Abstract
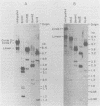
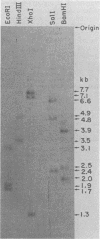
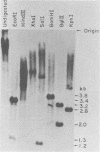
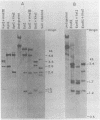
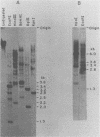
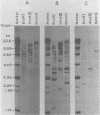
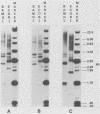
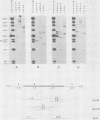
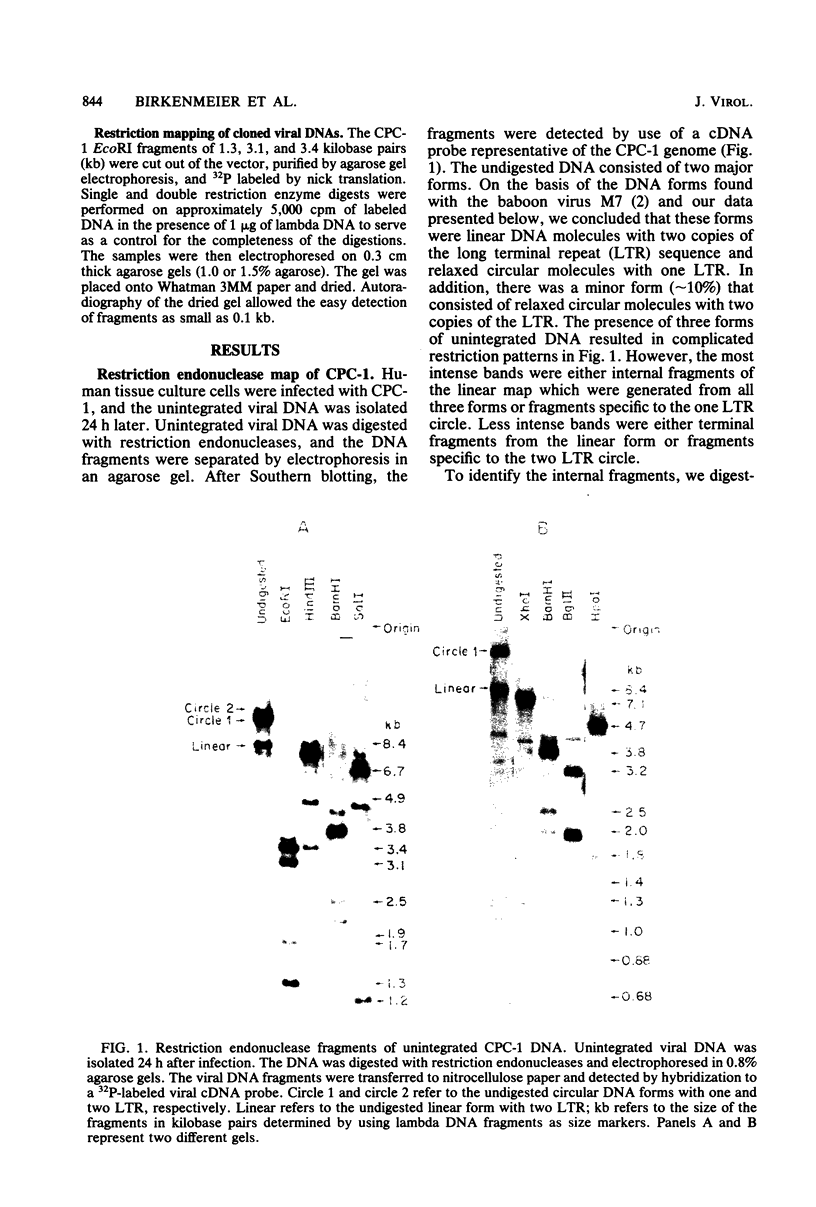
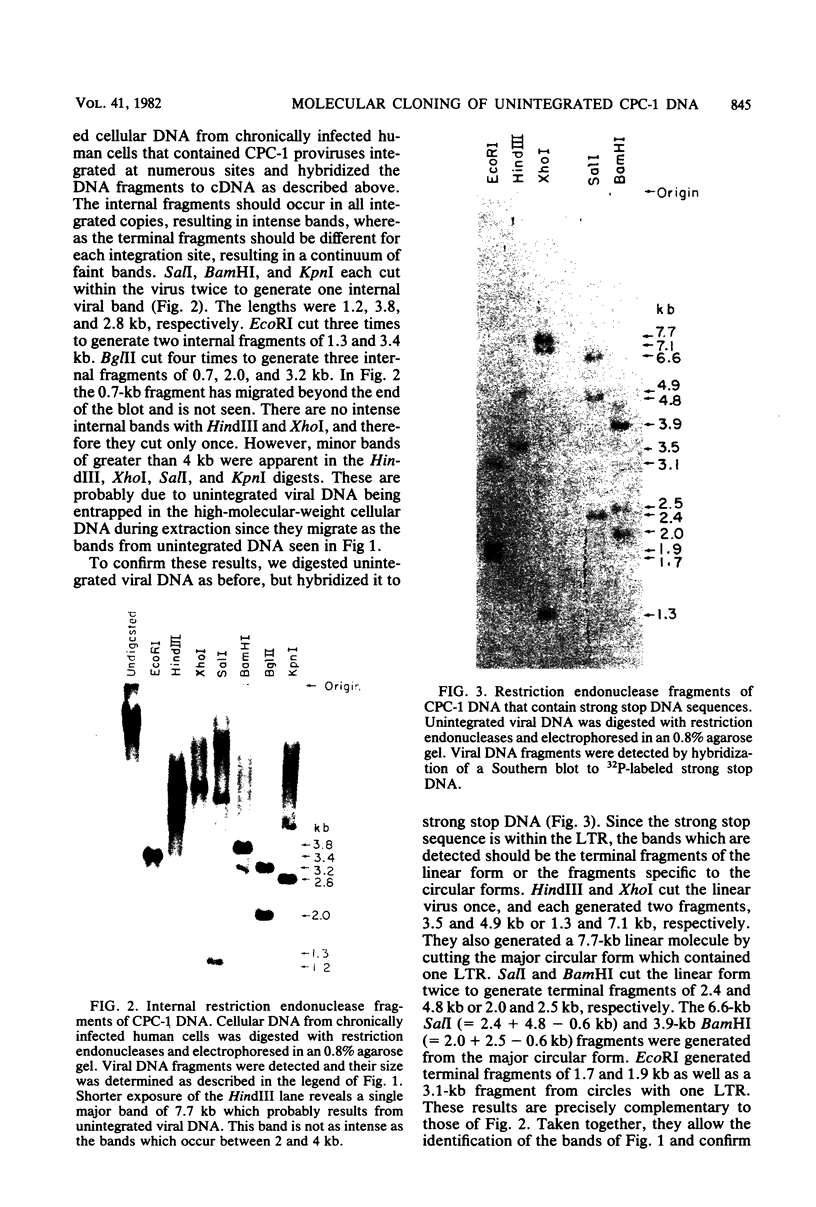
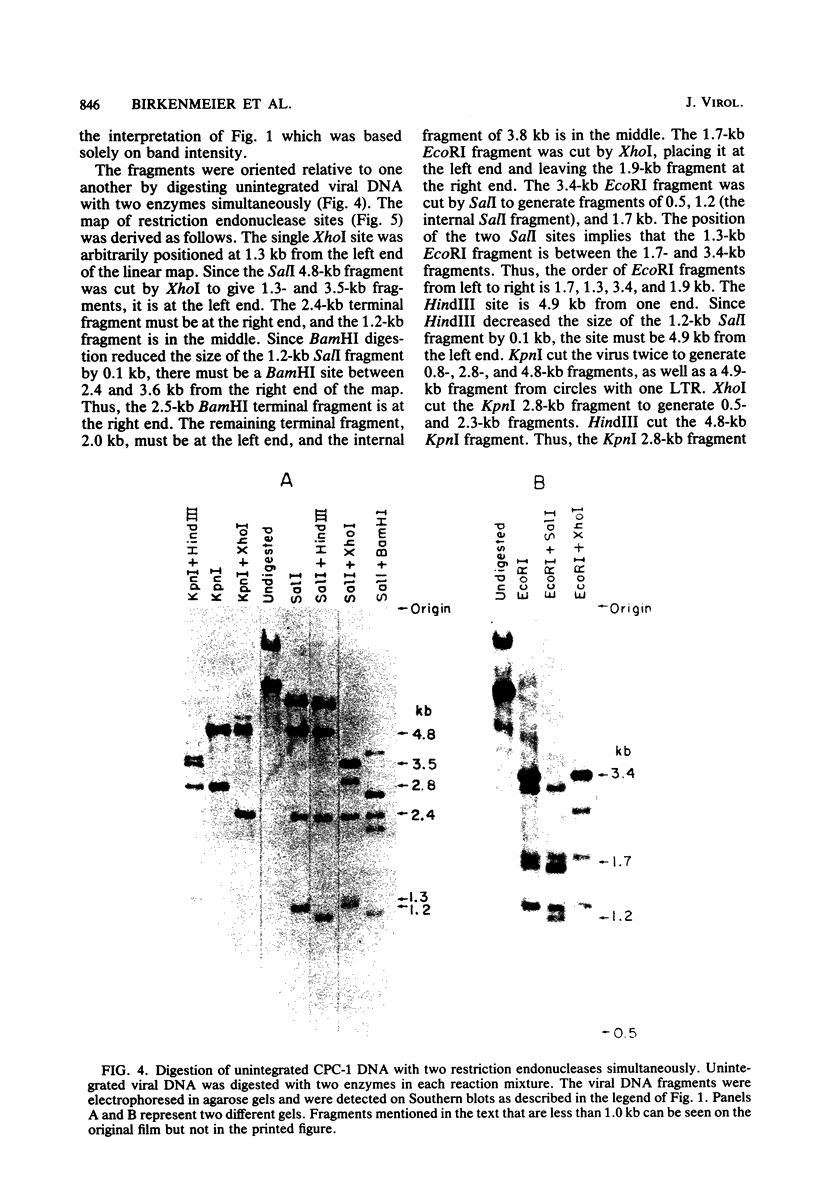
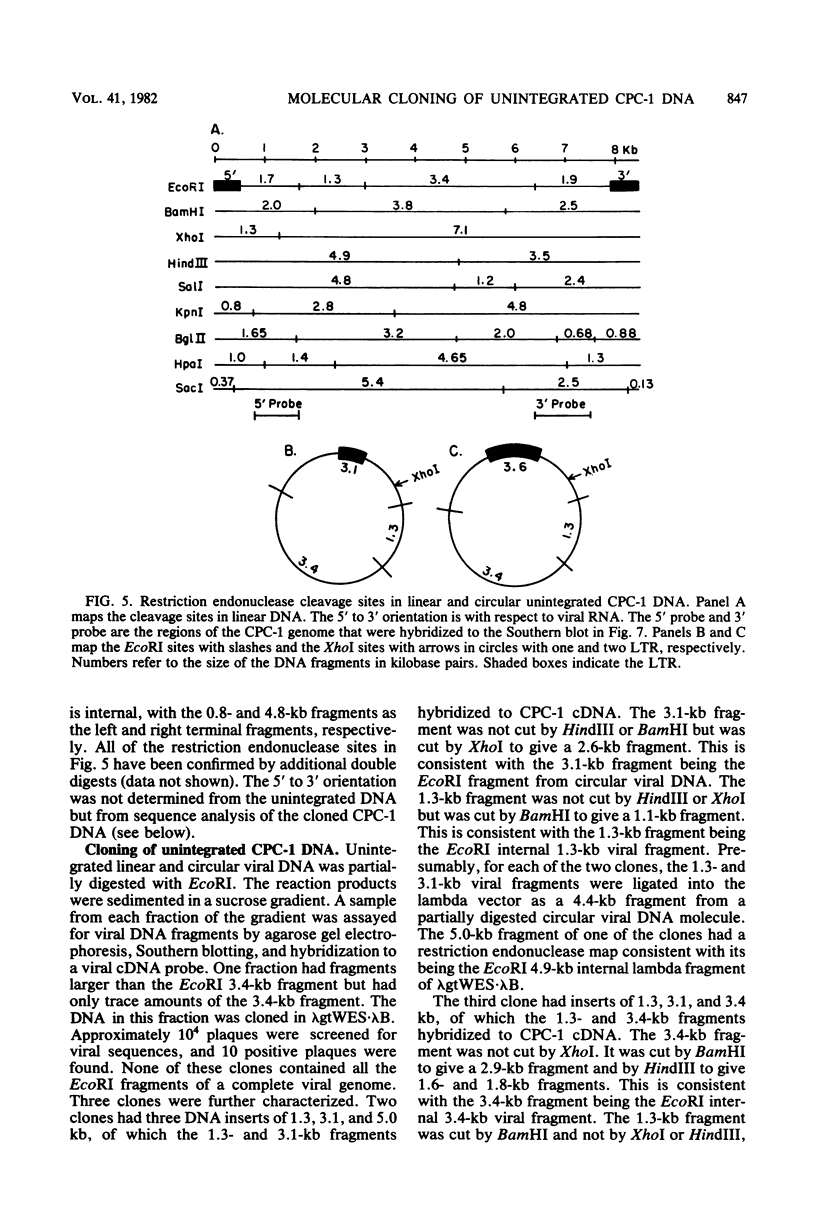
The unintegrated viral DNA intermediates of colobus type C virus (CPC-1) were isolated from infected human cells that were permissive for viral growth. There were two major species of DNA, linear molecules with two copies of the long terminal repeat and relaxed circles containing only a single long terminal repeat. In addition, there was a minor species (approximately 10%) composed of relaxed circles with two copies of the long terminal repeat. A restriction endonuclease map of the unintegrated DNA was constructed. The three EcoRI fragments of circular CPC-1 DNA were cloned in the EcoRI site of lambda gtWES . lambda B and then subcloned in the EcoRI site of pBR322. Using these subgenomic fragments as probes, we have characterized the endogenous viral sequences found in colobus cellular DNA. They are not organized in tandem arrays, as is the case in some other gene families. The majority of sequences detected in cellular DNA have the same map as the CPC-1 unintegrated DNA at 17 of 18 restriction endonuclease sites. There are, however, other sequences that are present in multiple copies and do not correspond to the CPC-1 map. They do not contain CPC-1 sequences either in an altered form or fused to common nonviral sequences. Instead, they appear to be derived from a distinct family of sequences that is substantially diverged from the CPC-1 family. This second family of sequences, CPC-2, is also different from the sequences related to baboon endogenous type C virus that forms a third family of virus-related sequences in the colobus genome.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore D. Gene conversion: some implications for immunoglobulin genes. Cell. 1981 Jun;24(3):592–594. doi: 10.1016/0092-8674(81)90082-9. [DOI] [PubMed] [Google Scholar]
- Battula N., Todaro G. J. Physical map of infectious baboon type C viral DNA and sites of integration in infected cells. J Virol. 1980 Dec;36(3):709–718. doi: 10.1128/jvi.36.3.709-718.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Todaro G. J. Evolution of type C viral genes: evidence for an Asian origin of man. Nature. 1976 May 13;261(5556):101–108. doi: 10.1038/261101a0. [DOI] [PubMed] [Google Scholar]
- Bonner T. I., Todaro G. J. The evolution of baboon endogenous type C virus: related sequences in the DNA of distant species. Virology. 1980 May;103(1):217–227. doi: 10.1016/0042-6822(80)90139-7. [DOI] [PubMed] [Google Scholar]
- Cohen M., Davidson N., Gilden R. V., McAllister R. M., Nicolson M. O., Stephens R. M. The baboon endogenous virus genome. II. Provirus sequence variations in baboon cell DNA. Nucleic Acids Res. 1980 Oct 10;8(19):4423–4440. doi: 10.1093/nar/8.19.4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M., Nicolson M. O., McAllister R. M., Shure M., Davidson N., Rice N., Gilden R. V. Baboon endogenous virus genome. I. Restriction enzyme map of the unintegrated DNA genome of a primate retrovirus. J Virol. 1980 Apr;34(1):28–39. doi: 10.1128/jvi.34.1.28-39.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn R. H., Lowry J. C., Kedes L. H. Histone genes of the sea urchin (S. purpuratus) cloned in E coli: order, polarity, and strandedness of the five histone-coding and spacer regions. Cell. 1976 Sep;9(1):147–161. doi: 10.1016/0092-8674(76)90060-x. [DOI] [PubMed] [Google Scholar]
- Enquist L., Sternberg N. In vitro packaging of lambda Dam vectors and their use in cloning DNA fragments. Methods Enzymol. 1979;68:281–298. doi: 10.1016/0076-6879(79)68020-5. [DOI] [PubMed] [Google Scholar]
- Forsheit A. B., Davidson N., Brown D. D. An electron microscope heteroduplex study of the ribosomal DNAs of Xenopus laevis and Xenopus mulleri. J Mol Biol. 1974 Dec 5;90(2):301–314. doi: 10.1016/0022-2836(74)90375-1. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager G. L., Chang E. H., Chan H. W., Garon C. F., Israel M. A., Martin M. A., Scolnick E. M., Lowy D. R. Molecular cloning of the Harvey sarcoma virus closed circular DNA intermediates: initial structural and biological characterization. J Virol. 1979 Sep;31(3):795–809. doi: 10.1128/jvi.31.3.795-809.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- Rabin H., Benton C. V., Tainsky M. A., Rice N. R., Gilden R. V. Isolation and characterization of an endogenous type C virus of rhesus monkeys. Science. 1979 May 25;204(4395):841–842. doi: 10.1126/science.87013. [DOI] [PubMed] [Google Scholar]
- Rice N. R., Bonner T. I., Gilden R. V. Nucleic acid homology between avian and mammalian type C viruses: relatedness of reticuloendotheliosis virus cdna to cloned proviral DNA of the endogenous Colobus virus CPC-1. Virology. 1981 Oct 15;114(1):286–290. doi: 10.1016/0042-6822(81)90279-8. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Sugden B., Sambrook J. Detection of two restriction endonuclease activities in Haemophilus parainfluenzae using analytical agarose--ethidium bromide electrophoresis. Biochemistry. 1973 Jul 31;12(16):3055–3063. doi: 10.1021/bi00740a018. [DOI] [PubMed] [Google Scholar]
- Sherwin S. A., Todaro G. J. A new endogenous primate type C virus isolated from the Old World monkey Colobus polykomos. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5041–5045. doi: 10.1073/pnas.76.10.5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimotohno K., Mizutani S., Temin H. M. Sequence of retrovirus provirus resembles that of bacterial transposable elements. Nature. 1980 Jun 19;285(5766):550–554. doi: 10.1038/285550a0. [DOI] [PubMed] [Google Scholar]
- Shoyab M., Sen A. A rapid method for the purification of extrachromosomal DNA from eukaryotic cells. J Biol Chem. 1978 Oct 10;253(19):6654–6656. [PubMed] [Google Scholar]
- Smith G. P. Evolution of repeated DNA sequences by unequal crossover. Science. 1976 Feb 13;191(4227):528–535. doi: 10.1126/science.1251186. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieslander L. A simple method to recover intact high molecular weight RNA and DNA after electrophoretic separation in low gelling temperature agarose gels. Anal Biochem. 1979 Oct 1;98(2):305–309. doi: 10.1016/0003-2697(79)90145-3. [DOI] [PubMed] [Google Scholar]