Abstract
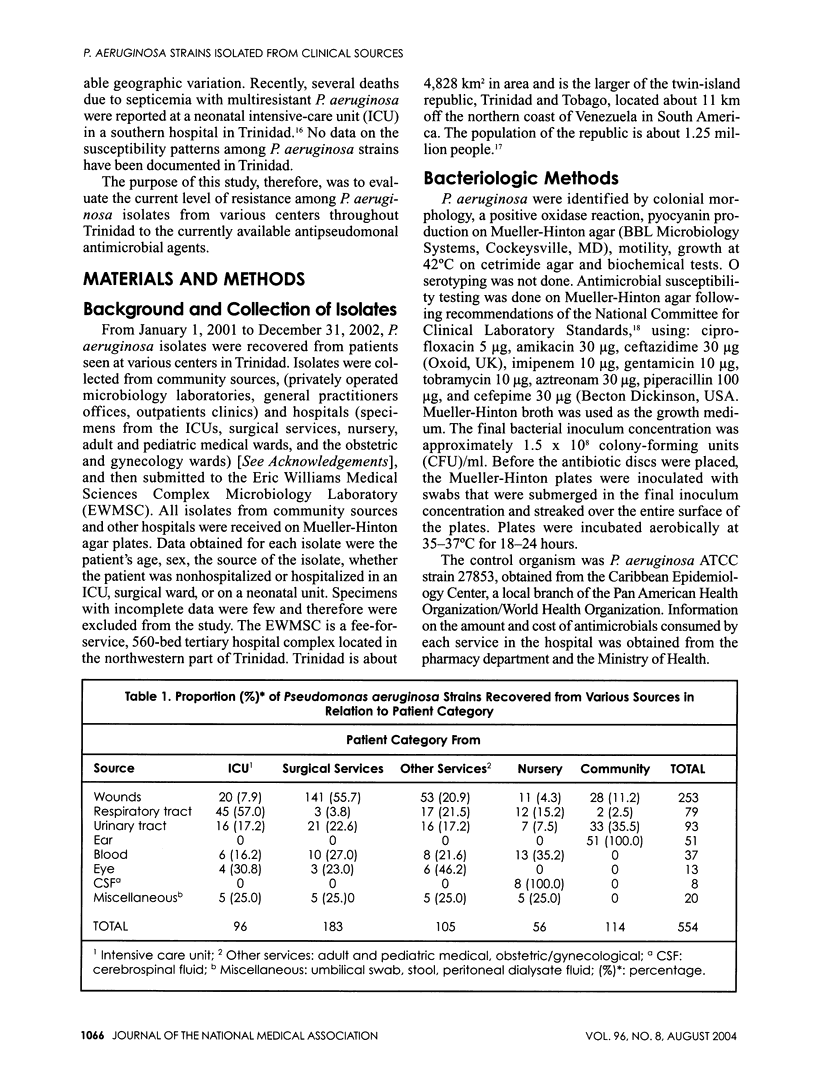
A two-year prospective study of 554 Pseudomonas aeruginosa isolates was recovered from various clinical sources throughout Trinidad, and their resistance patterns to antipseudomonal antimicrobial agents were determined. Of the 554 P. aeruginosa isolates, 20.6% (114/554) were community isolates, 17.3% (96/554) from the intensive care unit (ICU), 10.1% (56/554) from the nursery, and the remaining 52% (288/554) were from other hospital inpatient services. Respiratory tract infections were the predominant source of P. aeruginosa isolates from the ICU--46.9% (45/96)--and nursery--21.4% (12/56), whereas wounds were the principal source of P. aeruginosa from the surgical services--77.0% (141/183). Community isolates of P. aeruginosa were predominantly from ear--100% (51/51)--and urinary tract infections--35.5%, (33/93). The overall prevalence of resistance was low for both hospital isolates (13.9%) and community isolates (3.8%). All community isolates were fully sensitive to four of the nine antimicrobials tested. Resistance rates among community strains ranged from 2.6% (ciprofloxacin and ceftazidime) to 12.3% for piperacillin. All isolates from hospital were fully sensitive to imipenem, but resistance rates for the other drugs ranged between 2.5% and 27.3%. The study showed that the overall resistance pattern of P. aeruginosa was relatively low. This is an encouraging observation but invites caution since resistance to the newly introduced drug, cefepime, has now emerged within the hospital environment and may present serious therapeutic problems within the near future. Policies governing the use of antimicrobials in many institutions are lacking. Such policies must be instituted in order to limit the spread of resistance and also to reduce the emergence of resistance to newly commissioned drugs within the country.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansary S. P., Haque R., Faisal A. A., Chowdhury M. S. Resistance pattern of Pseudomonas aeruginosa occurring in northern part of Bangladesh. Trop Doct. 1994 Oct;24(4):188–188. doi: 10.1177/004947559402400428. [DOI] [PubMed] [Google Scholar]
- Bert F., Maubec E., Bruneau B., Berry P., Lambert-Zechovsky N. Multi-resistant Pseudomonas aeruginosa outbreak associated with contaminated tap water in a neurosurgery intensive care unit. J Hosp Infect. 1998 May;39(1):53–62. doi: 10.1016/s0195-6701(98)90243-2. [DOI] [PubMed] [Google Scholar]
- Büscher K. H., Cullmann W., Dick W., Opferkuch W. Imipenem resistance in Pseudomonas aeruginosa resulting from diminished expression of an outer membrane protein. Antimicrob Agents Chemother. 1987 May;31(5):703–708. doi: 10.1128/aac.31.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H. Y., Yuan M., Livermore D. M. Mechanisms of resistance to beta-lactam antibiotics amongst Pseudomonas aeruginosa isolates collected in the UK in 1993. J Med Microbiol. 1995 Oct;43(4):300–309. doi: 10.1099/00222615-43-4-300. [DOI] [PubMed] [Google Scholar]
- Faden H., Grossi M. Acute osteomyelitis in children. Reassessment of etiologic agents and their clinical characteristics. Am J Dis Child. 1991 Jan;145(1):65–69. doi: 10.1001/archpedi.1991.02160010071018. [DOI] [PubMed] [Google Scholar]
- Grayson M. L., Eliopoulos G. M. Antimicrobial resistance in the intensive care unit. Semin Respir Infect. 1990 Sep;5(3):204–214. [PubMed] [Google Scholar]
- Hiraoka M., Inoue M., Mitsuhashi S. Hydrolytic rate at low drug concentration as a limiting factor in resistance to newer cephalosporins. Rev Infect Dis. 1988 Jul-Aug;10(4):746–751. doi: 10.1093/clinids/10.4.746. [DOI] [PubMed] [Google Scholar]
- Komshian S. V., Tablan O. C., Palutke W., Reyes M. P. Characteristics of left-sided endocarditis due to Pseudomonas aeruginosa in the Detroit Medical Center. Rev Infect Dis. 1990 Jul-Aug;12(4):693–702. doi: 10.1093/clinids/12.4.693. [DOI] [PubMed] [Google Scholar]
- Liu P. Y., Gur D., Hall L. M., Livermore D. M. Survey of the prevalence of beta-lactamases amongst 1000 gram-negative bacilli isolated consecutively at the Royal London Hospital. J Antimicrob Chemother. 1992 Oct;30(4):429–447. doi: 10.1093/jac/30.4.429. [DOI] [PubMed] [Google Scholar]
- Livermore D. M. Interplay of impermeability and chromosomal beta-lactamase activity in imipenem-resistant Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1992 Sep;36(9):2046–2048. doi: 10.1128/aac.36.9.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis A. N., Karkavitsas C., Maniatis N. A., Tsiftsakis E., Genimata V., Legakis N. J. Pseudomonas aeruginosa folliculitis due to non-O:11 serogroups: acquisition through use of contaminated synthetic sponges. Clin Infect Dis. 1995 Aug;21(2):437–439. doi: 10.1093/clinids/21.2.437. [DOI] [PubMed] [Google Scholar]
- Miller G. H., Sabatelli F. J., Hare R. S., Glupczynski Y., Mackey P., Shlaes D., Shimizu K., Shaw K. J. The most frequent aminoglycoside resistance mechanisms--changes with time and geographic area: a reflection of aminoglycoside usage patterns? Aminoglycoside Resistance Study Groups. Clin Infect Dis. 1997 Jan;24 (Suppl 1):S46–S62. doi: 10.1093/clinids/24.supplement_1.s46. [DOI] [PubMed] [Google Scholar]
- Orrett F. A. Fatal multi-resistant Pseudomonas aeruginosa septicemia outbreak in a neonatal intensive care unit in Trinidad. Ethiop Med J. 2000 Apr;38(2):85–91. [PubMed] [Google Scholar]
- Orrett F. A. Prevalence of Proteus species in urinary tract infections in a regional hospital in Trinidad. Zhonghua Yi Xue Za Zhi (Taipei) 1999 Jul;62(7):438–442. [PubMed] [Google Scholar]
- Orrett F. A., Shurland S. M. Neonatal sepsis and mortality in a regional hospital in Trinidad: aetiology and risk factors. Ann Trop Paediatr. 2001 Mar;21(1):20–25. doi: 10.1080/02724930020028867. [DOI] [PubMed] [Google Scholar]
- Orrett F. A., Shurland S. M. Prevalence of bacterial pathogens and susceptibility patterns from clinical sources in Trinidad. West Indian Med J. 2000 Sep;49(3):205–209. [PubMed] [Google Scholar]
- Orrett F. A., Shurland S. M. Prevalence of resistance to antimicrobials of Escherichia coli isolates from clinical sources at a private hospital in Trinidad. Jpn J Infect Dis. 2001 Apr;54(2):64–68. [PubMed] [Google Scholar]
- Poole K., Krebes K., McNally C., Neshat S. Multiple antibiotic resistance in Pseudomonas aeruginosa: evidence for involvement of an efflux operon. J Bacteriol. 1993 Nov;175(22):7363–7372. doi: 10.1128/jb.175.22.7363-7372.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qadri S. M., Cunha B. A., Ueno Y., Abumustafa F., Imambaccus H., Tullo D. D., Domenico P. Activity of cefepime against nosocomial blood culture isolates. J Antimicrob Chemother. 1995 Sep;36(3):531–536. doi: 10.1093/jac/36.3.531. [DOI] [PubMed] [Google Scholar]
- Schaberg D. R., Weinstein R. A., Stamm W. E. Epidemics of nosocomial urinary tract infection caused by multiply resistant gram-negative bacilli: epidemiology and control. J Infect Dis. 1976 Mar;133(3):363–366. doi: 10.1093/infdis/133.3.363. [DOI] [PubMed] [Google Scholar]
- Shannon K., Phillips I. Mechanisms of resistance to aminoglycosides in clinical isolates. J Antimicrob Chemother. 1982 Feb;9(2):91–102. doi: 10.1093/jac/9.2.91. [DOI] [PubMed] [Google Scholar]
- Sofianou D., Tsakris A., Skoura L., Douboyas J. Extended high-level cross-resistance to antipseudomonal antibiotics amongst Pseudomonas aeruginosa isolates in a university hospital. J Antimicrob Chemother. 1997 Nov;40(5):740–742. doi: 10.1093/jac/40.5.740. [DOI] [PubMed] [Google Scholar]
- Sundström J., Jacobson K., Munck-Wikland E., Ringertz S. Pseudomonas aeruginosa in otitis externa. A particular variety of the bacteria? Arch Otolaryngol Head Neck Surg. 1996 Aug;122(8):833–836. doi: 10.1001/archotol.1996.01890200023004. [DOI] [PubMed] [Google Scholar]
- Toleman Mark A., Rolston Kenneth, Jones Ronald N., Walsh Timothy R. Molecular and biochemical characterization of OXA-45, an extended-spectrum class 2d' beta-lactamase in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2003 Sep;47(9):2859–2863. doi: 10.1128/AAC.47.9.2859-2863.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]


