving information (for review see Arimura and Kaibuchi, 2007). They arise from polarized neural progenitors that divide asymmetrically (for review see Solecki et al., 2006). One key question in neural development is how polarity is established and maintained. Neural polarity requires conserved machinery used in all animal cells, including Rho family and Par proteins. The Par3–Par6–aPKC complex is an apical marker in epithelial cells and neural precursors and plays critical roles in asymmetric distributing cell fate determinants and aligning mitotic spindles (for reviews see Watabe-Uchida et al., 2006; Goldstein and Macara, 2007).
One superb model of neuronal polarity is cultured hippocampal neurons (for review see Arimura and Kaibuchi, 2007). They undergo ordered changes from multipolar extension of many immature neurites to selection of one as the axon followed by rapid axon growth. Other neurites become dendrites. The Par3 complex localizes to nascent axon tips, and altering Par protein function disrupts polarity and thus axon outgrowth. To influence axon outgrowth, the axon selection machinery must influence the actin and microtubule (MT) cytoskeletons. The Par complex is proposed to regulate GSK3 kinase, which in turn regulates MT-associated proteins like Tau and adenomatous polyposis coli (APC), linking the Par complex, the cytoskeleton, and axon outgrowth (for reviews see Solecki et al., 2006; Arimura and Kaibuchi, 2007).
APC is an attractive candidate polarity regulator. APC and GSK3 negatively regulate the Wnt effector β-catenin (β-cat), and Wnt signaling can shape cell polarity (Näthke, 2006). APC plays a separate role in cytoskeletal regulation in part through effects on MT stability and cortical association (Näthke, 2006). One illustrative example involves polarized astrocyte migration in which APC is suggested to act at the end of a cdc42–GSK3–Par complex pathway (Etienne-Manneville and Hall, 2003). APCs may also regulate neural precursor polarity. In fly embryos, neural precursors (neuroblasts [NBs]) divide asymmetrically, whereas other ectodermal cells divide symmetrically. APC2 RNAi depletion led epidermal cells to divide asymmetrically like NBs (Lu et al., 2001). In other stem cell types, APCs help orient mitotic spindles (Yamashita et al., 2003) or regulate exit from the stem cell fate (van de Wetering et al., 2002), which are roles they might also play in neural precursors.
Several studies suggest that APC is essential for neuronal polarity and axon extension. The Snider and Jan laboratories provided the first connection (Shi et al., 2004; Zhou et al., 2004). NGF triggers GSK3 inactivation specifically at axon tips, allowing APC to localize there. When they misexpressed truncated APCs that they propose are dominant negative, axon outgrowth was significantly reduced. Zhou et al. (2004) proposed that local GSK3 inhibition in growth cones allows APC to regulate MTs. Shi et al. (2004) suggested that APC helps transport Par3 to the axon tip. Several papers extended these studies but suggested other mechanisms. One suggested that GSK3 and APC act, at least in part, through β-cat via direct cytoskeletal/adhesive effects and via Wnt signaling (Votin et al., 2005). The other suggested that GSK3 regulates membrane trafficking to neurites, perhaps via APC (Gartner et al., 2006; Collin et al., 2008). Finally, APC inactivation by CALI (chromophore-assisted laser inactivation) affects growth cone migration (Koester et al., 2007), suggesting an additional role in axon guidance. These studies suggest that APC is a GSK3 effector acting on the cytoskeleton to direct axon polarization, outgrowth, and guidance, a model now widely accepted in reviews (for reviews see Arimura and Kaibuchi, 2007; Goldstein and Macara, 2007) and textbooks (Squire et al., 2008).
These experiments have two important caveats. First, none used genetic loss of function to reduce or eliminate APC function; instead, they expressed truncated APC fragments, reasoning that these are dominant negative, or used CALI to inactivate APC and possibly its binding partners. Because APC has many cytoskeletal partners, truncated APCs may sequester these in inactive complexes and thus have indirect effects (McCartney et al., 2006). These issues are critical, as is shown by Par3. Although cell culture studies supported a Par3 role in neuronal polarity, (e.g., Shi et al., 2003) loss of function analysis in Drosophila melanogaster demonstrated that this is not an essential Par3 function (Rolls and Doe, 2004). Second, most studies did not consider the possibility that APC inactivation alters Wnt signaling. Because Wnts regulate asymmetric divisions, neuronal migration, and axon outgrowth in Caenorhabditis elegans (Silhankova and Korswagen, 2007) and inhibit NGF-induced neurite outgrowth in culture (Chou et al., 2000), APC might affect axon outgrowth by affecting Wnt signaling.
We tested the hypothesis that APCs play a fundamental cytoskeletal role in polarity of neuronal progenitors and neurons. In Drosophila, we have the unique ability to simultaneously eliminate function of both APCs using protein-null alleles, revealing how complete APC loss affects polarization of neural precursors and their neuronal progeny.
Results and discussion
APCs are not essential for asymmetric division or spindle orientation in central brain (CB) NBs
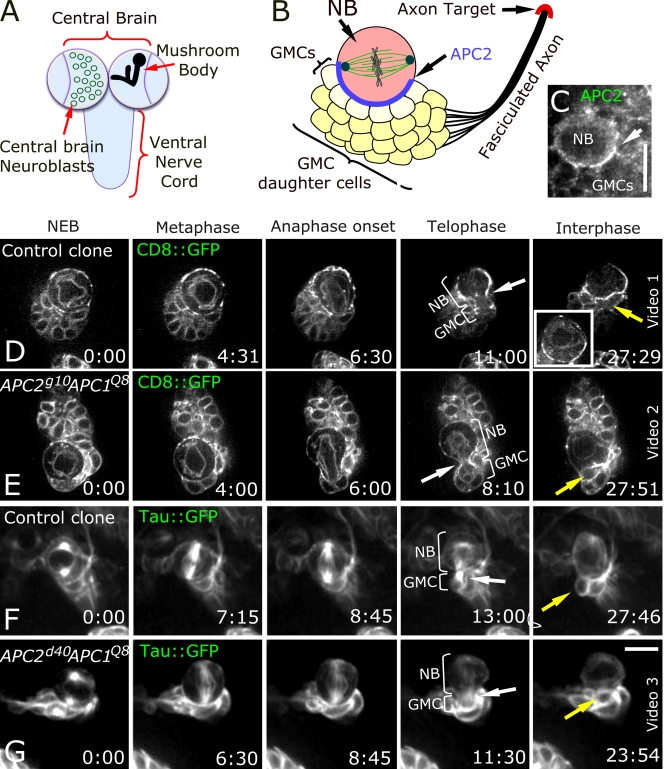
APC2 is asymmetrically localized in larval CB NBs (Fig. 1, A–C; Akong et al., 2002). These neural progenitors undergo a series of asymmetric divisions, giving rise to ganglion mother cells (GMCs) that remain associated with the NB (Fig. 1 B). Progeny of GMCs remain associated as a cluster and differentiate as neurons. CB NB asymmetric divisions occur with a persistent spindle orientation with each new daughter born next to the previous daughter (Akong et al., 2002). This persistent orientation is thought to involve a series of cues, including a fixed centrosome, cortical cues, and cortical interactions of astral MTs (Siller et al., 2005, 2006; Rebollo et al., 2007; Rusan and Peifer, 2007). APC2's striking asymmetric localization suggested it helps orient spindles as it does in the Drosophila male germline (Yamashita et al., 2003).
Figure 1.
CB NBs do not require APCs for asymmetric cell division. (A) Larval brain. (B) CB NB, GMCs, neurons, and their fasciculated axons and target. (C) Asymmetric APC2 localization in CB NB (arrow). (D–G) Time-lapse images, wild-type, or double mutant NBs from nuclear envelope breakdown through next interphase. In all panels, NB divides asymmetrically (fourth column, brackets). White arrows, contractile ring; yellow arrows, new GMC. (D) Wild-type control clone. CD8∷GFP marks cell membrane, nuclei, and spindle. (inset) Different plane of focus showing nuclear envelope. (E) APC2g10 APC1Q8, CD8∷GFP. (F) Control clone, Tau∷GFP marking MTs. (G) APC2d40 APC1Q8, Tau∷GFP. Time is given in minutes:seconds. Bars, 10 μm.
To test this hypothesis, we generated CB NBs double mutant for both APC proteins in otherwise wild-type hosts. Both flies and mammals have only two APC family members. Drosophila offers unique tools allowing us to completely eliminate APC function using well-characterized protein-null alleles of both genes. APC2g10 has a stop codon in the Arm repeats (Fig. S1 A, available at http://www.jcb.org/cgi/content/full/jcb.200807079/DC1) and is clearly protein null, as we demonstrated by immunoblotting with an N-terminal antibody; APC2g10 does not produce detectable truncated proteins, whereas alleles with later stop codons do so (McCartney et al., 2006). APC1Q8 also truncates APC1 very early in the second of seven Arm repeats (Fig. S1 A). It is protein null with APC1 antibody (Ahmed et al., 1998). Maternal-zygotic APC2g10 APC1Q8 double mutant embryos die with strongly activated Wnt signaling; cell fates and Arm levels are similar to those of null alleles of other destruction complex proteins (McCartney et al., 2006). We used mosaic analysis with a repressible cell marker (MARCM; Lee and Luo, 2001) to generate GFP-marked double null mutant cells (APC2g10 APC1Q8) with GFP-marked wild-type clones as controls. In some cases, we also generated cells null for APC1 and carrying APC2d40. APC2d40 produces strongly reduced levels of a truncated protein like those seen in human tumors and is strong but not null with respect to Wnt signaling. It also has dominant-negative effects on cytoskeletal events in syncytial embryos (McCartney et al., 2006). To ensure that all residual wild-type APC was depleted or diluted, we waited 3–4 d after induction; during this time, CB NBs undergo 30–50 divisions, and clone volume increases ∼20-fold. We confirmed APC2 depletion by APC2 antibody staining APC2d40 APC1Q8 clones; perdurant wild-type protein plus levels of truncated APC2d40 protein were reduced to background levels (Fig. S1, compare B with C). APC1 protein accumulates at low levels in NBs and high levels in neuronal axons; this staining is abolished in APC1Q8 mutants (Akong et al., 2002). To confirm that residual wild-type APC1 was depleted in APC2g10 APC1Q8 clones, we stained these with APC1 antibody (Hayashi et al., 1997). Staining was reduced to background levels (Fig. S2), which was especially apparent in CB neuron axons where staining is usually prominent.
We hypothesized that loss of both APCs would abrogate asymmetric division or eliminate persistent spindle alignment. Surprisingly, neither property was disrupted. We assessed asymmetric divisions using CD8-GFP, revealing membranes, nuclei, and spindles (Fig. 1, D and E), or Tau-GFP, marking spindles (Fig. 1, F and G). Double mutant NBs (both APC2g10 APC1Q8 and APC2d40 APC1Q8) exhibited apparently normal asymmetric divisions with high fidelity (Fig. 1, D–G; Table S1, and Videos 1–3, available at http://www.jcb.org/cgi/content/full/jcb.200807079/DC1). Furthermore, mutant NBs maintained a persistent division axis as in wild type, with the new daughter always born next to the previous daughter (Fig. 2, A–C; Table S1, and Videos 4–6). Because cortical polarity proteins regulate spindle alignment in many cell types, we examined the relationship between cortical polarity and spindle orientation. This was normal for both apical (aPKC; Fig. S3, A and B) and basal (Miranda; Fig. S3, C and D) markers. Finally, adherens junction proteins remained asymmetrically localized (Fig. S3, E–J). Thus, APCs are not essential for the decision between symmetric and asymmetric divisions here, as was suggested in embryos (Lu et al., 2001). Furthermore, APC loss must not eliminate MT–cortical interactions critical for CB NB spindle orientation (Siller et al., 2005, 2006), as loss of APC does in some cultured mammalian cells (Green and Kaplan, 2003; Caldwell et al., 2007).
Figure 2.
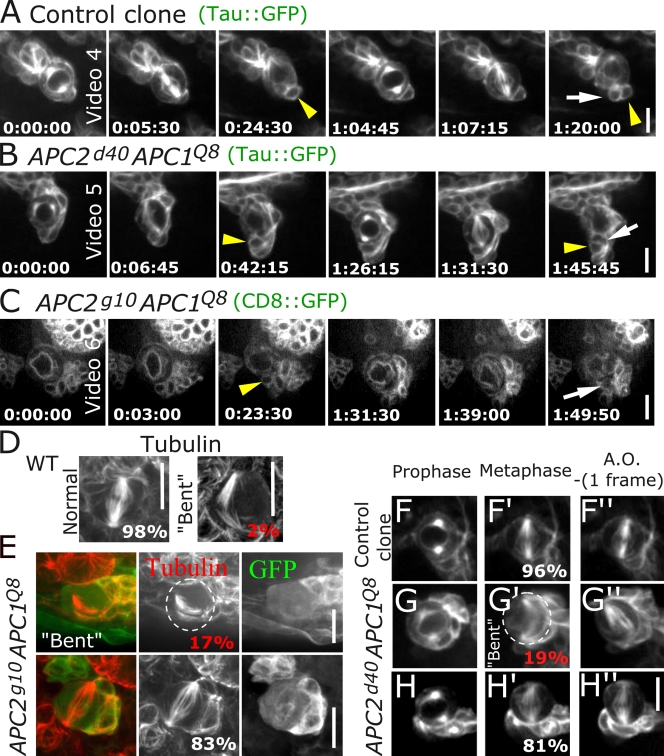
CB NBs do not require APCs for persistent spindle positioning. (A–C) Time-lapse images; NB clones followed for two rounds of division. Tau∷GFP (A and B) or CD8∷GFP (C). Arrowheads, GMCs from first round of division; arrows, GMCs from second round. (A) Control clone. (B) APC2d40APC1Q8 clone. (C) APC2g10 APC1Q8 clone. The GMC from the first round does not remain in focus during the second round. (D–H) Bent spindle phenotype. (D and E) Fixed samples. (D) Wild type (WT). (E) APC2g10APC1Q8. (F–H) Time lapse of NB clones, prophase, metaphase, and one frame before anaphase onset (AO). (F) Control. (G) APC2d40APC1Q8 transitioning through bent spindle intermediate. Dashed circles outline NB. (H) APC2d40APC1Q8 without bent spindle. Time is given in hours:minutes:seconds. Bars,10 μm.
APC loss affects spindle structure and the cell cycle
APC loss was not without consequence. Mammalian APC can regulate spindle structure, and APC loss can alter cell cycle progression, perhaps via the spindle assembly checkpoint (Dikovskaya et al., 2004, 2007). We examined both parameters in APC double mutants. Although spindle structure was grossly normal, a subset of cells exhibited spindle defects. Wild-type spindles are symmetric and straight along the axis between centrosomes (98% are symmetric, with only 2% (1/54) of spindles bent to follow the cortex; Fig. 2 D). In APC double mutants, bent spindle frequency was significantly increased; 17% of APC2g10 APC1Q8 NBs had bent spindles (4/24; P < 0.05; Fig. 2 E). Our live imaging revealed that this is transient and was corrected before mitotic exit (4/21 APC2d40 APC1Q8 NBs imaged live had bent spindles; Fig. 2 G).
APC mutant mammalian cells exhibit cell cycle alterations, although studies differ on whether anaphase onset is delayed (Draviam et al., 2006) or occurs prematurely (Dikovskaya et al., 2007). Thus, we explored cell cycle length in APC double mutant CB NBs. In APC2g10 APC1Q8 double null NBs, the cell cycle and mitosis duration were significantly prolonged (Table I), mimicking delays seen in some mammalian cells after APC loss (Draviam et al., 2006). APC2d40 APC1Q8 NBs had subtle changes in cell cycle length, whereas mitosis duration was slightly increased, consistent with this allele's hypomorphic nature (Table I). Thus, CB NBs completely lacking APC function have defects in cell cycle progression and spindle regulation, but these are not severe enough to disrupt asymmetric division.
Table I. Analysis of NB mitosis and cell cycle.
| Genotype | Duration of mitosis NEB–anaphase onset |
Cell cycle length NEB–NEB |
|---|---|---|
| min/s | min | |
| Wild-type clones | 7:41 ± 3:05 (n = 26) |
73 ± 16 (n = 11) |
| APC2d40 APC1q8 | 9:39 ± 4:13a (n = 21) |
87 ± 20 (n = 9) |
| Wild-type clones | 8:51 ± 3:29 (n = 24) |
79 ± 41 (n = 11) |
| APC2g10 APC1q8 | 11:33 ± 4:03bc (n = 35) |
127 ± 24bd (n = 14) |
All measurements include only the first and second imaged cell cycles per cell to reduce possible ambiguous results caused by light damage or prolonged culturing. NEB, nuclear envelope breakdown. n, number of clones analyzed.
P < 0.05 when compared with wild-type controls.
P < 0.01 when compared with wild-type controls.
P < 0.05 when compared with APC2d40 APC1Q8.
P < 0.001 when compared with APC2d40 APC1Q8.
CB neurons do not require APCs for polarization, axon outgrowth, or fasciculation
Many studies suggest that APCs play key roles in neural polarization and axon outgrowth (see Introduction). However, these studies involved dominant-negative rather than loss of function approaches. CB neurons provide a good place to test APC roles using loss of function. They form a cluster and extend axons in a single fasciculated nerve (Fig. 3, A [left] and B). These axons accumulate both APCs (Akong et al., 2002). Thus, we hypothesized that APCs might regulate neuronal polarization, axon outgrowth, or axon fasciculation.
Figure 3.
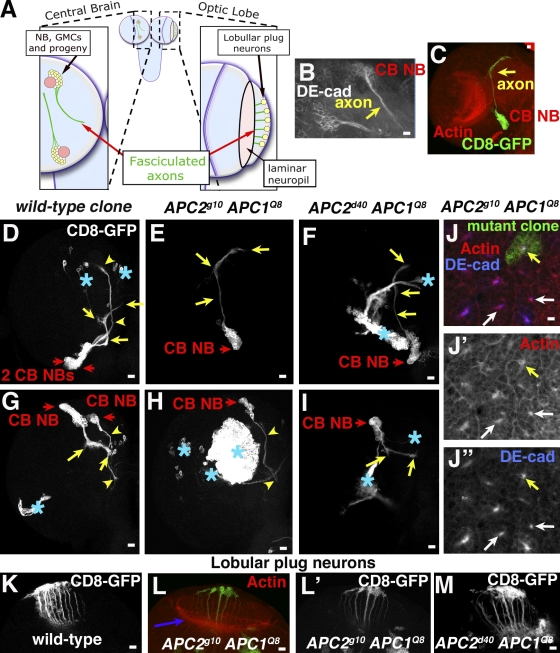
APCs are not required for axon outgrowth and fasciculation. (A) Cartoon of axon projections, CB NBs (left), and lobullar plug neurons (right). (B–N) Third instar brains, genotypes, and antigens as indicated. (B) Drosophila E-cadherin enriched in CB NB, neurons, and axons (yellow arrow). (C) APC2g10APC1Q8 clone in the context of the brain lobe. Axons, yellow arrow. (D–M) CB NB clones, clonal cells marked with CD8-GFP. (E–J) Red arrows, CB NBs; yellow arrows or arrowheads, axons of progeny of particular NBs; asterisks, GFP-labeled clones in other cell types. (J) Cross sections of fasciculated axons of several CB NBs; yellow arrows, APC2g10APC1Q8 mutant clone; white arrows, wild type. (K–M). Wild-type or mutant clones of lobular plug neurons. (L) Purple arrow indicates the axon target in laminar neuropil. Bars, 10 μm.
To test this, we generated CB neurons completely lacking both APCs or null for APC1 and carrying the truncation allele APC2d40 (clones were GFP marked by MARCM). In control clones, CB neurons remain associated and send out a single fasciculated nerve (Fig. 3, D and G). In striking contrast to predictions from dominant-negative studies in mammals (Shi et al., 2004; Zhou et al., 2004; Votin et al., 2005), double mutant CB neurons successfully initiate and extend an axon (Fig. 3, C, E, F, H, and I). Furthermore, their axons fasciculate to form a single nerve. In wild type, actin and Drosophila E-cadherin accumulate at high levels in fasciculated axons (Fig. 3, B [glancing section along fasciculated axons] and J, [white arrows, axon in cross section]). In APC2g10 APC1Q8 double null axons, both proteins had a similar polarized accumulation (Fig. 3 J, yellow arrows). Thus, in these neurons, APCs are dispensable for axon polarization and outgrowth, suggesting APCs do not regulate these processes in all neurons.
APCs are not essential for axon outgrowth or targeting in other larval neurons
APCs are also suggested to regulate growth cone guidance in culture (Koester et al., 2007). Thus, we tested whether APCs play general roles in this process. One disadvantage of CB NBs is that there are ∼100 CB NBs and neuronal progeny of each have distinct axon projection patterns (Pereanu and Hartenstein, 2006), making it very difficult to assess precision of axon targeting in clonally derived mutant neurons. Thus, we turned to two other larval neuronal populations with stereotyped axon projection patterns. We first examined lobular plug neurons, which have cell bodies at the lateral edge of the optic lobe and axons projecting medially to the laminar neuropil (Fig. 3 A, right). Clonal lobular neurons double null for both APCs had axonal projections indistinguishable from control wild-type clones; they polarized and sent out axons that projected medially to the neuropil (Fig. 3, K–M). Thus, APCs are not essential for axon outgrowth or guidance decisions of these neurons with relatively simple axon trajectories.
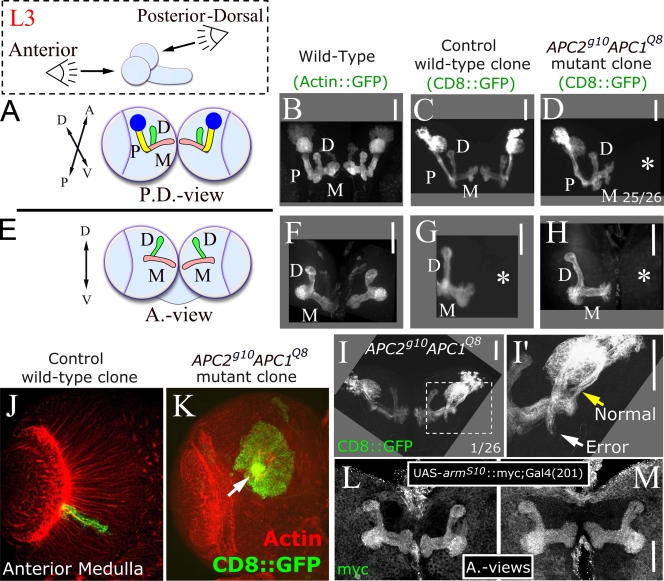
Our final test used some of the best characterized larval neurons, the mushroom body (MB; Heisenberg, 2003). They have highly reproducible axon and dendrite projections with fasciculated axons that first project ventrally and then turn and bifurcate in separate dorsal and medial projections (we visualized MBs using the MB-specific GAL4201Y driver; Fig. 4, A, B, E, and F; Yang et al., 1995). We generated control and APC2g10 APC1Q8 double null mutant clones and specifically visualized clones arising in MBs (see Materials and methods). In control clones, MB axon projections were normal (n = 12; Fig. 4, C and G). In APC2g10 APC1Q8 double null mutant clones, MB neurons differentiated, polarized, and sent out axons and dendrites normally. 25/26 clones had normal axon architecture (Fig. 4, D and H). One clone made an axon outgrowth error (Fig. 4, I and I′), which may reflect a modest role for APCs in this complex guidance decision or may be a random error unrelated to APC.
Figure 4.
APCs are not required for MB axon targeting. (A and E) Posterior-dorsal (A) and anterior views (E) of third instar MB. Cell body cluster plus dendritic region (blue), peduncle (P, yellow), and medial (M, pink) and dorsal (D, green) axon projections. (B and F) Wild type. (C and G) Control wild-type clones. (D and H) APC2g10APC1Q8 clones. Asterisks, brain hemispheres where a clone was not induced. (I and I′) Pathfinding error. (I′) Enlargement of area is shown in dashed square. White arrow indicates error, and yellow arrow indicates normal axon trajectory. (J and K) Wild-type and APC double null medullar neurons. Arrow indicates axon outgrowth defect. (L and M) MBs expressing armS10. Bars, 50 μm.
There are larval neurons where APC does regulate axon outgrowth, those in the anterior medulla. However, medullar axon outgrowth defects result from activated Wnt signaling caused by APC loss rather than APC cytoskeletal roles (Fig. 4, J and K; Hayden et al., 2007). Thus, we tested whether activated Wnt signaling alters MB axons by expressing activated Armadillo, the Wnt effector. This had no effect (Fig. 4, L and M), suggesting MB neurons do not respond to Wnt signaling. Activated Wnt signaling also does not alter CB neuron axon outgrowth (Akong et al., 2002). Thus, our data suggest that in axons where Wnt signaling is not crucial, axon outgrowth and targeting do not require APC.
Together, our data provide the first direct test of the hypothesis that APC is a universal mediator of symmetric versus asymmetric divisions or axon polarization and outgrowth in vivo. APCs play key roles in asymmetric divisions in male germline stem cells (Yamashita et al., 2003). RNAi in the embryonic ectoderm also suggested a role in division symmetry (Lu et al., 2001), although a subsequent loss of function study called this into question (McCartney et al., 2006). CB NBs do not require APCs for division asymmetry or persistent spindle orientation despite APC2's striking asymmetric localization. APC-null cells also undergo asymmetric divisions in the larval medulla (Hayden et al., 2007), whose architecture is epithelial and thus more like the mammalian cortex. Thus, APCs do not play a key instructive role in matching cortical polarity to spindle cues in CB NBs. We cannot rule out a supporting role in CB NBs, which helps to ensure extremely high fidelity asymmetric division, perhaps acting in parallel with other pathways like those mediated by Mud or the apical polarity cues. Perhaps in different tissues, different pathways work additively but the contributions of each vary. Clearly, in the male germline, loss of APCs disrupts the fidelity of asymmetric division, but even in that tissue APC mutant spindles are mispositioned in only a subset of cells (Yamashita et al., 2003). This could be tested by double mutant analysis.
APC loss did prolong the cell cycle, as occurs in mammalian cells (Draviam et al., 2006), but this delay did not prevent successful completion of mitosis. Spindle structure was also affected with an increased frequency of bent spindles, but these defects did not disrupt asymmetric division.
We next tested the hypothesis that APCs are key effectors of neuronal polarity, axon outgrowth, and growth cone guidance (Shi et al., 2004; Zhou et al., 2004; Votin et al., 2005; Koester et al., 2007). However, in three different neuronal populations, axon outgrowth and fasciculation were normal in APC's absence, and in lobular plug and MB neurons, axon targeting was also unaffected. These data compellingly demonstrate that APCs do not play conserved roles in axon polarization and outgrowth in all neurons.
How do we explain the discrepancies between our and earlier data? First, APCs may not play a universal role in neuronal polarity in either flies or mammals, with effects in hippocampal neurons the result of methods used to disrupt APC function. Because GSK3 has many substrates, including MAPs like Tau, GSK3 manipulation may affect substrates other than APC. Misexpression of APC fragments may sequester binding partners (e.g., GSK3, ASEF, and KIF3) with important roles in cytoskeletal regulation, blocking polarization even if APCs themselves play no essential role. Consistent with this, truncated APC2 has dominant-negative effects on cytoskeletal events in early embryos in which APC2 plays only a modest role (McCartney et al., 2006). Second, effects in hippocampal neurons could reflect APC's role in Wnt signaling rather than a direct role in polarization. Wnts regulate axon outgrowth in C. elegans, flies, and mammals (Yoshikawa et al., 2003; Lu et al., 2004; Hayden et al., 2007; Silhankova and Korswagen, 2007). Perhaps Wnts regulate hippocampal axon outgrowth. Interestingly, while our manuscript was under review, a study appeared suggesting that Wnt signals regulate growth cone structure and migration in cultured dorsal root ganglion neurons, perhaps via changes in APC localization (Purro et al., 2008). Of course, it is possible that machinery regulating neuronal polarity differs between flies and mammals, although this is not the case for epithelial polarity (for review see Goldstein and Macara, 2007).
These possibilities can now be tested in mice. Conditional APC knockout in the developing brain would directly test whether APC is critical for axon outgrowth. Furthermore, if effects are seen, one can test whether these are mediated by Wnt signaling using activated β-cat and dominant-negative TCF (T cell factor) to activate or block Wnt signaling. Similar work revealed that APC's role in chromosome instability is mediated, at least in part, via Wnt signaling (Aoki et al., 2007). Our results suggest that these experiments should be a high priority for future research.
Materials and methods
Fly strain and genetic crosses
Genes and alleles are described at Flybase.org. Experiments were performed at 25°C. Control and mutant clones were generated using the MARCM technique (Lee and Luo, 2001). In brief, we used a 3-h heat shock at 37°C to induce expression of a heat shock promoter-driven flippase 48 h after egg laying. This induces mitotic recombination to remove the repressor Gal80 and generate homozygous mutant cells that express GFP. Wild-type clones or clones doube mutant for APC2 and APC1 were generated by crossing hsFlp,mCD8∷GFP;;tubGal4,FRT82B Gal80/TM6 Tb or hsFlp,mCD8∷GFP;UAS-Tau∷GFP;tubGal4,FRT82B Gal80/TM6 Tb males to FRT82B or FRT82B APC2(d40 or g10) APC1Q8/TM6 Tb females. Non-Tb female crawling third instar larvae were dissected for both live and fixed analysis. For MB clones, we replaced the tubGal4 driver with the MB-specific driver Gal4(201) to visualize GFP market clones in the MBs only. Thus, the males from the previous cross were replaced with hsFlp,mCD8∷GFP;Gal4-201Y/+;FRT82B Gal80/+. In the MB scheme, we were able to select for Gal80-expressing brains and against a wild-type brain because Gal4-201 drives expression in a subset of cells in the ventral nerve cord. Any brain that expressed GFP in these cells had received a wild-type third chromosome from the father and was not analyzed.
Live cell imaging of NBs
Crawling third instar larvae were dissected in Schneider's Drosophila medium (Invitrogen). The entire brain was explanted and placed in a 40-μl drop of Schneider's Drosophila medium in the middle of a gas-permeable membrane (petriPERM; Sigma-Aldrich). This drop was surrounded by four drops of Halocarbon oil 700 (Halocarbon Products Corp.) to form the shape of the five on dice. A #1.5 glass coverslip was placed on top of the five drops. A kimwipe was used to wick away oil and media from the edge of the coverslip until the coverslip came in full contact with the brain. The brain was left intact and was not allowed to explode under the weight of the coverslip.
Samples were imaged using a spinning-disk confocal (Yokogawa; PerkinElmer) mounted on a microscope (Eclipse TE300; Nikon), which is equipped with an interline transfer-cooled charge-coupled device camera (ORCA-ER; Hamamatsu Photonics), a z-focus motor (Prior Scientific), an excitation and emission wheel controlled by the Lambda 10–2 controller (Sutter Instrument Co.), and emission filters (Semrock). 100× 1.4 NA, 60× 1.4 NA, and 40× oil 1.3 NA objectives were used. All microscope hardware was controlled by MetaMorph (MDS Analytical Technologies). All images were processed for brightness and contrast and prepared for publication using Photoshop (CS3; Adobe Systems, Inc.).
Immunostaining
Brains were fixed in 3.7% formaldehyde in PBS for 15 min, blocked in 1% normal goat serum for 3 h, and stained in a microcentrifuge tube in primary antibody and 1% normal goat serum in PTA (PBS + 0.1% Tween 20 + 0.2 g/liter sodium azide) overnight at 4°C. Brains were washed and incubated in secondary antibodies for 2 h at RT. The following antibodies were used: E7 mouse anti–α-tubulin (1:250; Developmental Studies Hybridoma Bank [DSHB]), DCAD2 rat monoclonal anti–DE-cadherin (1:200; DSHB), N271A mouse anti-Arm monoclonal (1:200; DSHB), rat polyclonal anti-APC2 (1:1,000; McCartney et al., 2006), rabbit anti-APC1 (1:500; Y. Ahmed, Dartmouth Medical School, Hanover, NH; Hayashi et al., 1997), rabbit anti-Miranda (1:2,000; F. Matsuzaki, RIKEN, Kobe, Japan; Ikeshima-Kataoka et al., 1997), rabbit anti-aPKC (1:2,000; Santa Cruz Biotechnology, Inc.), and Alexa-phalloidin (1:500; Invitrogen). Secondary antibodies were Alexa 488 or 546 (Invitrogen) and were used at a final concentration of 1:500. Images were acquired on the same system as mentioned in the Live cell imaging of NBs section.
Statistical methods
A Fisher's exact test was used to analyze the mutant bent spindle phenotype. An unpaired two-sample Student's t test was used to compare cell cycle and mitosis durations.
Online supplemental material
Fig. S1 and Fig. S2 present data that APC double mutant clones show only background levels of APC2 and APC1 staining, respectively. Fig. S3 shows that APC mutant NBs exhibit normal polarity. Table S1 shows NB asymmetric and persistent division. Video 1 shows a wild-type NB clone undergoing asymmetric division. Video 2 shows a APC2g10 APC1Q8 mutant NB undergoing asymmetric division. Video 3 shows an APC2d40 APC1Q8 mutant NB undergoing asymmetric division. Video 4 shows a control NB clone through two rounds of mitosis showing a persistent division axis. Video 5 shows an APC2d40 APC1Q8 mutant NB through two rounds of mitosis showing a persistent division axis. Video 6 shows an APC2g10 APC1Q8 mutant NB through two rounds of mitosis showing a persistent division axis. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200807079/DC1.
Supplementary Material
Acknowledgments
We thank F. Matsuzaki and Y. Ahmed for reagents; M. Hayden for data collection and discussions; and S. Wheeler, B. Goldstein and his laboratory, B. McCartney, G. Shemer, D. Roberts, B. Duronio, and J. Sawyer for helpful suggestions.
N.M. Rusan is supported by American Cancer Society grant PF-06-108-CCG. This work was supported by National Institutes of Health grant GM67236.
K. Akong's present address is Dept. of Pediatrics, University of California, San Diego, La Jolla, CA 92093.
Abbreviations used in this paper: APC, adenomatous polyposis coli; CB, central brain; GMC, ganglion mother cell; MARCM, mosaic analysis with a repressible cell marker; MB, mushroom body; MT, microtubule; NB, neuroblast.
References
- Ahmed, Y., S. Hayashi, A. Levine, and E. Wieschaus. 1998. Regulation of Armadillo by a Drosophila APC inhibits neuronal apoptosis during retinal development. Cell. 93:1171–1182. [DOI] [PubMed] [Google Scholar]
- Akong, K., B. McCartney, and M. Peifer. 2002. Drosophila APC2 and APC1 have overlapping roles in the larval brain despite their distinct intracellular localizations. Dev. Biol. 250:71–90. [DOI] [PubMed] [Google Scholar]
- Aoki, K., M. Aoki, M. Sugai, N. Harada, H. Miyoshi, T. Tsukamoto, T. Mizoshita, M. Tatematsu, H. Seno, T. Chiba, et al. 2007. Chromosomal instability by beta-catenin/TCF transcription in APC or beta-catenin mutant cells. Oncogene. 26:3511–3520. [DOI] [PubMed] [Google Scholar]
- Arimura, N., and K. Kaibuchi. 2007. Neuronal polarity: from extracellular signals to intracellular mechanisms. Nat. Rev. Neurosci. 8:194–205. [DOI] [PubMed] [Google Scholar]
- Caldwell, C.M., R.A. Green, and K.B. Kaplan. 2007. APC mutations lead to cytokinetic failures in vitro and tetraploid genotypes in Min mice. J. Cell Biol. 178:1109–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou, A.H., S. Zheng, T. Itsukaichi, and B.D. Howard. 2000. Wnt-1 inhibits nerve growth factor-induced differentiation of PC12 cells by preventing the induction of some but not all late-response genes. Brain Res. Mol. Brain Res. 77:232–245. [DOI] [PubMed] [Google Scholar]
- Collin, L., K. Schlessinger, and A. Hall. 2008. APC nuclear membrane association and microtubule polarity. Biol. Cell. 100:243–252. [DOI] [PubMed] [Google Scholar]
- Dikovskaya, D., I.P. Newton, and I.S. Nathke. 2004. The adenomatous polyposis coli protein is required for the formation of robust spindles formed in CSF Xenopus extracts. Mol. Biol. Cell. 15:2978–2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikovskaya, D., D. Schiffmann, I.P. Newton, A. Oakley, K. Kroboth, O. Sansom, T.J. Jamieson, V. Meniel, A. Clarke, and I.S. Nathke. 2007. Loss of APC induces polyploidy as a result of a combination of defects in mitosis and apoptosis. J. Cell Biol. 176:183–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draviam, V.M., I. Shapiro, B. Aldridge, and P.K. Sorger. 2006. Misorientation and reduced stretching of aligned sister kinetochores promote chromosome missegregation in EB1- or APC-depleted cells. EMBO J. 25:2814–2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne-Manneville, S., and A. Hall. 2003. Cdc42 regulates GSK-3beta and adenomatous polyposis coli to control cell polarity. Nature. 421:753–756. [DOI] [PubMed] [Google Scholar]
- Gartner, A., X. Huang, and A. Hall. 2006. Neuronal polarity is regulated by glycogen synthase kinase-3 (GSK-3beta) independently of Akt/PKB serine phosphorylation. J. Cell Sci. 119:3927–3934. [DOI] [PubMed] [Google Scholar]
- Goldstein, B., and I.G. Macara. 2007. The PAR proteins: fundamental players in animal cell polarization. Dev. Cell. 13:609–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green, R.A., and K.B. Kaplan. 2003. Chromosome instability in colorectal tumor cells is associated with defects in microtubule plus-end attachments caused by a dominant mutation in APC. J. Cell Biol. 163:949–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi, S., B. Rubinfeld, B. Souza, P. Polakis, E. Wieschaus, and A. Levine. 1997. A Drosophila homolog of the tumor suppressor gene adenomatous polyposis coli down-regulates β-catenin but its zygotic expression is not essential for the regulation of Armadillo. Proc. Natl. Acad. Sci. USA. 94:242–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden, M.A., K. Akong, and M. Peifer. 2007. Novel roles for APC family members and Wingless/Wnt signaling during Drosophila brain development. Dev. Biol. 305:358–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisenberg, M. 2003. Mushroom body memoir: from maps to models. Nat. Rev. Neurosci. 4:266–275. [DOI] [PubMed] [Google Scholar]
- Ikeshima-Kataoka, H., J.B. Skeath, Y. Nabeshima, C.Q. Doe, and F. Matsuzaki. 1997. Miranda directs Prospero to a daughter cell during Drosophila. Nature. 390:625–629. [DOI] [PubMed] [Google Scholar]
- Koester, M.P., O. Muller, and G.E. Pollerberg. 2007. Adenomatous polyposis coli is differentially distributed in growth cones and modulates their steering. J. Neurosci. 27:12590–12600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, T., and L. Luo. 2001. Mosaic analysis with a repressible cell marker (MARCM) for Drosophila neural development. Trends Neurosci. 24:251–254. [DOI] [PubMed] [Google Scholar]
- Lu, B., F. Roegiers, L.Y. Jan, and Y.N. Jan. 2001. Adherens junctions inhibit asymmetric division in the Drosophila epithelium. Nature. 409:522–525. [DOI] [PubMed] [Google Scholar]
- Lu, W., V. Yamamoto, B. Ortega, and D. Baltimore. 2004. Mammalian Ryk is a Wnt coreceptor required for stimulation of neurite outgrowth. Cell. 119:97–108. [DOI] [PubMed] [Google Scholar]
- McCartney, B.M., M.H. Price, R.L. Webb, M.A. Hayden, L.M. Holot, M. Zhou, A. Bejsovec, and M. Peifer. 2006. Testing hypotheses for the functions of APC family proteins using null and truncation alleles in Drosophila. Development. 133:2407–2418. [DOI] [PubMed] [Google Scholar]
- Näthke, I. 2006. Cytoskeleton out of the cupboard: colon cancer and cytoskeletal changes induced by loss of APC. Nat. Rev. Cancer. 6:967–974. [DOI] [PubMed] [Google Scholar]
- Pereanu, W., and V. Hartenstein. 2006. Neural lineages of the Drosophila brain: a three-dimensional digital atlas of the pattern of lineage location and projection at the late larval stage. J. Neurosci. 26:5534–5553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purro, S.A., L. Ciani, M. Hoyos-Flight, E. Stamatakou, E. Siomou, and P.C. Salinas. 2008. Wnt regulates axon behavior through changes in microtubule growth directionality: a new role for adenomatous polyposis coli. J. Neurosci. 28:8644–8654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebollo, E., P. Sampaio, J. Januschke, S. Llamazares, H. Varmark, and C. Gonzalez. 2007. Functionally unequal centrosomes drive spindle orientation in asymmetrically dividing Drosophila neural stem cells. Dev. Cell. 12:467–474. [DOI] [PubMed] [Google Scholar]
- Rolls, M.M., and C.Q. Doe. 2004. Baz, Par-6 and aPKC are not required for axon or dendrite specification in Drosophila. Nat. Neurosci. 7:1293–1295. [DOI] [PubMed] [Google Scholar]
- Rusan, N.M., and M. Peifer. 2007. A role for a novel centrosome cycle in asymmetric cell division. J. Cell Biol. 177:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, S.H., L.Y. Jan, and Y.N. Jan. 2003. Hippocampal neuronal polarity specified by spatially localized mPar3/mPar6 and PI 3-kinase activity. Cell. 112:63–75 [DOI] [PubMed] [Google Scholar]
- Shi, S.H., T. Cheng, L.Y. Jan, and Y.N. Jan. 2004. APC and GSK-3beta are involved in mPar3 targeting to the nascent axon and establishment of neuronal polarity. Curr. Biol. 14:2025–2032. [DOI] [PubMed] [Google Scholar]
- Silhankova, M., and H.C. Korswagen. 2007. Migration of neuronal cells along the anterior-posterior body axis of C. elegans: Wnts are in control. Curr. Opin. Genet. Dev. 17:320–325. [DOI] [PubMed] [Google Scholar]
- Siller, K.H., M. Serr, R. Steward, T.S. Hays, and C.Q. Doe. 2005. Live imaging of Drosophila brain neuroblasts reveals a role for Lis1/dynactin in spindle assembly and mitotic checkpoint control. Mol. Biol. Cell. 16:5127–5140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siller, K.H., C. Cabernard, and C.Q. Doe. 2006. The NuMA-related Mud protein binds Pins and regulates spindle orientation in Drosophila neuroblasts. Nat. Cell Biol. 8:594–600. [DOI] [PubMed] [Google Scholar]
- Solecki, D.J., E.E. Govek, T. Tomoda, and M.E. Hatten. 2006. Neuronal polarity in CNS development. Genes Dev. 20:2639–2647. [DOI] [PubMed] [Google Scholar]
- Squire, L.R., D. Berg, F.E. Bloom, S. du Lac, A. Ghosh, and N.C. Spitzer. 2008. Fundamental Neuroscience. Third Edition. Academic Press, Amsterdam/Boston. 1256 pp.
- van de Wetering, M., E. Sancho, C. Verweij, W. de Lau, I. Oving, A. Hurlstone, K. van der Horn, E. Batlle, D. Coudreuse, A.P. Haramis, et al. 2002. The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell. 111:241–250. [DOI] [PubMed] [Google Scholar]
- Votin, V., W.J. Nelson, and A.I. Barth. 2005. Neurite outgrowth involves adenomatous polyposis coli protein and beta-catenin. J. Cell Sci. 118:5699–5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabe-Uchida, M., E.E. Govek, and L. Van Aelst. 2006. Regulators of Rho GTPases in neuronal development. J. Neurosci. 26:10633–10635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita, Y.M., D.L. Jones, and M.T. Fuller. 2003. Orientation of asymmetric stem cell division by the APC tumor suppressor and centrosome. Science. 301:1547–1550. [DOI] [PubMed] [Google Scholar]
- Yang, M.Y., J.D. Armstrong, I. Vilinsky, N.J. Strausfeld, and K. Kaiser. 1995. Subdivision of the Drosophila mushroom bodies by enhancer-trap expression patterns. Neuron. 15:45–54. [DOI] [PubMed] [Google Scholar]
- Yoshikawa, S., R.D. McKinnon, M. Kokel, and J.B. Thomas. 2003. Wnt-mediated axon guidance via the Drosophila Derailed receptor. Nature. 422:583–588. [DOI] [PubMed] [Google Scholar]
- Zhou, F.Q., J. Zhou, S. Dedhar, Y.H. Wu, and W.D. Snider. 2004. NGF-induced axon growth is mediated by localized inactivation of GSK-3beta and functions of the microtubule plus end binding protein APC. Neuron. 42:897–912. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.