Abstract
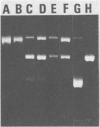
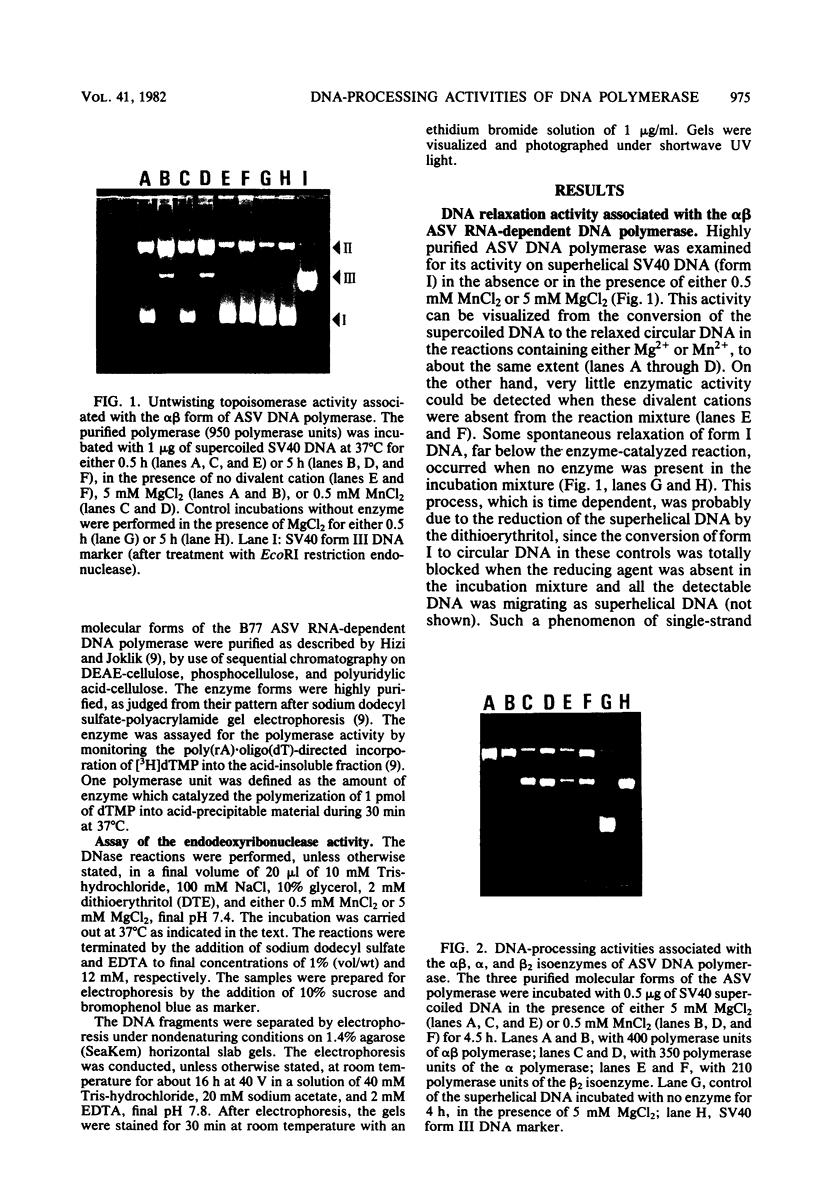
The RNA-dependent DNA polymerase purified from B77 avian sarcoma virus exhibited two distinct DNA-processing activities. The alpha and beta 2 isoenzymes possessed an endodeoxyribonuclease activity capable of nicking simian virus 40 superhelical DNA, whereas the alpha beta isoenzyme performed as an untwisting topoisomerase. Both activities associated with the three molecular forms of the retroviral DNA polymerase were dependent on the presence of either Mn2+ or Mg2+ ions. From analysis of the denaturated DNA products, it is apparent that the alpha and beta 2 isoenzymes introduced two nicks, one per each strand in the superhelical simian virus 40 DNA molecules, whereas the alpha beta polymerase converted these supercoiled molecules to the relaxed covalently closed circular form. The notion that the DNA-processing activities are located on the DNA polymerase molecules was supported by the following: (i) the three isoenzymes were of a high purity; (ii) the activities cosedimented in glycerol gradients with the DNA polymerase activities of the alpha, beta 2, and alpha beta molecular forms; and (iii) immunoglobulin directed against the purified polymerase immunoprecipitated the DNA-processing activities. Chemical treatments of the DNA polymerase molecules (with pyridoxalphosphate, iodoacetamide, and sulfhydryl reagents), which inhibited the polymerase activity, also suppressed the endonucleolytic and topoisomerase activities, suggesting that cystein and amino groups play an important role in the active sites of the DNA-processing activities as well.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bode V. C. Single-strand scissions induced in circular and linear lambda DNA by the presence of dithiothreitol and other reducing agents. J Mol Biol. 1967 May 28;26(1):125–129. doi: 10.1016/0022-2836(67)90266-5. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Leis J. P., Smith M. S., Faras A. J. Unwinding-like activity associated with avian retrovirus RNA-directed DNA polymerase. J Virol. 1978 May;26(2):498–509. doi: 10.1128/jvi.26.2.498-509.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson W., Verma I. M. Studies on the reverse transcriptase of RNA tumor viruses. Structural relatedness of two subunits of avian RNA tumor viruses. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4991–4994. doi: 10.1073/pnas.71.12.4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golomb M., Grandgenett D. P. Endonuclease activity of purified RNA-directed DNA polymerase from avian myeloblastosis virus. J Biol Chem. 1979 Mar 10;254(5):1606–1613. [PubMed] [Google Scholar]
- Gorecki M., Panet A. Discrimination of DNA polymerase and RNase H activities in reverse transcriptase of avian myeloblastosis virus. Biochemistry. 1978 Jun 13;17(12):2438–2442. doi: 10.1021/bi00605a030. [DOI] [PubMed] [Google Scholar]
- Grandgenett D. P., Golomb M., Vora A. C. Activation of an Mg2+-dependent DNA endonuclease of avian myeloblastosis virus alpha beta DNA polymerase by in vitro proteolytic cleavage. J Virol. 1980 Jan;33(1):264–271. doi: 10.1128/jvi.33.1.264-271.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guntaka R. V., Richards O. C., Shank P. R., Kung H. J., Davidson N. Covalently closed circular DNA of avian sarcoma virus: purification from nuclei of infected quail tumor cells and measurement by electron microscopy and gel electrophoresis. J Mol Biol. 1976 Sep 15;106(2):337–357. doi: 10.1016/0022-2836(76)90090-5. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Hizi A., Joklik W. K. RNA-dependent DNA polymerase of avian sarcoma virus B77. I. Isolation and partial characterization of the alpha, beta2, and alphabeta forms of the enzyme. J Biol Chem. 1977 Apr 10;252(7):2281–2289. [PubMed] [Google Scholar]
- Hizi A., Leis J. P., Joklik W. K. RNA-dependent DNA polymerase of avian sarcoma virus B77. II. Comparison of the catalytic properties of the alpha, beta2, and alphabeta enzyme forms. J Biol Chem. 1977 Apr 10;252(7):2290–2295. [PubMed] [Google Scholar]
- Hizi A., Leis J. P., Joklik W. K. The RNA-dependent DNA polymerase of avian sarcoma virus B77. Binding of viral and nonviral ribonucleic acids to the alpha, beta2, and alphabeta forms of the enzyme. J Biol Chem. 1977 Oct 10;252(19):6878–6884. [PubMed] [Google Scholar]
- Hizi A., McCrae M. A., Joklik W. K. Studies on the amino acid sequence content of proteins specified by the gag and pol genes of avian sarcoma virus B77. Virology. 1978 Aug;89(1):272–284. doi: 10.1016/0042-6822(78)90059-4. [DOI] [PubMed] [Google Scholar]
- Hoffmann P. J., Cheng Y. C. DNase induced after infection of KB cells by herpes simplex virus type 1 or type 2. II. Characterization of an associated endonuclease activity. J Virol. 1979 Nov;32(2):449–457. doi: 10.1128/jvi.32.2.449-457.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modak M. J. Observations on the pyridoxal 5'-phosphate inhibition of DNA polymerases. Biochemistry. 1976 Aug 10;15(16):3620–3626. doi: 10.1021/bi00661a033. [DOI] [PubMed] [Google Scholar]
- Papas T. S., Pry T. W., Marciani D. J. Inactivation of avian myeloblastosis virus DNA polymerase by specific binding of pyridoxal 5'-phosphate to deoxynucleoside triphosphate binding site. J Biol Chem. 1977 Feb 25;252(4):1425–1430. [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff R. D., Grandgenett D. P. Virus-coded origin of a 32,000-dalton protein from avian retrovirus cores: structural relatedness of p32 and the beta polypeptide of the avian retrovirus DNA polymerase. J Virol. 1978 Oct;28(1):279–291. doi: 10.1128/jvi.28.1.279-291.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seal G., Loeb L. A. On the fidelity of DNA replication. Enzyme activities associated with DNA polymerases from RNA tumor viruses. J Biol Chem. 1976 Feb 25;251(4):975–981. [PubMed] [Google Scholar]
- Verma I. M. The reverse transcriptase. Biochim Biophys Acta. 1977 Mar 21;473(1):1–38. doi: 10.1016/0304-419x(77)90005-1. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A. Structure of the intermediates leading to the integrated provirus. Biochim Biophys Acta. 1977 Mar 21;473(1):39–55. doi: 10.1016/0304-419x(77)90006-3. [DOI] [PubMed] [Google Scholar]