Abstract
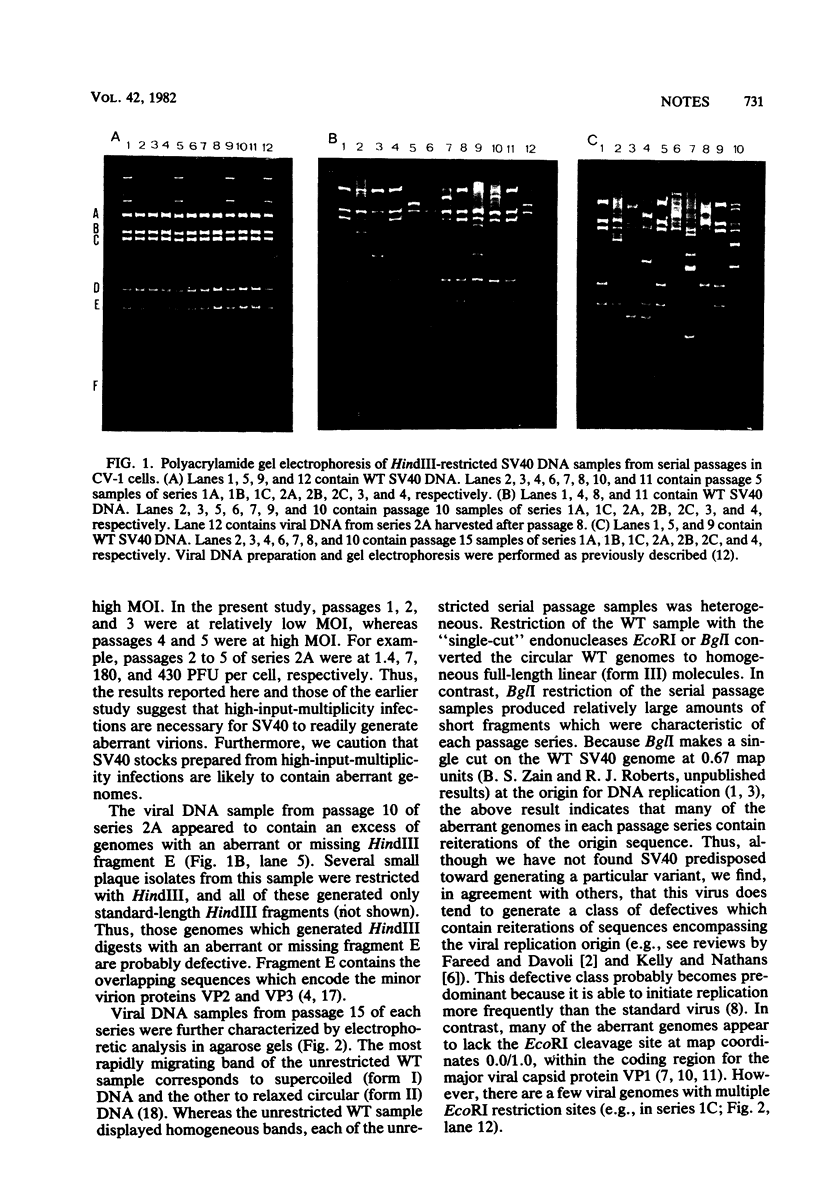
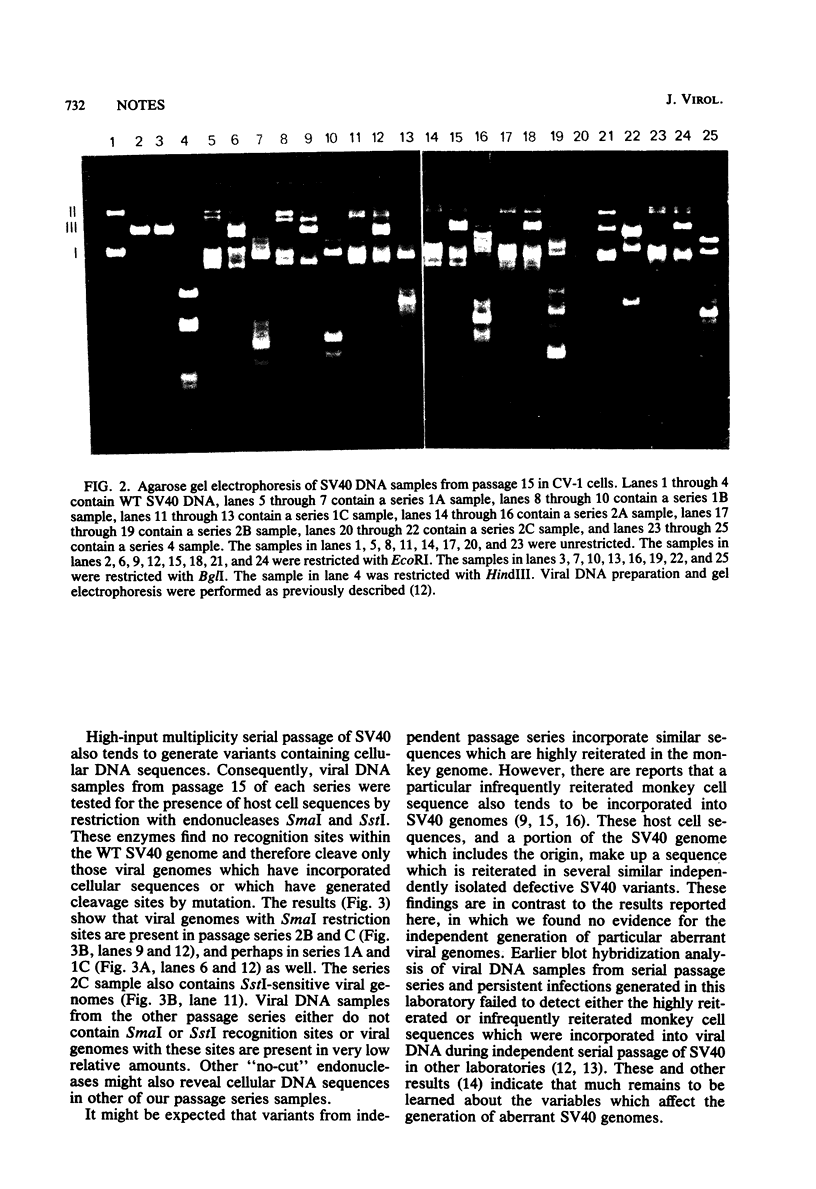
Three serial passage series of simian virus 40 (SV40) in CV-1 cells were initiated by infection directly from the same wild-type plaque isolate, three series were initiated by infection with another plaque isolate, and two series were initiated with each of two other plaque isolates. Aberrant SV40 genomes were not detected in any of the passage series until after the fifty undiluted passage, and each series generated a different array of variant genomes. The results show that the variants were not present in the original plaque isolates but, instead, were randomly generated during subsequent high-input multiplicity passages. Although many of the aberrant viral genomes in each passage series contained reiterations of the SV40 origin of replication and some also contained host cell sequences, there was no indication that SV40 is predisposed toward generating any particular variant.
Full text
PDF
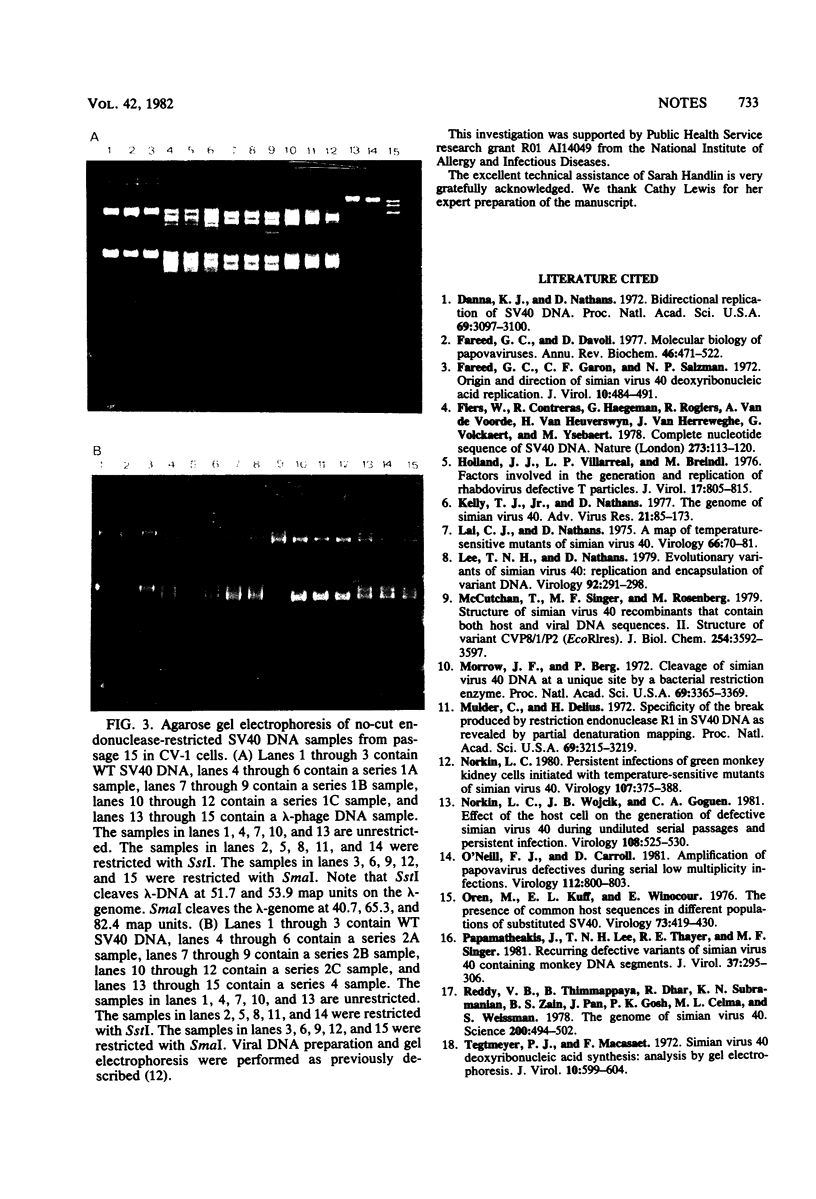
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Danna K. J., Nathans D. Bidirectional replication of Simian Virus 40 DNA. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3097–3100. doi: 10.1073/pnas.69.11.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareed G. C., Davoli D. Molecular biology of papovaviruses. Annu Rev Biochem. 1977;46:471–522. doi: 10.1146/annurev.bi.46.070177.002351. [DOI] [PubMed] [Google Scholar]
- Fareed G. C., Garon G. F., Salzman N. P. Origin and direction of simian virus 40 deoxyribonucleic acid replication. J Virol. 1972 Sep;10(3):484–491. doi: 10.1128/jvi.10.3.484-491.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiers W., Contreras R., Haegemann G., Rogiers R., Van de Voorde A., Van Heuverswyn H., Van Herreweghe J., Volckaert G., Ysebaert M. Complete nucleotide sequence of SV40 DNA. Nature. 1978 May 11;273(5658):113–120. doi: 10.1038/273113a0. [DOI] [PubMed] [Google Scholar]
- Holland J. J., Villarreal L. P., Breindl M. Factors involved in the generation and replication of rhabdovirus defective T particles. J Virol. 1976 Mar;17(3):805–815. doi: 10.1128/jvi.17.3.805-815.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly T. J., Jr, Nathans D. The genome of simian virus 40. Adv Virus Res. 1977;21:85–173. doi: 10.1016/s0065-3527(08)60762-9. [DOI] [PubMed] [Google Scholar]
- Lai C. J., Nathans D. A map of temperature-sensitive mutants of simian virus 40. Virology. 1975 Jul;66(1):70–81. doi: 10.1016/0042-6822(75)90179-8. [DOI] [PubMed] [Google Scholar]
- Lee T. N., Nathans D. Evolutionary variants of simian virus 40: replication and encapsidation of variant DNA. Virology. 1979 Jan 30;92(2):291–298. doi: 10.1016/0042-6822(79)90134-x. [DOI] [PubMed] [Google Scholar]
- McCutchan T., Singer M., Rosenberg M. Structure of simian virus 40 recombinants that contain both host and viral DNA sequences. II. The structure of variant 1103 and its comparison to variant CVPS/1P2 (EcoRI res). J Biol Chem. 1979 May 10;254(9):3592–3597. [PubMed] [Google Scholar]
- Morrow J. F., Berg P. Cleavage of Simian virus 40 DNA at a unique site by a bacterial restriction enzyme. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3365–3369. doi: 10.1073/pnas.69.11.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder C., Delius H. Specificity of the break produced by restricting endonuclease R 1 in Simian virus 40 DNA, as revealed by partial denaturation mapping. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3215–3219. doi: 10.1073/pnas.69.11.3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norkin L. C. Persistent infections of green monkey kidney cells initiated with temperature-sensitive mutants of simian virus 40. Virology. 1980 Dec;107(2):375–388. doi: 10.1016/0042-6822(80)90305-0. [DOI] [PubMed] [Google Scholar]
- Norkin L. C., Wojcik J. B., Goguen C. A. Effect of the host cell on the generation of defective Simian Virus 40 during undiluted serial passages and persistent infection. Virology. 1981 Jan 30;108(2):525–530. doi: 10.1016/0042-6822(81)90462-1. [DOI] [PubMed] [Google Scholar]
- O'Neill F. J., Carroll D. Amplification of papovavirus defectives during serial low multiplicity infections. Virology. 1981 Jul 30;112(2):800–803. doi: 10.1016/0042-6822(81)90330-5. [DOI] [PubMed] [Google Scholar]
- Oren M., Kuff E. L., Winocour E. The presence of common host sequences in different populations of substituted SV40 DNA. Virology. 1976 Sep;73(2):419–430. doi: 10.1016/0042-6822(76)90403-7. [DOI] [PubMed] [Google Scholar]
- Papamatheakis J., Lee T. N., Thayer R. E., Singer M. F. Recurring defective variants of simian virus 40 containing monkey DNA segments. J Virol. 1981 Jan;37(1):295–306. doi: 10.1128/jvi.37.1.295-306.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy V. B., Thimmappaya B., Dhar R., Subramanian K. N., Zain B. S., Pan J., Ghosh P. K., Celma M. L., Weissman S. M. The genome of simian virus 40. Science. 1978 May 5;200(4341):494–502. doi: 10.1126/science.205947. [DOI] [PubMed] [Google Scholar]
- Tegtmeyer P., Macasaet F. Simian virus 40 deoxyribonucleic acid synthesis: analysis by gel electrophoresis. J Virol. 1972 Oct;10(4):599–604. doi: 10.1128/jvi.10.4.599-604.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]