Abstract
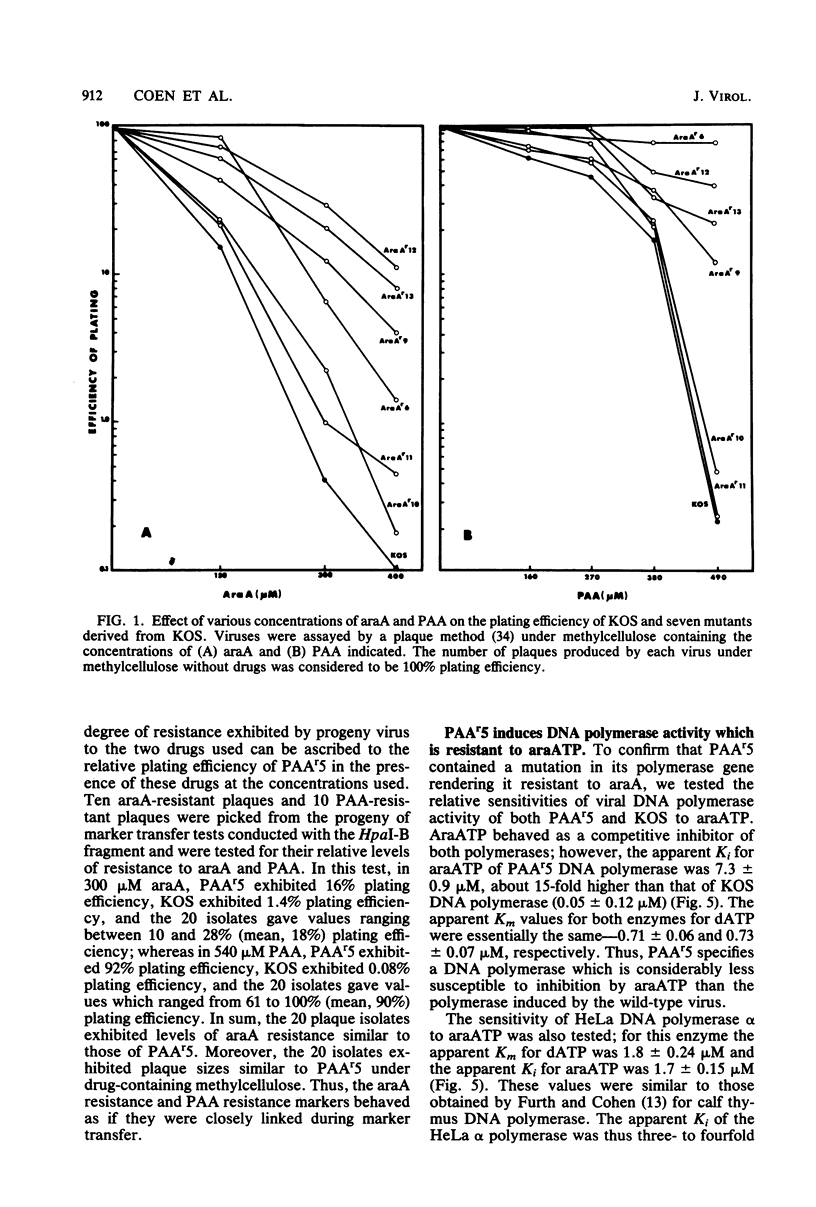
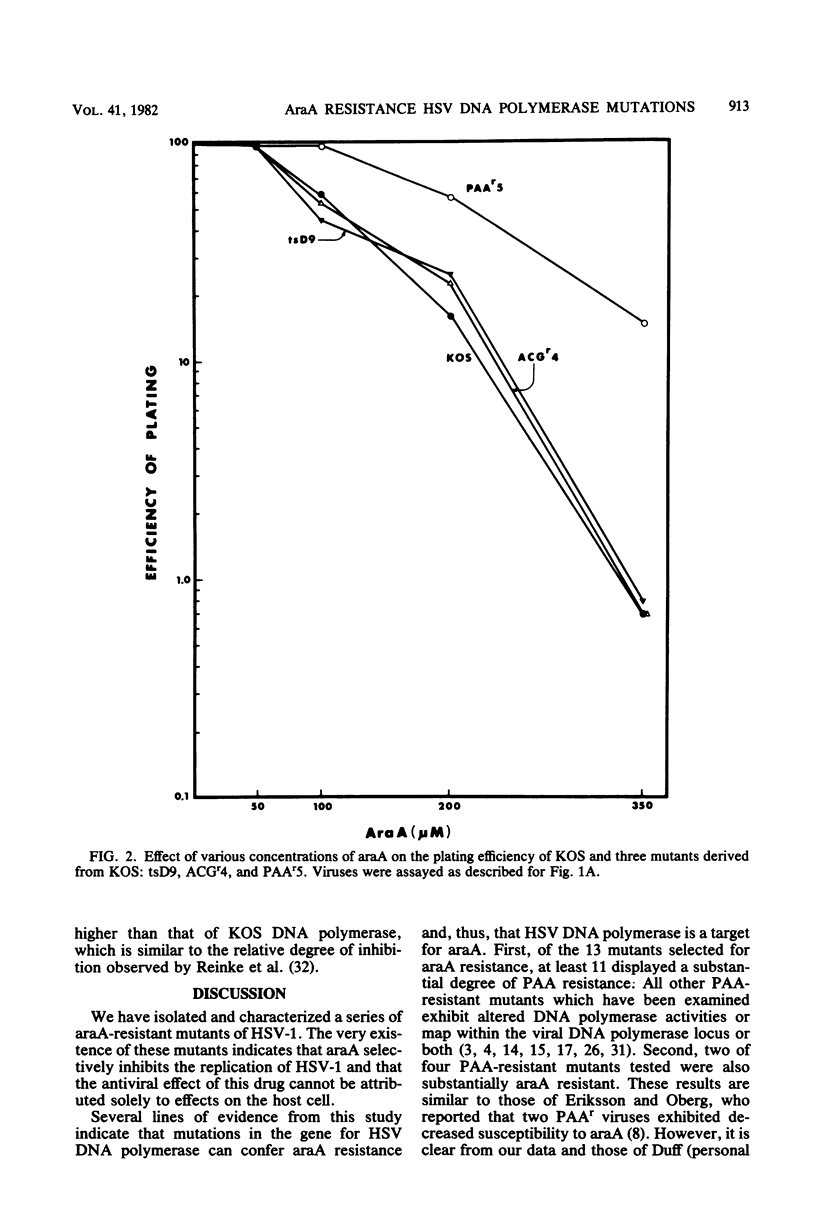
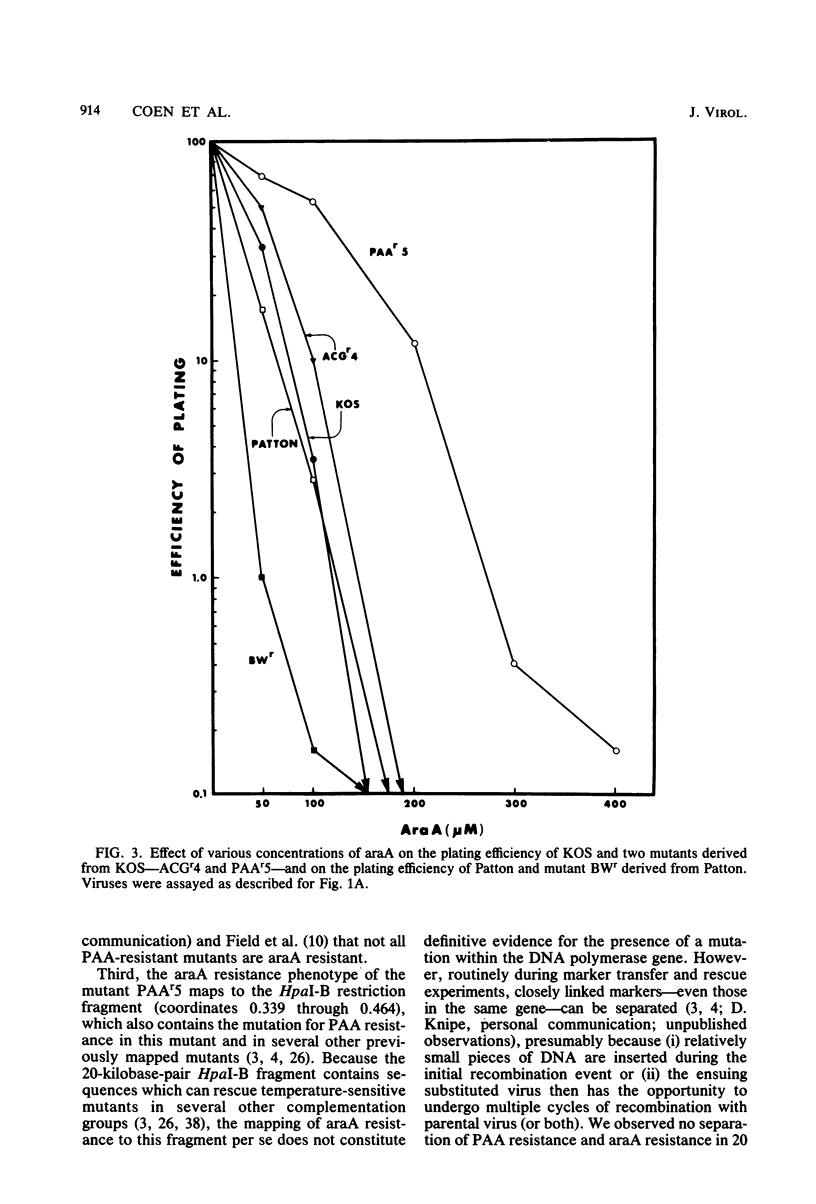
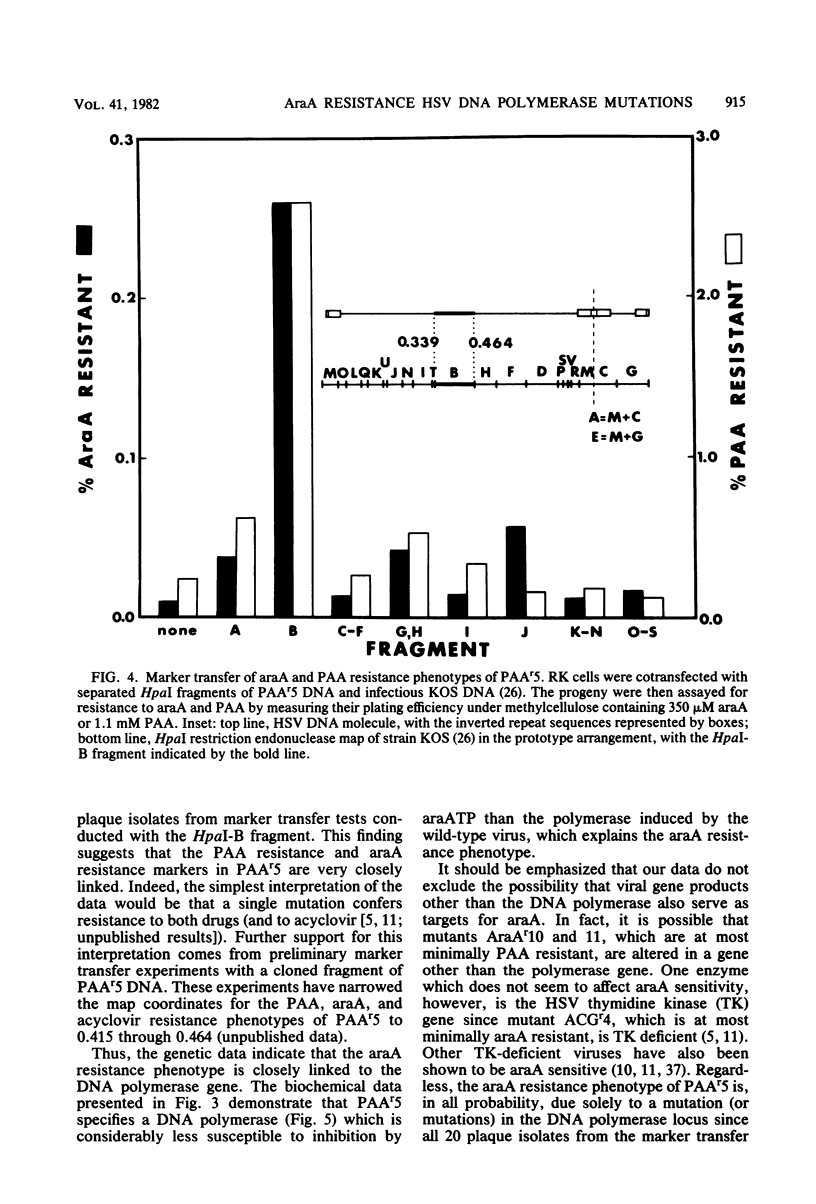
Mutants of herpes simplex virus type 1 resistant to the antiviral drug 9-beta-D-arabinofuranosyladenine (araA) have been isolated and characterized. AraA-resistant mutants can be isolated readily and appear at an appreciable frequency in low-passage stocks of wild-type virus. Of 13 newly isolated mutants, at least 11 were also resistant to phosphonoacetic acid (PAA). Of four previously described PAA-resistant mutants, two exhibited substantial araA resistance. The araA resistance phenotype of one of these mutants, PAAr5, has been mapped to the HpaI-B fragment of herpes simplex virus DNA by marker transfer, and araA resistance behaved in marker transfer experiments as if it were closely linked to PAA resistance, a recognized marker for the viral DNA polymerase locus. PAAr5 induced viral DNA polymerase activity which was much less susceptible to inhibition by the triphosphate derivative of araA than was wild-type DNA polymerase. These genetic and biochemical data indicate that the herpes simplex virus DNA polymerase gene is a locus which, when mutated, can confer resistance to araA and thus that the herpes simplex virus DNA polymerase is a target for this antiviral drug.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aron G. M., Purifoy D. J., Schaffer P. A. DNA synthesis and DNA polymerase activity of herpes simplex virus type 1 temperature-sensitive mutants. J Virol. 1975 Sep;16(3):498–507. doi: 10.1128/jvi.16.3.498-507.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett L. L., Jr, Shannon W. M., Allan P. W., Arnett G. Studies on the biochemical basis for the antiviral activities of some nucleoside analogs. Ann N Y Acad Sci. 1975 Aug 8;255:342–358. doi: 10.1111/j.1749-6632.1975.tb29242.x. [DOI] [PubMed] [Google Scholar]
- Chartrand P., Crumpacker C. S., Schaffer P. A., Wilkie N. M. Physical and genetic analysis of the herpes simplex virus DNA polymerase locus. Virology. 1980 Jun;103(2):311–326. doi: 10.1016/0042-6822(80)90190-7. [DOI] [PubMed] [Google Scholar]
- Chartrand P., Stow N. D., Timbury M. C., Wilkie N. M. Physical mapping of paar mutations of herpes simplex virus type 1 and type 2 by intertypic marker rescue. J Virol. 1979 Aug;31(2):265–276. doi: 10.1128/jvi.31.2.265-276.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen D. M., Schaffer P. A. Two distinct loci confer resistance to acycloguanosine in herpes simplex virus type 1. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2265–2269. doi: 10.1073/pnas.77.4.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. H. Ribonucleotide reductase activity of synchronized KB cells infected with herpes simplex virus. J Virol. 1972 Mar;9(3):408–418. doi: 10.1128/jvi.9.3.408-418.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq E., Descamps J., Verhelst G., Walker R. T., Jones A. S., Torrence P. F., Shugar D. Comparative efficacy of antiherpes drugs against different strains of herpes simplex virus. J Infect Dis. 1980 May;141(5):563–574. doi: 10.1093/infdis/141.5.563. [DOI] [PubMed] [Google Scholar]
- Eriksson B., Oberg B. Characteristics of herpesvirus mutants resistant to phosphonoformate and phosphonoacetate. Antimicrob Agents Chemother. 1979 Jun;15(6):758–762. doi: 10.1128/aac.15.6.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field H. J., Darby G. Pathogenicity in mice of strains of herpes simplex virus which are resistant to acyclovir in vitro and in vivo. Antimicrob Agents Chemother. 1980 Feb;17(2):209–216. doi: 10.1128/aac.17.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field H., McMillan A., Darby G. The sensitivity of acyclovir-resistant mutants of herpes simplex virus to other antiviral drugs. J Infect Dis. 1981 Feb;143(2):281–285. doi: 10.1093/infdis/143.2.281. [DOI] [PubMed] [Google Scholar]
- Furman P. A., Coen D. M., St Clair M. H., Schaffer P. A. Acyclovir-resistant mutants of herpes simplex virus type 1 express altered DNA polymerase or reduced acyclovir phosphorylating activities. J Virol. 1981 Dec;40(3):936–941. doi: 10.1128/jvi.40.3.936-941.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman P. A., St Clair M. H., Fyfe J. A., Rideout J. L., Keller P. M., Elion G. B. Inhibition of herpes simplex virus-induced DNA polymerase activity and viral DNA replication by 9-(2-hydroxyethoxymethyl)guanine and its triphosphate. J Virol. 1979 Oct;32(1):72–77. doi: 10.1128/jvi.32.1.72-77.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furth J. J., Cohen S. S. Inhibition of mammalian DNA polymerase by the 5'-triphosphate of 1-beta-d-arabinofuranosylcytosine and the 5'-triphosphate of 9-beta-d-arabinofuranoxyladenine. Cancer Res. 1968 Oct;28(10):2061–2067. [PubMed] [Google Scholar]
- Hay J., Subak-Sharpe J. H. Mutants of herpes simplex virus types 1 and 2 that are resistant to phosphonoacetic acid induce altered DNA polymerase activities in infected cells. J Gen Virol. 1976 Apr;31(1):145–148. doi: 10.1099/0022-1317-31-1-145. [DOI] [PubMed] [Google Scholar]
- Honess R. W., Watson D. H. Herpes simplex virus resistance and sensitivity to phosphonoacetic acid. J Virol. 1977 Feb;21(2):584–600. doi: 10.1128/jvi.21.2.584-600.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huszar D., Bacchetti S. Partial purification and characterization of the ribonucleotide reductase induced by herpes simplex virus infection of mammalian cells. J Virol. 1981 Feb;37(2):580–588. doi: 10.1128/jvi.37.2.580-588.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jofre J. T., Schaffer P. A., Parris D. S. Genetics of resistance to phosphonoacetic acid in strain KOS of herpes simplex virus type 1. J Virol. 1977 Sep;23(3):833–836. doi: 10.1128/jvi.23.3.833-836.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R. J., Friedman-Kien A. E. Phosphonoacetic acid-resistant herpes simplex virus infection in hairless mice. Antimicrob Agents Chemother. 1975 Mar;7(3):289–293. doi: 10.1128/aac.7.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langelier Y., Déchamps M., Buttin G. Aanlysis of dCMP deaminase and CDP reductase levels in hamster cells infected by herpes simplex virus. J Virol. 1978 Jun;26(3):547–553. doi: 10.1128/jvi.26.3.547-553.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore E. C., Cohen S. S. Effects of arabinonucleotides on ribonucleotide reduction by an enzyme system from rat tumor. J Biol Chem. 1967 May 10;242(9):2116–2118. [PubMed] [Google Scholar]
- Müller W. E., Zahn R. K., Bittlingmaier K., Falke D. Inhibition of herpesvirus DNA synthesis by 9-beta-D-arabinofuranosyladenine in cellular and cell-free systems. Ann N Y Acad Sci. 1977 Mar 4;284:34–48. doi: 10.1111/j.1749-6632.1977.tb21935.x. [DOI] [PubMed] [Google Scholar]
- North T. W., Cohen S. S. Aranucleosides and aranucleotides in viral chemotherapy. Pharmacol Ther. 1979;4(1):81–108. doi: 10.1016/0163-7258(79)90016-0. [DOI] [PubMed] [Google Scholar]
- Ostrander M., Cheng Y. C. Properties of herpes simplex virus type 1 and type 2 DNA polymerase. Biochim Biophys Acta. 1980 Sep 19;609(2):232–245. doi: 10.1016/0005-2787(80)90234-8. [DOI] [PubMed] [Google Scholar]
- Parris D. S., Courtney R. J., Schaffer P. A. Temperature-sensitive mutants of herpes simplex virus type 1 defective in transcriptional and post-transcriptional functions required for viral DNA synthesis. Virology. 1978 Oct 15;90(2):177–186. doi: 10.1016/0042-6822(78)90301-x. [DOI] [PubMed] [Google Scholar]
- Parris D. S., Dixon R. A., Schaffer P. A. Physical mapping of herpes simplex virus type 1 ts mutants by marker rescue: correlation of the physical and genetic maps. Virology. 1980 Jan 30;100(2):275–287. doi: 10.1016/0042-6822(80)90519-x. [DOI] [PubMed] [Google Scholar]
- Pedersen M., Talley-Brown S., Millette R. L. Gene expression of herpes simplex virus. III. Effect of arabinosyladenine on viral polypeptide synthesis. J Virol. 1981 May;38(2):712–719. doi: 10.1128/jvi.38.2.712-719.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelling J. C., Drach J. C., Shipman C., Jr Internucleotide incorporation of arabinosyladenine into herpes simplex virus and mammalian cell DNA. Virology. 1981 Mar;109(2):323–335. doi: 10.1016/0042-6822(81)90503-1. [DOI] [PubMed] [Google Scholar]
- Potuzak H., Wintersberger U. DNA covalently linked to carboxymethyl-cellulose and its application in affinity chromatography. FEBS Lett. 1976 Mar 15;63(1):167–170. doi: 10.1016/0014-5793(76)80218-9. [DOI] [PubMed] [Google Scholar]
- Purifoy D. J., Lewis R. B., Powell K. L. Identification of the herpes simplex virus DNA polymerase gene. Nature. 1977 Oct 13;269(5629):621–623. doi: 10.1038/269621a0. [DOI] [PubMed] [Google Scholar]
- Reinke C. M., Drach J. C., Shipman C., Jr, Weissbach A. Differential inhibition of mammalian DNA polymerases alpha, beta and gamma and herpes simplex virus-induced DNA polymerase by the 5'-triphosphates of arabinosyladenine and arabinosylcytosine. IARC Sci Publ. 1978;(24 Pt 2):999–1005. [PubMed] [Google Scholar]
- Ruyechan W. T., Morse L. S., Knipe D. M., Roizman B. Molecular genetics of herpes simplex virus. II. Mapping of the major viral glycoproteins and of the genetic loci specifying the social behavior of infected cells. J Virol. 1979 Feb;29(2):677–697. doi: 10.1128/jvi.29.2.677-697.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer P. A., Tevethia M. J., Benyesh-Melnick M. Recombination between temperature-sensitive mutants of herpes simplex virus type 1. Virology. 1974 Mar;58(1):219–228. doi: 10.1016/0042-6822(74)90156-1. [DOI] [PubMed] [Google Scholar]
- Schnipper L. E., Crumpacker C. S. Resistance of herpes simplex virus to acycloguanosine: role of viral thymidine kinase and DNA polymerase loci. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2270–2273. doi: 10.1073/pnas.77.4.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipman C., Jr, Smith S. H., Carlson R. H., Drach J. C. Antiviral activity of arabinosyladenine and arabinosylhypoxanthine in herpes simplex virus-infected KB cells: selective inhibition of viral deoxyribonucleic acid synthesis in synchronized suspension cultures. Antimicrob Agents Chemother. 1976 Jan;9(1):120–127. doi: 10.1128/aac.9.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. O., Kennell W. L., Poirier R. H., Lynd F. T. In vitro and in vivo resistance of herpes simplex virus to 9-(2-hydroxyethoxymethyl)guanine (acycloguanosine). Antimicrob Agents Chemother. 1980 Feb;17(2):144–150. doi: 10.1128/aac.17.2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stow N. D., Wilkie N. M. Physical mapping of temperature-sensitive mutations of herpes simplex virus type 1 by intertypic marker rescue. Virology. 1978 Oct 1;90(1):1–11. doi: 10.1016/0042-6822(78)90327-6. [DOI] [PubMed] [Google Scholar]
- Tenser R. B., Miller R. L., Rapp F. Trigeminal ganglion infection by thymidine kinase-negative mutants of herpes simplex virus. Science. 1979 Aug 31;205(4409):915–917. doi: 10.1126/science.224454. [DOI] [PubMed] [Google Scholar]
- Weissbach A., Hong S. C., Aucker J., Muller R. Characterization of herpes simplex virus-induced deoxyribonucleic acid polymerase. J Biol Chem. 1973 Sep 25;248(18):6270–6277. [PubMed] [Google Scholar]


