Abstract
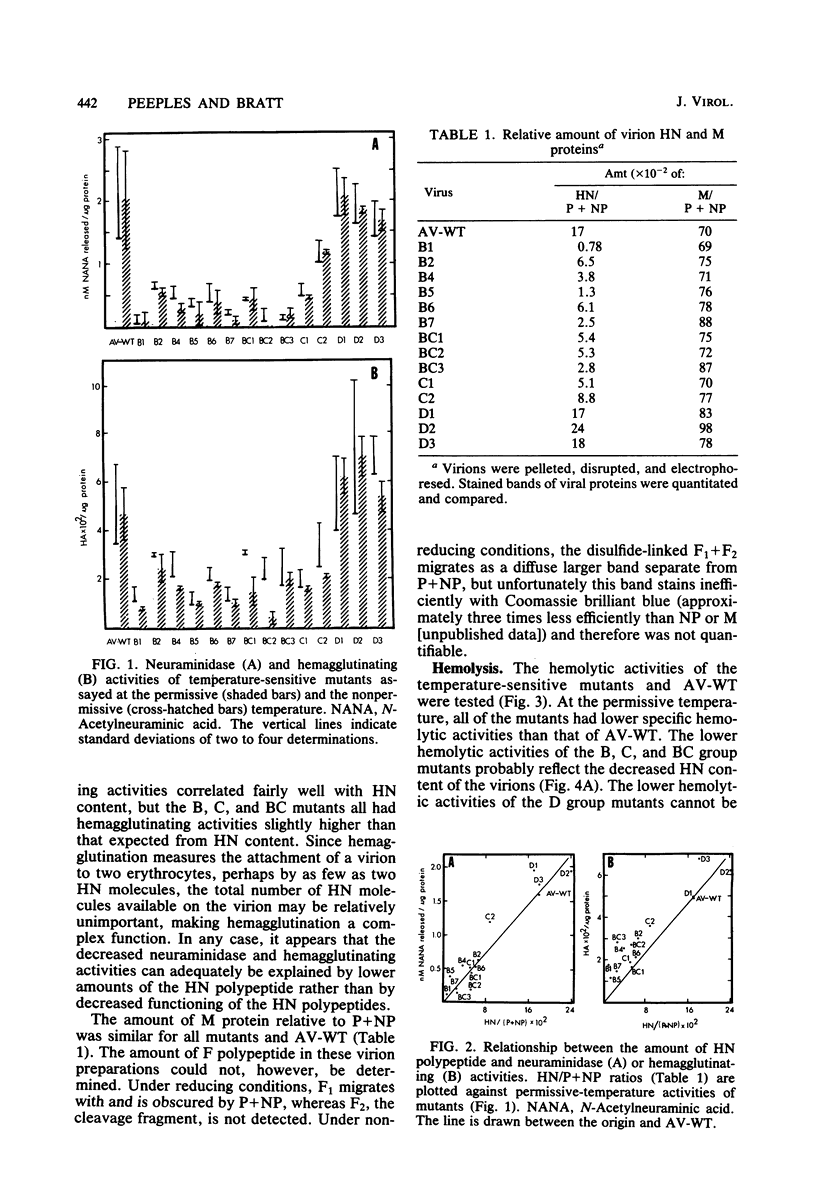
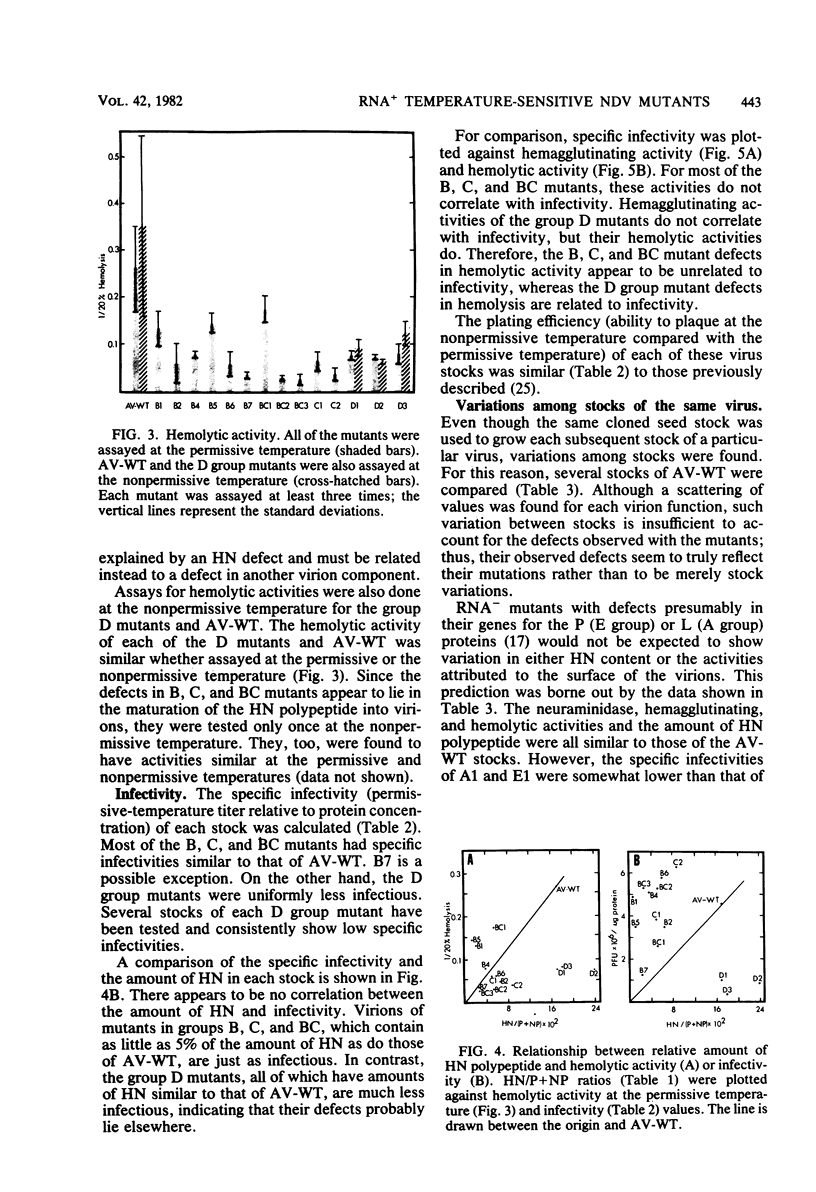
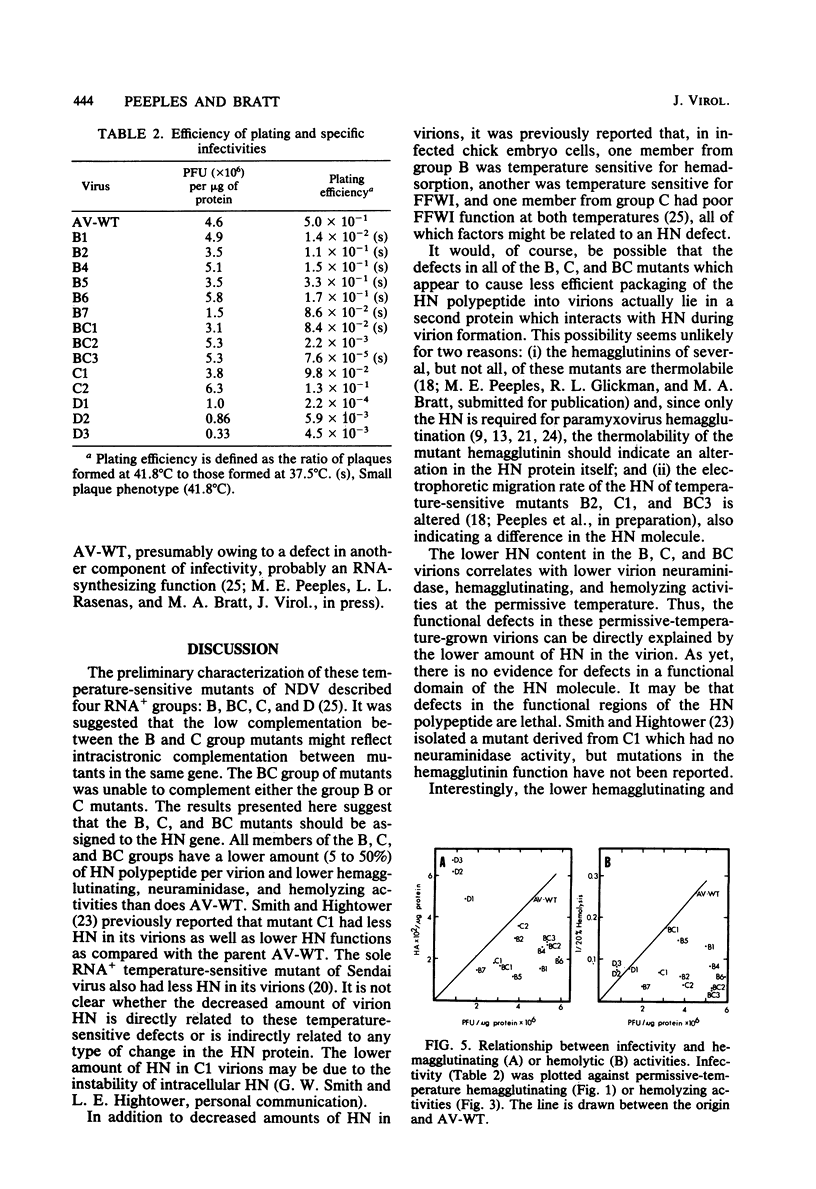
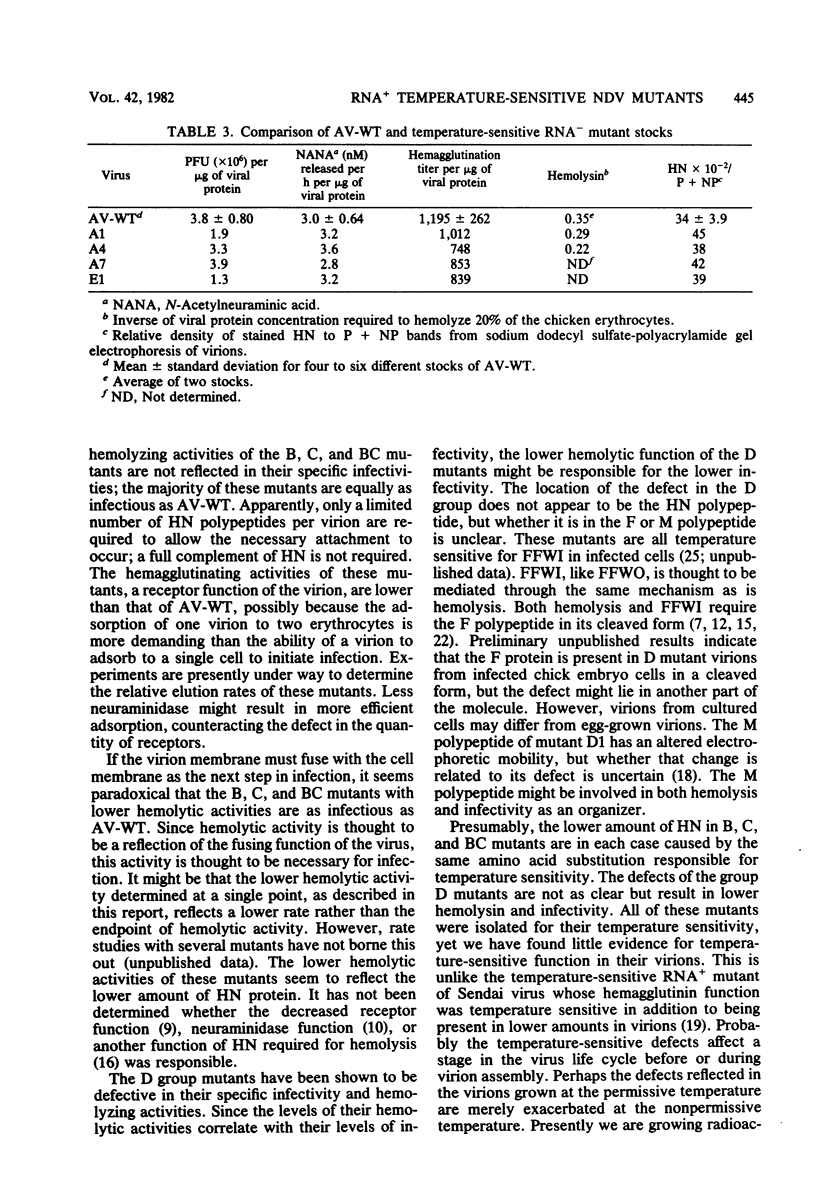
Virions from Newcastle disease virus mutants in four temperature-sensitive RNA+ groups were grown in embryonated hen eggs at the permissive temperature, purified, and then analyzed for biological properties at both the permissive and nonpermissive temperatures. At the permissive temperature, virions of mutants in groups B, C, and BC (11 mutants) were all lower in specific (per milligram of protein) hemagglutination, neuraminidase, and hemolysis activities compared with the wild type. These deficiencies were related to decreased amounts of hemagglutinin-neuraminidase glycoprotein in the virions. Activities of these mutant virions at both the permissive and nonpermissive temperatures were similar, indicating that hemagglutinin-neuraminidase synthesized at the permissive temperature was not temperature sensitive in function. The three group D mutants displayed a different pattern. At the permissive temperature, they had wild-type hemagglutination and neuraminidase activities but were deficient compared with the wild type in hemolysis. Again, functions were similar at both temperatures. Most of the B, C, and BC mutants had specific infectivities similar to that of the wild type despite lower hemagglutination, neuraminidase, and hemolysis functions. However, the D mutants were all less infectious. This evidence is consistent with a shared hemagglutinin-neuraminidase defect in the B, C, and BC mutants and a defect in either the F glycoprotein or the M protein in the D mutants.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMINOFF D. Methods for the quantitative estimation of N-acetylneuraminic acid and their application to hydrolysates of sialomucoids. Biochem J. 1961 Nov;81:384–392. doi: 10.1042/bj0810384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratt M. A., Clavell L. A. Hemolytic interaction of Newcastle disease virus and chicken erythrocytes. I. Quantitative comparison procedure. Appl Microbiol. 1972 Mar;23(3):454–460. doi: 10.1128/am.23.3.454-460.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratt M. A., Gallaher W. R. Preliminary analysis of the requirements for fusion from within and fusion from without by Newcastle disease virus. Proc Natl Acad Sci U S A. 1969 Oct;64(2):536–543. doi: 10.1073/pnas.64.2.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavell L. A., Bratt M. A. Hemolytic interaction of Newcastle disease virus and chicken erythrocytes. II. Determining factors. Appl Microbiol. 1972 Mar;23(3):461–470. doi: 10.1128/am.23.3.461-470.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HORSFALL F. L., Jr, TAMM I. Fractional dilution procedure for precise titration of hemagglutinating viruses and hemagglutination-inhibiting antibodies. J Immunol. 1953 Mar;70(3):253–259. [PubMed] [Google Scholar]
- Hightower L. E., Bratt M. A. Protein synthesis in Newcastle disease virus-infected chicken embryo cells. J Virol. 1974 Apr;13(4):788–800. doi: 10.1128/jvi.13.4.788-800.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma M., Ouchi M. Trypsin action on the growth of Sendai virus in tissue culture cells. 3. Structural difference of Sendai viruses grown in eggs and tissue culture cells. J Virol. 1973 Dec;12(6):1457–1465. doi: 10.1128/jvi.12.6.1457-1465.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu M. C., Scheid A., Choppin P. W. Reconstitution of membranes with individual paramyxovirus glycoproteins and phospholipid in cholate solution. Virology. 1979 Jun;95(2):476–491. doi: 10.1016/0042-6822(79)90502-6. [DOI] [PubMed] [Google Scholar]
- Huang R. T., Rott R., Wahn K., Klenk H. D., Kohama T. The function of the neuraminidase in membrane fusion induced by myxoviruses. Virology. 1980 Dec;107(2):313–319. doi: 10.1016/0042-6822(80)90299-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Madansky C. H., Bratt M. A. Relationships among virus spread, cytopathogenicity, and virulence as revealed by the noncytopathic mutants of Newcastle disease virus. J Virol. 1981 Dec;40(3):691–702. doi: 10.1128/jvi.40.3.691-702.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz D. C., Scheid A., Choppin P. W. Immunological studies of the functions of paramyxovirus glycoproteins. Virology. 1981 Feb;109(1):94–105. doi: 10.1016/0042-6822(81)90474-8. [DOI] [PubMed] [Google Scholar]
- Nagai Y., Klenk H. D. Activation of precursors to both glycoporteins of Newcastle disease virus by proteolytic cleavage. Virology. 1977 Mar;77(1):125–134. doi: 10.1016/0042-6822(77)90412-3. [DOI] [PubMed] [Google Scholar]
- Nagai Y., Klenk H. D., Rott R. Proteolytic cleavage of the viral glycoproteins and its significance for the virulence of Newcastle disease virus. Virology. 1976 Jul 15;72(2):494–508. doi: 10.1016/0042-6822(76)90178-1. [DOI] [PubMed] [Google Scholar]
- Ozawa M., Asano A., Okada Y. Biological activities of glycoproteins of HVJ (Sendai virus) studied by reconstitution of hybrid envelope and by concanavalin A-mediated binding: a new function of HANA protein and structural requirement of F protein in hemolysis. Virology. 1979 Nov;99(1):197–202. doi: 10.1016/0042-6822(79)90055-2. [DOI] [PubMed] [Google Scholar]
- Peeples M. E., Bratt M. A. UV irradiation analysis of complementation between, and replication of, RNA-negative temperature-sensitive mutants of Newcastle disease virus. J Virol. 1982 Mar;41(3):965–973. doi: 10.1128/jvi.41.3.965-973.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portner A., Marx P. A., Kingsbury D. W. Isolation and characterization of Sendai virus temperature-sensitive mutants. J Virol. 1974 Feb;13(2):298–304. doi: 10.1128/jvi.13.2.298-304.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portner A., Scroggs R. A., Marx P. S., Kingsbury D. W. A temperature-sensitive mutant of Sendai virus with an altered hemagglutinin-neuraminidase polypeptide: consequences for virus assembly and cytopathology. Virology. 1975 Sep;67(1):179–187. doi: 10.1016/0042-6822(75)90415-8. [DOI] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Identification of biological activities of paramyxovirus glycoproteins. Activation of cell fusion, hemolysis, and infectivity of proteolytic cleavage of an inactive precursor protein of Sendai virus. Virology. 1974 Feb;57(2):475–490. doi: 10.1016/0042-6822(74)90187-1. [DOI] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Isolation and purification of the envelope proteins of Newcastle disease virus. J Virol. 1973 Feb;11(2):263–271. doi: 10.1128/jvi.11.2.263-271.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozawa H., Watanabe M., Ishida N. Structural components of Sendai virus. Serological and physicochemical characterization of hemagglutinin subunit associated with neuraminidase activity. Virology. 1973 Sep;55(1):242–253. doi: 10.1016/s0042-6822(73)81027-x. [DOI] [PubMed] [Google Scholar]
- Tsipis J. E., Bratt M. A. Isolation and preliminary characterization of temperature-sensitive mutants of Newcastle disease virus. J Virol. 1976 Jun;18(3):848–855. doi: 10.1128/jvi.18.3.848-855.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. R., Bratt M. A. Polyadenylate sequences on Newcastle disease virus mRNA synthesized in vivo and in vitro. J Virol. 1974 Jun;13(6):1220–1230. doi: 10.1128/jvi.13.6.1220-1230.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]


