Abstract
One pathway in forming synaptic-like microvesicles (SLMV) involves direct budding from the plasma membrane, requires adaptor protein 2 (AP2) and is brefeldin A (BFA) resistant. A second route leads from the plasma membrane to an endosomal intermediate from which SLMV bud in a BFA-sensitive, AP3-dependent manner. Because AP3 has been shown to bind to a di-leucine targeting signal in vitro, we have investigated whether this major class of targeting signals is capable of directing protein traffic to SLMV in vivo. We have found that a di-leucine signal within the cytoplasmic tail of human tyrosinase is responsible for the majority of the targeting of HRP-tyrosinase chimeras to SLMV in PC12 cells. Furthermore, we have discovered that a Met-Leu di-hydrophobic motif within the extreme C terminus of synaptotagmin I supports 20% of the SLMV targeting of a CD4-synaptotagmin chimera. All of the traffic to the SLMV mediated by either di-Leu or Met-Leu is BFA sensitive, strongly suggesting a role for AP3 and possibly for an endosomal intermediate in this process. The differential reduction in SLMV targeting for HRP-tyrosinase and CD4-synaptotagmin chimeras by di-alanine substitutions or BFA treatment implies that different proteins use the two routes to the SLMV to differing extents.
INTRODUCTION
The efficient sorting of many transmembrane proteins to a variety of post-Golgi destinations is controlled by short specific sequences located within their cytoplasmic domains, sorting signals (for review, see Trowbridge et al., 1993; Sandoval and Bakke, 1994). At present, two major groups of sorting signals have been identified. The first group comprises tyrosine-based signals, which usually conform to the consensus YXXØ (where X is any amino acid, and Ø is a strong hydrophobic amino acid) or FXNPXY. The second group of sorting signals contains di-leucine/di-hydrophobic signals, in which one of the leucines can be substituted by isoleucine, methionine, or valine without loss of function (Letourner and Klausner, 1992; Bremnes et al., 1994; Sandoval and Bakke, 1994; Pond et al., 1995). Sorting signals falling outside these groups include the amphipathic α-helixes, which can adopt a supercoiled conformation and were found in the cytoplasmic domains of vesicle-associated membrane protein II (VAMPII) and the β-chain of the interleukin-1 receptor (Grote et al., 1995; Subtil et al., 1997). In addition, clusters of acidic residues in the context of a casein kinase II recognition site were shown to facilitate intracellular sorting of both furin and the mannose-6-phosphate receptor (Schafer et al., 1995; Voorhees et al., 1995; Mauxion et al., 1996). The functioning of sorting signals requires their direct (or possibly indirect) interaction with adaptor protein (AP) complexes, such as AP1, AP2, and AP3, which assemble with clathrin during vesicular budding (for review, see Kirchhausen et al., 1997; Odorizzi et al., 1998), or with arrestins, which function as adaptors for G-protein–coupled receptors (Ferguson et al., 1996; Goodman et al., 1996).
Whereas the signals involved in the variety of sorting steps leading membrane proteins to the compartments of the endosomal–lysosomal system are being actively characterized, less is known about targeting requirements which direct proteins to the specialized organelles that arise from the endocytic pathway within some cell types. Addressing this problem is important both for understanding the biogenesis of these specialized organelles and for providing clues as to how the cell could modify a pathway universally present in all cell types to form tissue-specific organelles.
In terms of signal-mediated trafficking, a preliminary analysis of several such organelles, including the melanosomes and the synaptic-like microvesicles (SLMV), has been carried out. Melanosomes store the pigment melanin, synthesis of which is catalyzed by a tyrosinase (Hearing and Tsukamoto, 1991). Tyrosinase is a type I membrane protein with a cytoplasmic domain of 30 amino acids (Kwon et al., 1987). Mutations in this enzyme lead to loss of pigmentation (oculocutaneous albinism) both in the mouse and in humman (Beermann et al., 1990). For example, a spontaneous mutation, platinum, results in deletion of the cytoplasmic domain of tyrosinase and causes a rerouting of the tailless protein to the cell surface in mice homozygous for this allele (Beermann et al., 1995). Mutations of subunits of mouse AP3 are also reported to affect the formation of pigment granules and can lead to phenotypes similar to those caused by mutations of tyrosinase. The same mutations within AP3 also affect the functioning of lysosomes and synaptic vesicles (for review see Odorizzi et al., 1998). An explanation for the role of AP3 in the biogenesis of pigment granules is provided by an in vitro demonstration of the binding of AP3 to the cytoplasmic tail of tyrosinase. The critical determinant responsible for this interaction is found to be a di-leucine signal proximal to the membrane bilayer within the cytoplasmic domain of mouse tyrosinase (Höning et al., 1998). In addition, di-leucine motifs are found in the short cytoplasmic tails of other melanosomal proteins, and targeting of some of these proteins, such as tyrosinase-related protein, to melanosomes and lysosomes have also been documented to be dependent on di-leucine signals (Vijayasaradhi et al., 1995). These observations together strongly suggest that the di-leucine motifs of melanosomal proteins may be a general prerequisite for their efficient sorting to the melanosomes in an AP3-dependent manner.
The mechanisms of protein sorting to the SLMV are more elaborate than those for melanosomal proteins. One of the reasons for this complexity is that transport to the SLMV can occur by two routes: directly from the plasma membrane and/or via an endosomal intermediate. The first route was suggested by morphological studies in nerve terminals (Takei et al., 1996), and was then supported by both biochemical experiments in PC12 cells (Schmidt et al., 1997) and analyses of vesicle recycling (Murthy and Stevens, 1998). This process was found to be AP2, clathrin, and dynamin dependent (Takei et al., 1996; Cremona and De Camilli, 1997; Shupliakov et al., 1997). A second group of observations suggests that SLMV originate from endosomal intermediate(s), which contain transferrin, rab5, and the fluid phase endocytic tracer HRP (Clift-O’Grady et al., 1990; Cameron et al., 1991; Bauerfeind et al., 1993; Mundigl and De Camilli, 1994; Fischer von Mollard et al., 1994; Norcott et al., 1996; Lichtenstein et al., 1998; Blagoveshchenskaya et al., 1999; Strasser et al., 1999). This pathway is dependent on both the small GTPase ADP ribosylation factor 1 (ARF1) (Faundez et al., 1997) and on AP3 (Faundez et al., 1998). However, recent data on VAMPII provide evidence that both pathways may be used simultaneously in the same cell (Shi et al., 1998). Importantly, the direct pathway of SLMV formation from the plasma membrane was found to be brefeldin A (BFA) resistant, whereas that involving an endosomal intermediate is BFA sensitive (Shi et al., 1998; Blagoveshchenskaya et al., 1999) reflecting the recruitment of AP3 by ARF1 (Ooi et al., 1998).
Very little is known about the targeting signals that are used by SLMV membrane proteins. To date, only one endogenous protein, VAMPII, has been characterized in detail. In this protein, an amphipathic α-helix was found to promote SLMV targeting (Grote et al., 1995) and to bind AP3 (Salem et al., 1998). A second resident SLMV protein for which adaptor binding has been established is synaptotagmin I, a key member of the docking and fusion machinery controlling neurotransmission (Schiavo et al., 1995; Schiavo et al., 1996). Synaptotagmin I has a large cytoplasmic tail, which includes two C2 domains, C2A and C2B, as well as a short sequence at the extreme C terminus (Perin et al., 1990). C2A is involved in a Ca2+-dependent interaction with negative phospholipids (Davletov and Südhof, 1993) and syntaxin (Chapman et al., 1995; Li et al., 1995; Kee and Scheller, 1996). Ca2+ binding by the C2B domain alters its specificity for inositol polyphosphates (Schiavo et al., 1996; Sugita et al., 1996). C2B can also interact independently of Ca2+ with both β-soluble N-ethyl-maleimide-sensitive factor attachment protein (Schiavo et al., 1995) and AP2 (Zhang et al., 1994). The latter finding strongly suggests that synaptotagmin is capable of using the direct route to SLMV from the plasma membrane. However, given the data of Shi et al. (1998), it is likely that this protein will also be delivered to SLMV via the BFA-sensitive, AP3-dependent route.
Given the binding of AP3 to di-leucine signals in both higher eukaryotes and yeast (Odorizzi et al., 1998) and the involvement of AP3 in SLMV formation (Faundez et al., 1998), an AP3-dependent route to SLMV might well be expected to be di-leucine mediated. In this work, we attempted to determine whether any sorting of proteins to the SLMV is dependent on di-leucine signals. Our analyses of targeting of HRP-tyrosinase chimeras show that a di-leucine signal within the cytoplasmic tail of tyrosinase is capable of directing this chimera to the SLMV within PC12 cells. We have also found that the di-hydrophobic sequence Met-Leu within the C-terminal portion of the cytoplasmic tail of synaptotagmin can support targeting of CD4-synaptotagmin chimeras to SLMV. In the case of tyrosinase, the di-leucine motif is responsible for the vast majority (80%) of the SLMV targeting, whereas the Met-Leu within synaptotagmin supports only 20% of trafficking to this organelle. The extent to which SLMV targeting is reduced after substitution of di-leucine or Met-Leu by di-alanine is equivalent to that caused by BFA treatment of both wild-type chimeras, strongly, albeit indirectly, suggesting a role for AP3 in sorting to SLMV. Because the ablation of this Leu-Leu/Met-Leu-dependent, BFA-sensitive pathway differentially affects the SLMV targeting of HRP-tyrosinase and CD4-synaptotagmin, we conclude that the proportion of traffic to the SLMV taking this route can vary between individual proteins.
MATERIALS AND METHODS
Materials and Reagents
Murine antihuman monoclonal (clone Q4120) antibody against CD4 was obtained from the Medical Research Council AIDS Reagents Program (National Institute for Biological Standards and Control, South Mimms, Potters Bar, United Kingdom). Rabbit polyclonal 729 antiserum against the cytoplasmic domain of synaptotagmin I/p65 was kindly provided by Dr. G.E. Dean (Cincinnati, OH). Rabbit polyclonal antiserum against synaptophysin/p38 was as described (Cutler and Cramer, 1990). ECL substrates were purchased from Amersham Pharmacia Biotech (Buckinghamshire, United Kingdom). Other chemicals were purchased from Sigma (Poole, United Kingdom).
Q4120 was iodinated using 125I-3-(p-hydroxyphenyl)-propionic acid N-hydroxy-succinimide ester (Bolton and Hunter reagent) as described (Pelchen-Matthews et al., 1998). Specific activities were typically 40,000–80,000 cpm/ng.
Cell Culture and Transfections
The rat pheochromocytoma cell line PC12 (CCL23; American Type Culture Collection, Manassas, VA) was cultivated and transiently transfected as described previously (Norcott et al., 1996). Cells expressing chimeras were used for analyses 2–3 d after transfection. Where stated, cells were treated with 10 μg/ml BFA for 1 h at 37°C.
Constructs
CD4-Synaptotagmin Chimeras.
The CD4 open reading frame (ORF) was cloned as an EcoRI–BamHI fragment from pSG5-CD4 (Pitcher et al., 1999) into the same sites of the expression vector pGW1 (Blackstone et al., 1992). The CD4 ORF was then subcloned as an EcoRI–HindIII fragment into the same sites of pGEM3Zf(+) (Promega, Madison, WI). In the resulting plasmid pGEM3Zf(+)-CD4 the NarI site immediately after the stop transfer sequence in the CD4 cytoplasmic tail is unique. Regions of the bovine synaptotagmin I cytoplasmic sequence (Davletov et al., 1993) were amplified by PCR and cloned into pGEM3Zf(+)-CD4 as NarI/HindIII fragments using this unique NarI such that the synaptotagmin-derived ORFs replaced the CD4 cytoplasmic tail. The entire predicted cytoplasmic sequence of synaptotagmin (aa 81–422, C2AB) was amplified using the oligonucleotides SYT81 and SYT422; the C-terminal 173 aa (aa 249–422, C2B) was amplified using the oligonucleotides SYT249 and SYT422; the N-terminal cytoplasmic region (aa 81–265, C2A) was amplified using the oligonucleotides SYT81 and SYT265; and the C-terminal 28 aa (395–422, C terminus) were amplified using SYT395 and SYT422. The resultant constructs were sequenced, and the CD4-synaptotagmin chimera ORFs were cloned into the expression vector pRK34 (Norcott et al., 1996) using EcoRI and HindIII to allow expression in PC12 cells under the cytomegalovirus promoter. CD4-tailless was generated by site-directed mutagenesis of pGW1-CD4 by introducing a stop codon immediately after the stop transfer sequence. Mutagenesis was performed using the Stratagene (La Jolla, CA) QuickChange site-directed mutagenesis kit as per manufacturer’s instructions. The sense oligonucleotide used was CD4 STOP; the antisense primer was the exact complement. CD4-C2AB/AA, CD4-C2B/AA, and CD4-C-terminus/AA were also generated by site directed mutagenesis using pRK34-CD4-C2AB, pRK34-CD4-C2B, and pRK34-CD4-C-terminus as templates, respectively. The Met-Leu motif (aa 417–418) in each was substituted with a di-alanine using the sense oligonucleotide SYTAA; the antisense was the exact compliment.
HRP-Tyrosinase Chimeras.
The tyrosinase tail was generated from a series of overlapping oligonucleotides (TYR1, TYR2 REV, TYR3, and TYR4), which together encode the entire predicted cytoplasmic tail of human tyrosinase (aa 505–529; Shibahara et al., 1988). The oligonucleotides were phosphorylated with polynucleotide kinase and ligated together, and the product used as a template for PCR amplified using the primers TYR1 + TYR4 REV. The ORF of the wild-type HRP-P-selectin chimera up to the stop transfer sequence was amplified using the vector specific oligonucleotide PRK5-EcoRI and TYR1 REV using pRK34-HRP-P-selectin as the template (Norcott et al., 1996). The 3′ end of TYR1 REV was complementary to the HRP-P-selectin construct, and its 5′ end was complementary to the tyrosinase tail PCR product; therefore, the two PCR products had complementary 3′ and 5′ termini. Amplification with oligonucleotides PRK5 EcoRI and TYR4 REV using the two PCR products as templates spliced the two reading frames together to generate an HRP-tyrosinase tail chimera. The PCR product was cloned directly into pCR2.1 (Invitrogen, San Diego, CA), and after sequencing, the HRP-tyrosinase chimera was cloned as a BamHI fragment into pRK34 for expression in PC12 cells. The chimera HRP-tyrosinase/AA was generated by site directed mutagenesis such that the di-leucine in the tyrosinase cytoplasmic tail was replaced with di-alanine. pRK34-HRP-tyrosinase was used as the template for the reaction with the mutagenic oligonucleotide TYRAA and its exact complement.
Oligonucleotides used: SYT81, ACACGGCGCCTTAAGAAATGCTTATTCAAAAAG; SYT 249, ACACGGCGCCTTAACACGGTGGATTTCGGTCAC; SYT265, GTGTAAGCTTTATGCACTTTGCAGATCACGCCA; SYT395, ATATGGCGCCTTGCTAACCCCCGGC-GACCCATCGCCCAGTGG; SYT422, GTGTAAGCTTTTACTTCTTGACTGCCAGCAT; SYTAA, GAGGTTGACGCCGCAGCTGCCGTCAAGAAGTAA; CD4 STOP, CGGCACCGAAGGCGCTGAGCAGAGCGGATGTCT; TYR1, CTGGCTTTGCTAAGAAAGCGTAA-GCAGCTTCCTGAAGAAAA; TYR1 REV, TTTTCTTCAGGAAGC-TGCTTACGCTTTCTTAGCAAAGCCAG; TYR2 REV, TCTCCATGAGGAGTGGCTGCTTTTCTTCAGGAAGCTGCTT; TYR3, GCA-GCCACTCCTCATGGAGAAAGAGGATTACCACAGCTTG; TYR4 REV, ATATCTTAAGTTATAAATGGCTCTGATACAAGCTGTG-GTAATCCTCTT; and PRK5 EcoRI, ACTGCACCTCGGTTCTATCG-ATTG; TYRAA, GAAGAAAAGCAGCCAGCAGCAATGGAGAA-AGAGGAT.
Subcellular Fractionation and Quantitation of Data
PC12 cells expressing CD4-synaptotagmin chimeras (grown on 9-cm plates) were fed with 100 ng/ml 125I-Q4120 for 1 h at 37°C in the growth medium, washed twice, and scraped into 1.5 ml of buffer A (150 mM NaCl, 0.1 mM MgCl2, 1 mM EGTA, and 10 mM HEPES, pH 7.3). Cells expressing HRP-tyrosinase chimeras were washed and scraped into 1.5 ml of buffer A. Cell suspensions were homogenized by passing nine times through a ball-bearing homogenizer with a 0.009-mm clearance. The homogenate was then centrifuged for 15 min at 13,000 × g in a microfuge. The postnuclear supernatant (PNS) was then layered on top of the 11-ml 5–25% preformed glycerol gradients made in buffer A and centrifuged in an SW40Ti rotor (Beckman Instruments, Palo Alto, CA) for 2 h 50 min and fractionated in 0.5-ml fractions from the top of the tube using an Autodensi-Flow IIC (Buchler Instruments, Kansas City, MO). The efficiency of SLMV targeting of CD4-synaptotagmin chimeras was calculated as the amount of 125I-Q4120 radioactivity present within the SLMV peak normalized to the total radioactivity in the homogenate to take into account variations in the level of expression for different chimeras. SLMV targeting of HRP-tyrosinase chimeras was analyzed with a standard HRP assay carried out in triplicate using o-phenylene-diamine as described previously (Norcott et al., 1996).
Internalization Assay
The endocytosis of CD4-synaptotagmin chimeras was analyzed by uptake of 125I-Q4120. Cells grown to confluence on six-well plates were washed with cold growth medium and incubated with 100 ng/ml 125I-Q4120 in the growth medium containing 10 mM HEPES, pH 7.2, for 1 h at 4°C. After three thorough washes with fresh medium to remove any unbound ligand, the cells were allowed to internalize the prebound 125I-Q4120 at 37°C for 5, 10, and 15 min or left on ice. 125I-Q4120 present on the plasma membrane were removed using two washes (3 min each) with acetic buffer (20 mM acetic acid and 50 mM NaCl, pH 3.5) on ice. Amounts of intracellular ligand were calculated as the proportion of acid-resistant cell-associated radioactivity for 0, 5, 10, and 15 min at 37°C and expressed as percent of total bound radioactivity for each chimera. A background level of acid-resistant counts in the cells incubated at 4°C only (∼10% of total bound radioactivity) was subtracted from each value. Initial internalization rates (percent per minute) were calculated by linear regression after the first 5 min of warm-up at 37°C.
RESULTS
Role of Di-Leucine Sorting Signals in Sorting of HRP-Tyrosinase to the SLMV
To determine whether di-leucine sorting signals function in directing proteins to the SLMV, we have constructed a chimera comprising the cytoplasmic domain of human tyrosinase fused to the transmembrane domain from another type-1 membrane protein, P-selectin, with its lumenal portion replaced by HRP to provide an enzymatic reporter (Figure 1). The tailless HRP-P-selectin chimera was previously shown to accumulate at the plasma membrane in PC12 cells (Norcott et al., 1996).
Figure 1.
Schematic illustration showing wild-type and mutant HRP-tyrosinase chimeras. The top line shows the components used for constructions as follows: hGH, human growth hormone signal; HRP, enzimatically active domain of HRP; P-Sel, the transmembrane domain of P-selectin; and Tyrosinase, the cytoplasmic domain of human tyrosinase. Boxes show the sequence boundaries of individual components. Sequences outside boxes show the components added during construction. The middle line shows the full amino acid sequence of the cytoplasmic tail of tyrosinase as used in wild-type HRP-tyrosinase. The bottom line shows the sequence of the cytoplasmic domain of tyrosinase with di-alanine substitution of di-leucine; HRP-tyrosinase/AA. Amino acid numbers from human tyrosinase are shown.
When heterologously expressed in nonmelanocytic cell lines, tyrosinase was shown to be sorted to lysosomal compartments using a di-leucine signal (Calvo et al., 1999; Simmen et al., 1999), which binds to the AP3 adaptor complex in vitro (Höning et al., 1998). In this study, we transfected neuroendocrine PC12 cells to transiently express wild-type HRP-tyrosinase and analyzed by subcellular fractionation whether this chimera is found within SLMV. PC12 cells expressing HRP-tyrosinase were homogenized, and a PNS was centrifuged on 5–25% Glycerol gradients as described (see MATERIALS AND METHODS). This well-established subcellular fractionation procedure is specifically designed for isolation of SLMV (Clift-O’Grady et al., 1990, Norcott et al., 1996; West et al., 1997; Clift-O’Grady et al., 1998; Blagoveshchenskaya et al., 1999; Strasser et al., 1999), which are contaminated neither with early endosomes (Blagoveshchenskaya et al., 1999) nor with late endosomes or lysosomes (Blagoveshchenskaya and Cutler, unpublished observations). After fractionation, a significant proportion of HRP activity was present within a peak in the middle of the gradient which corresponds to SLMV, as shown by the distribution of immunoreactivity of endogenous SLMV markers such as synaptophysin/p38 and synaptotagmin/p65 (Figure 2). These data indicate that the cytoplasmic tail of tyrosinase is both necessary and sufficient to promote SLMV targeting in PC12 cells.
Figure 2.
Targeting of wild-type and mutant HRP-tyrosinase chimeras to SLMV in PC12 cells. (A) PC12 cells expressing either wild-type HRP-tyrosinase or HRP-tyrosinase/AA were homogenized, and PNS was then fractionated on 5–25% glycerol gradients to isolate SLMV. HRP activity for wild-type (●) and for mutant (∗) chimeras is expressed in arbitrary units representing the amount of HRP activity in each fraction divided by that in the homogenate. (B) Cells expressing wild-type HRP-tyrosinase were incubated in the presence (10 μg/ml; ○) or absence (●) of BFA for 1 h at 37°C and processed by subcellular fractionation on glycerol gradients. HRP activity is expressed in arbitrary units as indicated in A. Aliquots from each fraction across the gradient shown in B (●) were separated by 10% SDS-PAGE and Western blotted with polyclonal antibodies against synaptophysin/p38 (C) or against synaptotagmin/p65 (D). The left tracks on both blots represent p38 or p65 in the homogenate.
We have further examined whether the di-leucine located within the cytoplasmic domain is responsible for mediating the targeting of HRP-tyrosinase to SLMV. A mutant chimera in which Leu-514 and Leu-515 were both altered to alanine has been constructed (HRP-tyrosinase/AA; Figure 1), and its targeting to SLMV has been analyzed. After fractionation of a PNS from PC12 cells expressing HRP-tyrosinase/AA on 5–25% glycerol gradients, in two independent experiments targeting to SLMV was reduced by 73 and 85% compared with that for wild-type HRP-tyrosinase (Figure 2A). This suggests that Leu-514 and Leu-515 are the critical residues for targeting HRP-tyrosinase to SLMV.
SLMV Targeting of Wild-Type HRP-Tyrosinase is BFA Sensitive
Shi et al. (1998) have recently documented that sorting to SLMV can occur by two routes. The first route is AP2, dynamin, and clathrin dependent and is BFA resistant, whereas the second route ending in SLMV formation requires ARF1 and AP3 and is BFA sensitive (Shi et al., 1998). By analyzing the targeting of HRP-tyrosinase in the presence of BFA, we have tested which pathway to SLMV is taken by this chimera. The high rate of constitutive fusion and recycling of SLMV in PC12 cells enables almost the entire population of this organelle to be affected by the drug in 1 h of treatment (Blagoveshchenskaya et al., 1999). PC12 cells transiently expressing wild-type HRP-tyrosinase were therefore treated with BFA for 1 h at 37°C, and the PNS from these cells was subjected to subcellular fractionation using glycerol gradients for SLMV isolation. Figure 2B shows that after pretreatment with BFA, a drastic fall of HRP activity within the SLMV peak is found. The magnititude of this decrease (71 and 77% in two independent experiments) was similar to that found for HRP-tyrosinase/AA in untreated cells (73 and 85%) (Figure 2A). These results, showing that SLMV targeting of HRP-tyrosinase is blocked to a similar extent by both inactivation of the di-leucine signal and by BFA, argue in favor of AP3 being involved in delivery of HRP-tyrosinase to this organelle.
Effect of BFA on SLMV Targeting of CD4-Synaptotagmin Chimeras
Having established that a di-leucine signal mediates targeting of HRP-tyrosinase to SLMV in a process that is similarly affected by BFA, we then determined whether this is also the case for an endogenous SLMV membrane protein. One candidate protein is synaptotagmin I/p65. We have constructed a chimera in which the cytoplasmic domain of synaptotagmin is attached to the transmembrane and lumenal domains of CD4, CD4-C2AB (Figure 3). We have chosen CD4 as a reporter because of the ease with which its traffic from the plasma membrane to the SLMV can be followed; the endocytosis of this protein has been extensively characterized (for review, see Marsh and Pelchen-Matthews, 1996), and there are many available antibodies against CD4 that do not affect internalization.
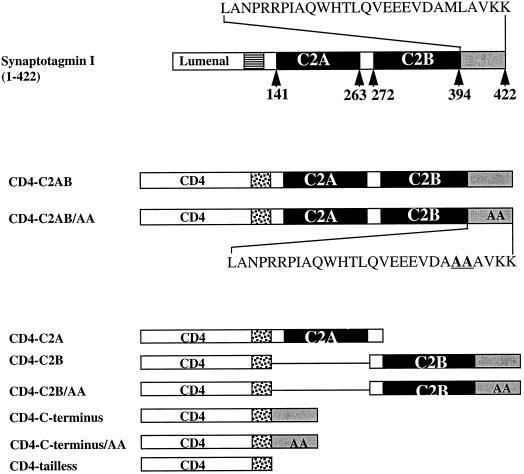
Figure 3.
Schematic illustration showing the CD4-synaptotagmin chimeras. The top line shows the structure of synaptotagmin I, which consists of a lumenal domain (empty box), transmembrane domain (striped box), and cytoplasmic tail comprising C2A, C2B (filled boxes), and the C terminus (gray box). The full amino acid sequence of the C terminus is indicated above. The middle section shows the CD4-synaptotagmin chimeras in which lumenal and transmembrane domains were those from CD4 (empty and dotted boxes, respectively), followed by the entire (wild-type CD4-C2AB) cytoplasmic tail of synaptotagmin I. The position of the di-alanine substitution of Met-Leu within the C terminus is shown in the insert for CD4-C2AB/AA. The lower section illustrates those chimeras in which deletions of the cytoplasmic domain of synaptotagmin have been fused with CD4. CD4-C2A, chimera with truncation both of C2B and the C terminus; CD4-C2B, chimera with a deletion of C2A; CD4-C-terminus, chimera with deletion both of C2A and C2B; CD4-tailless, chimera in which the whole cytoplasmic tail of synaptotagmin has been removed. Positions of di-Ala substitutions within the C terminus are shown within the gray boxes and are reflected in the chimera’s name.
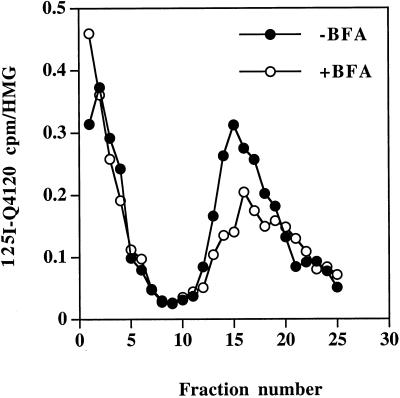
We initially set out to determine whether the cytoplasmic domain of synaptotagmin is sufficient to cause the BFA-sensitive targeting of a CD4-synaptotagmin chimera to SLMV. PC12 cells transiently expressing the wild-type CD4-C2AB chimera (Figure 3) were incubated with 100 ng/ml 125I-Q4120 in the growth medium at 37°C in the presence or absence of 10 μg/ml BFA. PNSs obtained from these cells were then fractionated on glycerol gradients, and the amount of 125I-radioactivity was determined in each fraction. The distribution of 125I-Q4120 on such a gradient is shown in Figure 4. Quantitation of the amount of 125I-Q4120 recovered within the SLMV peak indicates that 33 ± 3% (n = 4) of the total cell-associated radioactivity was recovered within the SLMV in untreated cells compared with 26 ± 2% (n = 4) in the presence of BFA. This partial inhibition of the SLMV targeting of CD4-C2AB by BFA, under conditions where trafficking of HRP-tyrosinase is inhibited by 80%, most likely reflects the use of both routes to the SLMV by CD4-C2AB, as seen previously with VAMPII (Shi et al., 1998). This phenomenon could be accounted for by the presence of more than one targeting signal to SLMV, one responsible for the BFA-sensitive and another one for BFA-resistant targeting of CD4-synaptotagmin.
Figure 4.
Targeting of CD4-C2AB to SLMV in PC12 cells. PC12 cells expressing wild-type CD4-C2AB were fed with 100 ng/ml 125I-Q4120 in the presence (○) or absence (●) of 10 μg/ml BFA and fractionated on 5–25% glycerol gradients to isolate SLMV. The efficiency of SLMV targeting is expressed as the amount of radioactivity in each fraction across the gradient normalized to that in the homogenate.
To determine in which domain the information responsible for BFA-sensitive SLMV targeting is present, we divided the cytoplasmic tail of this protein into three domains: C2A, C2B, and a C-terminal stretch. The divisions were based on analyses by Dr. Paul Driscoll (Department of Biochemistry, University College London) of the C2B domain using the known crystal structure of C2A (Sutton et al., 1995), which suggested that the last 28 amino acids of the cytoplasmic domain (C terminus) would fall outside the predicted C2B structure. A series of chimeras comprising the lumenal and transmembrane domains of CD4 fused to different portions of the cytoplasmic tail of synaptotagmin I were then constructed (Figure 3). PC12 cells expressing CD4-C2AB, CD4-C2A, CD4-C2B, CD4-C-terminus, or CD4-tailless were fed with 100 ng/ml 125I-Q4120 for 1 h in the presence or absence of 10 μg/ml BFA at 37°C, and SLMV were then isolated by subcellular fractionation. The targeting data shown in Figure 5A represent the amount of 125I-Q4120 within the SLMV peak normalized by that present in the homogenate to take into account variation in the levels of expression of different chimeras. The efficiency of targeting of each chimera is expressed on a scale of 0–1, where 1 corresponds to the targeting efficiency of wild-type CD4-C2AB, and 0 represents the basal level exhibited by CD4-tailless. The latter chimera was previously shown to be incapable of internalization and accumulates on the plasma membrane (Pelchen-Matthews et al., 1991). The results (Figure 5A) show a complex pattern. Deletion of both C2B and the C terminus, as in the CD4-C2A chimera, caused a loss of SLMV targeting to a basal level, whereas a chimera having the C terminus alone (CD4-C-terminus) is able to restore 20% of the wild-type phenotype. These results suggest that both C2B and the C terminus are needed for SLMV targeting and that the C terminus may be responsible for the 20% of SLMV targeting that is BFA sensitive, although the effect of the C terminus is modified by being in the intact cytoplasmic tail (see DISCUSSION).
Figure 5.
SLMV targeting of CD4-synaptotagmin chimeras. PC12 cells expressing the chimera indicated were fed with 100 ng/ml 125I-Q4120 in the presence or absence of BFA and fractionated on glycerol gradients. The efficiency of targeting to SLMV was calculated as the amount of 125I-Q4120 radioactivity within SLMV peak divided by that in the homogenate. (A) Targeting efficiency is expressed on a scale related to the wild-type CD4-C2AB (1) and the CD4-tailless chimera (0). Each bar represents the mean ± SE of five independent experiments. Deviations of <0.015 are not displayed. (B) The targeting efficencies of CD4-C2AB, CD4-C2B, and CD4-C-terminus are expressed such that each individual chimera in the absence of BFA has an efficiency of 1.
The effect of BFA on SLMV targeting of the different chimeras was very varied; the extent to which this drug affects SLMV trafficking ranges from 20 to 100% (Figure 5A). For ease of comparison, we have also expressed the data as SLMV targeting efficiency in the presence of BFA for each chimera on a scale where 1 represents the efficiency of SLMV targeting of that chimera in the absence of BFA (Figure 5B). The targeting of the wild-type chimera CD4-C2AB to SLMV was reduced by ∼20% (a statistically significant result with probability of arising by chance of <0.001 using Student’s t test) after treatment of cells with BFA, whereas CD4-C2B exhibited an inhibition of 50% (Figure 5B). However, the most dramatic fall (100%) was observed for CD4-C-terminus (Figure 5B). Together, these data imply that the C terminus contains the targeting signal which is responsible for the delivery of chimera via a BFA-sensitive route to the SLMV, and that the C2A domain can modulate this trafficking.
SLMV Targeting of CD4-Synaptotagmin Chimeras with Di-Alanine Substitutions
We examined the C terminus of the cytoplasmic domain of synaptotagmin for the presence of di-leucine signals, which might promote SLMV trafficking of CD4-synaptotagmin chimeras. We have found that the C terminus does contain a degenerate di-leucine signal: Met-Leu (Met-417, Leu-418 within the sequence of full-length synaptotagmin I; Davletov et al., 1993). Modified di-leucine, di-hydrophobic signals have previously been shown to mediate the delivery of proteins to the endocytic pathway directly from the trans-Golgi network or from the plasma membrane via the endosomes in nonpolarized cell lines (Sandoval and Bakke, 1994), as well as to promote basolateral targeting in polarized cells (Odorizzi and Trowbridge, 1997). We therefore constructed a series of CD4-synaptotagmin chimeras in which we have replaced the Met-Leu with alanine residues (Figure 3) and tested whether they reveal the same phenotype in terms of SLMV targeting as their unaltered analogues in the presence of BFA. PC12 cells were transfected with the chimeras indicated in Figure 6, and the efficiency of their SLMV targeting was then quantitated as described above. In each case, di-alanine substitution had the same effect on SLMV targeting as had BFA treatment on SLMV targeting of unaltered analogues (Figure 6). In addition, BFA did not cause any further significant decrease in SLMV targeting for those chimeras with di-alanine replacements, strongly implying that Met-417/Leu-418 provides all of the information responsible for BFA-sensitive SLMV trafficking of the CD4-synaptotagmin chimeras.
Figure 6.
The efficiency of SLMV targeting of CD4-synaptotagmin chimeras with di-alanine substitutions. PC12 cells expressing the indicated chimera were incubated with 100 ng/ml 125I-Q4120 in the presence or absence of BFA and fractionated to determine SLMV targeting as described in the legend for Figure 4. The efficiency of SLMV targeting for each chimera was calculated as the amount of 125I-Q4120 radioactivity within the SLMV peak normalized to that in the homogenate, expressed on a scale where 1 corresponds to the targeting efficiency of wild-type CD4-C2AB in the absence of BFA and 0 corresponds to that of CD4-tailless. Each bar represents the mean ± SE of three independent experiments. Deviations of <0.015 are not displayed.
Met-417/Leu-418 within Synaptotagmin I Does Not Operate as an Internalization Signal at the Plasma Membrane
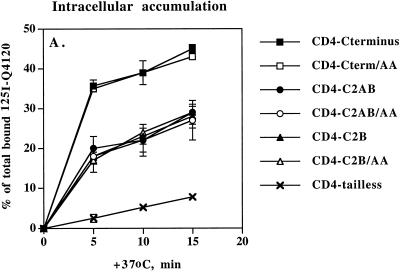
Many di-leucine sorting signals mediating indirect trafficking to lysosomes via the plasma membrane also serve as internalization signals. To determine whether Met-417/Leu-418 could promote internalization, we measured the kinetics of internalization of 125I-Q4120 in PC12 cells expressing CD4-synaptotagmin chimeras (Figure 7). Antibody prebound to the cells for 1 h at 4°C was allowed to internalize at 37°C for different time points followed by removal of surface-bound ligand with an acid wash on ice. Rates of internalization for the first 5 min after warm-up at 37°C were then calculated. In previous studies (Pelchen-Matthews et al., 1991; Pitcher et al., 1999), CD4-tailless was internalized at 0.5%/min, which is in agreement with our data using PC12 cells (Figure 7). Under the same conditions, CD4-C2AB and CD4-C2B were internalized eight times more efficiently, suggesting that a strong internalization signal is located within C2B and/or the C terminus of synaptotagmin. Importantly, di-alanine replacement of Met-417/Leu-418 did not affect internalization rates, as seen by CD4-C2AB/AA and CD4-C2B/AA (Figure 7), indicating that the Met-Leu motif does not promote internalization. Likewise, no difference in internalization rates was observed for CD4-C-terminus and CD4-C-terminus/AA (Figure 7). Interestingly, these chimeras were internalized twice as efficiently as wild-type CD4-C2AB, implying that some residues within the C terminus, other than Met-417/Leu-418, are capable of supporting efficient internalization of CD4-synaptotagmin chimeras. These data are also in agreement with the SLMV targeting data indicating that the C terminus may function differently within the context of the intact cytoplasmic domain.
Figure 7.
Endocytosis of 125I-Q4120 in PC12 cells expressing CD4-synaptotagmin chimeras. Cells expressing CD4-C2AB (●), CD4-C2AB/AA (○), CD4-C2B (▴), CD4-C2B/AA (▵), CD4-C-terminus (▪), CD4-C-terminus/AA (□), or CD4-tailless (X) were incubated with 100 ng/ml 125I-Q4120 at 4°C for 1 h and allowed to internalize the ligand for 0, 5, 10, or 15 min at 37°C. Antibodies remaining on the cell surface were then removed by washing with acetic buffer at 4°C. Intracellular radioactivity was normalized to the total radioactivity bound to the cells and expressed as percentages.
DISCUSSION
A large body of evidence has accumulated indicating that di-leucine signals located within the cytoplasmic domains of transmembrane proteins can mediate internalization, lysosomal targeting, and sorting of these proteins at the level of the trans-Golgi network within nonpolarized cells (for review, see Trowbridge et al., 1993; Sandoval and Bakke, 1994; Kirchhausen et al., 1997) as well as supporting basolateral sorting in polarized cells (Hunziker and Fumey, 1994). In the present work, we show that a di-leucine signal is also capable of directing proteins to a regulated secretory organelle; the SLMV within PC12 cells. Analysis of SLMV targeting of HRP-tyrosinase and CD4-synaptotagmin chimeras revealed that a classical di-leucine or the related di-hydrophobic Met-Leu can respectively promote SLMV targeting of a heterologously expressed transmembrane protein (tyrosinase) and of an endogenous SLMV membrane protein (synaptotagmin). This is a novel finding, because, despite our substantial knowledge of the molecular mechanisms underlying SLMV recycling, very little is known about the structural determinants responsible for targeting of proteins to the SLMV. Until now, the SLMV targeting signals of only two proteins have been characterized in detail: first, the amphipathic α-helix within the cytoplasmic domain of VAMPII (Grote et al., 1995; Grote and Kelly, 1996); and second, the tyrosine-based motif YGVF, Lys-768, and DPSP, all of which cytoplasmic sequences act to promote SLMV targeting of P-selectin (Blagoveshchenskaya et al., 1999). Whether di-leucine signals are used in SLMV targeting by other proteins has yet to be established.
In principle, SLMV targeting signals could also operate as internalization signals at the plasma membrane, as was found for VAMPII (Grote and Kelly, 1996). However, the Met-Leu motif of synaptotagmin is most likely to be involved in the budding of SLMV from endosomes but not from the plasma membrane. This conclusion arises from measuring the kinetics of internalization of CD4-synaptotagmin chimeras with intact and substituted Met-417/Leu-418 (Figure 7). Because we did not detect any reduction of internalization rates for those chimeras with the di-alanine substitutions, the di-hydrophobic motif does not mediate internalization and is therefore likely to be responsible for SLMV targeting at the endosomal level.
Recent studies have established that the di-leucine signal present within the cytoplasmic domain of tyrosinase is necessary to promote the targeting of this protein to lysosomes in nonpigmented cells (Calvo et al., 1999; Simmen et al., 1999). Because the melanosomes, in which tyrosinase is normally found, share common characteristics such as a low intraorganellar pH and a subset of marker proteins with lysosomes (Bhatnagar et al., 1993; Diment et al., 1995; Orlow, 1995), they are generally believed to be an evolutionary adaptation of the late endosomal–lysosomal pathway in melanocytic cells to allow the development of tissue type–specific organelles. Likewise, synaptic vesicles in neurons as well as SLMV in neuroendocrine cells are thought to represent an evolutionary adaptation of a prototypic endosomal–recycling pathway (Clift-O’Grady et al., 1990; Cameron et al., 1991; Mundigl and De Camilli, 1994). This point of view is also supported by our recent data (Blagoveshchenskaya et al., 1999; Strasser et al., 1999) and by those of Lichtenstein et al. (1998), which provide direct evidence that SLMV biogenesis involves an endosomal intermediate in PC12 cells. Interestingly, although the SLMV and melanosomes have a distinct protein composition, we show that when heterologously expressed in PC12 cells, HRP-tyrosinase is efficiently targeted to the SLMV. This finding could reflect the common origin in endosomes of melanosomes and SLMV and could also account for the use of the same determinant, i.e., the di-leucine motif within the cytoplasmic tail of tyrosinase to direct this protein to both organelles.
The efficiency of SLMV targeting of a tyrosinase chimera with di-alanine replacements (HRP-tyrosinase/AA) was dramatically reduced (by 80%) compared with that for the wild-type chimera, implying that only 20% of SLMV traffic is not mediated by this di-leucine signal. These data are in agreement with the findings of others (Calvo et al., 1999; Simmen et al., 1999), who found that although the di-leucine signal of tyrosinase is of primary importance in lysosomal targeting within nonmelanocytic cells, two tyrosine-based signal signals, YHSL and/or YQSHL, play a secondary auxiliary role in supporting this traffic. In contrast, in the case of synaptotagmin, Ala substitution of the di-hydrophobic motif Met-417/Leu-418 caused only a 20% reduction in SLMV targeting of CD4-synaptotagmin, suggesting that additional signals located elsewhere within the cytoplasmic tail of synaptotagmin are needed to mediate the other 80% of SLMV traffic. Our analysis of CD4-synaptotagmin chimeras (Figure 5, −BFA bars) indicates that the C2B domain contributes to efficient SLMV targeting. Although the C2A domain does not contain any SLMV targeting information (the efficiency of SLMV targeting of CD4-C2A was as low as for CD4-tailless; Figure 5), it is capable of modulating the functioning of C2B and the C terminus because its deletion increases SLMV trafficking of CD4-synaptotagmin chimeras. This increase in SLMV targeting caused by deletion of C2A suggests that the signals within the C terminus are partially masked in the context of the intact cytoplasmic tail. We speculate that the proportion of C terminus-dependent targeting of synaptotagmin might be altered by changing physiological conditions. In this work we measured targeting under resting conditions, but the use of different signals and therefore different pathways to SLMV could be affected by, e.g., chronic stimulation. This would provide for yet another control on formation of this organelle.
To ascertain which of the two pathways to the SLMV taken by HRP-tyrosinase and CD4-synaptotagmin is supported by di-leucine and Met-Leu, respectively, we analyzed the BFA-sensitivity of their trafficking to the SLMV. Because only one of the two (as yet described) pathways to the SLMV is BFA sensitive, we have used this drug to distinguish between them. The SLMV targeting of wild-type HRP-tyrosinase after pretreatment with BFA was diminished by 74%, whereas for CD4-synaptotagmin only a 20% decrease was found. The reduction in SLMV targeting of both wild-type chimeras after BFA treatment is therefore similar to that caused by di-alanine substitutions (Figures 2, A and B, and 6). These data strongly suggest that di-leucine (or di-hydrophobic) motifs may be responsible for the BFA-sensitive SLMV trafficking of these proteins. However, although the route taken by the majority of the HRP-tyrosinase to SLMV is BFA sensitive and therefore most likely involves an endosomal intermediate and is AP3 dependent, we find only a 20% decrease in SLMV targeting of the CD4-synaptotagmin after BFA treatment or after di-alanine substitution of Met-417/Leu-418 (Figures 2, 5, and 6). This implies the presence of additional targeting signals responsible for BFA-resistant, Met-417/Leu-418-independent targeting of CD4-synaptotagmin to the SLMV. As mentioned in INTRODUCTION, such a pathway to SLMV operates directly from the plasma membrane in an AP2-dependent manner. Synaptotagmin has been reported to be a high-affinity receptor for AP2 (Zhang et al., 1994), and Lys-326/Lys-327 within the C2B domain were found to be required for AP2 association (Chapman et al., 1998). We therefore presume that synaptotagmin travels to SLMV by two routes and that at least one step of the BFA-sensitive route is controlled by the Met-Leu targeting signal, which is not involved in the BFA-resistant pathway. The use of two routes has also been observed by Shi et al. (1998), who concluded that although VAMPII is mainly transported to the SLMV by the AP3-dependent, BFA-sensitive pathway, a minority travels via the AP2-dependent, BFA-resistant route.
Our findings are in agreement with the in vitro analysis of AP3 binding to the cytoplasmic tails of tyrosinase or Limp-II immobilized on a biosensor support (Höning et al., 1998). These authors have shown that substitution of the proximal di-leucine motif within the cytoplasmic tail of mouse tyrosinase (essentially corresponding to Leu-514/Leu-515 within HRP-tyrosinase) or of the LeuIle motif within Limp-II significantly reduced adaptor binding, thus suggesting that di-leucine signals within the cytoplasmic domains of these proteins are crucial determinants for AP3 binding. In addition, in vivo studies have also documented that a mutation in the protein product of the Drosophila garnet gene, which is homologous to the delta subunit of AP3, causes deficient eye pigmentation, thereby suggesting a role of AP3 in the biogenesis of pigment granules (Ooi et al., 1997; Simpson et al., 1997). Moreover, analyses of the mouse mutant mocha indicate that AP3 is responsible for cargo selection of melanosomes, platelet dense granules, and synaptic vesicles (Kantheti et al., 1998). Because AP3 was also found to be involved in the BFA-sensitive SLMV budding of VAMPII from endosomes in vitro (Faundez et al., 1997; Faundez et al., 1998) as well as to interact directly with VAMPII (Salem et al., 1998), we argue that the BFA sensitivity of SLMV trafficking represents the AP3-mediated pathway to SLMV. However, we cannot rule out some other trafficking routes to SLMV, which require, e.g., COPI or AP1, both of which are located on endosomes and are affected by BFA (Futter et al., 1998). Interestingly, we have recently shown that another resident SLMV membrane protein, synaptophysin, is delivered to SLMV mainly via a BFA-resistant route (Blagoveshchenskaya et al., 1999). Altogether these data imply that the choice of pathway to SLMV is likely to be protein-specific and that different SLMV proteins use one of the two routes to the SLMV in different ratios. The physiological significance of this phenomenon as well as the controls, which might lead to discrimination between these pathways, are not yet understood at more than a superficial level. Clearly, further investigation will be needed to answer the questions raised by these complexities of SLMV trafficking.
ACKNOWLEDGMENTS
We are grateful to the Medical Research Council AIDS Reagents Program for providing us with Q4120 antibody, to Dr. G.E. Dean for 729 antiserum against synaptotagmin I, to Dr. A. Pelchen-Matthews for advice on iodination of Q4120; to Drs. A. Knight and M. Marsh for constructs and advice, and Dr. M. Arribas for critical reading of the manuscript. We are deeply indebted to Dr. P. Driscoll who carried out the sequence analyses that led to our design of the CD4-synaptotagmin chimeras. This work was funded by the Medical Research Council.
Abbreviations used:
- AP
adaptor protein
- ARF
ADP ribosylation factor
- BFA
brefeldin A
- ORF
open reading frame
- PNS
postnuclear supernatant
- SLMV
synaptic-like microvesicles
- VAMP
vesicle-associated membrane protein
REFERENCES
- Bauerfeind RA, Regnier-Vigouroux A, Flatmark T, Huttner WB. Selective storage of acetylcholine, but not catecholamines, in neuroendocrine synaptic-like microvesicles of early endosomal origin. Neuron. 1993;11:105–121. doi: 10.1016/0896-6273(93)90275-v. [DOI] [PubMed] [Google Scholar]
- Beermann F, Orlow SJ, Boissy RE, Schmidt A, Boissy YL, Lamoreux ML. Misrouting of tyrosinase with a truncated cytoplasmic tail as a result of the murine platinum (Cp) mutation. Exp Eye Res. 1995;61:599–607. doi: 10.1016/s0014-4835(05)80053-3. [DOI] [PubMed] [Google Scholar]
- Beermann F, Ruppert S, Hummler E, Bosch FX, Müller G, Rüther U, Schütz G. Rescue of the albino phenotype by introduction of a functional tyrosinase gene into mice. EMBO J. 1990;9:2819–2826. doi: 10.1002/j.1460-2075.1990.tb07470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar V, Anjaiah S, Puri N, Darshanam BA, Ramaiah A. pH of melanosomes of B16 murine melanoma is acidic: its physiologic importance in the regulation of melanin biosynthesis. Arch Biochem Biophys. 1993;307:183–192. doi: 10.1006/abbi.1993.1577. [DOI] [PubMed] [Google Scholar]
- Blackstone CD, Moss SJ, Martin LJ, Levey AI, Price DL, Huganir RL. Biochemical characterization and localization of a non-N-methyl-d-aspartate glutamate receptor in rat brain. J Neurochem. 1992;58:1118–1126. doi: 10.1111/j.1471-4159.1992.tb09370.x. [DOI] [PubMed] [Google Scholar]
- Blagoveshchenskaya AD, Hewitt EW, Cutler DF. A complex web of signal-dependent trafficking underlies the triorganellar distribution of P-selectin in neuroendocrine PC12 cells. J Cell Biol. 1999;145:1419–1433. doi: 10.1083/jcb.145.7.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremnes B, Madsen T, Gedde-Dahl M, Bakke O. A LI and ML motif in the cytoplasmic tail of the MHC-associated invariant chain mediate rapid internalization. J Cell Sci. 1994;107:2021–2032. doi: 10.1242/jcs.107.7.2021. [DOI] [PubMed] [Google Scholar]
- Calvo PA, Franks DW, Bieler BM, Berson JF, Marks MS. A cytoplasmic sequence in human tyrosinase defines a second class of di-leucine-based sorting signals for late endosomal and lysosomal delivery. J Biol Chem. 1999;274:12780–12789. doi: 10.1074/jbc.274.18.12780. [DOI] [PubMed] [Google Scholar]
- Cameron PL, Südhof TC, Jahn R, De Camilli P. Colocalization of synaptophysin with transferrin receptors: implications for synaptic vesicle biogenesis. J Cell Biol. 1991;115:151–164. doi: 10.1083/jcb.115.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman ER, Desai RC, Davis AF, Tornehl CK. Delineation of the oligomerization, AP2 binding, and synprint binding region of the C2B domain of synaptotagmin. J Biol Chem. 1998;273:32966–32972. doi: 10.1074/jbc.273.49.32966. [DOI] [PubMed] [Google Scholar]
- Chapman ER, Hanson PI, An P, Jahn R. Ca2+ regulates the interaction between synaptotagmin and syntaxin 1. J Biol Chem. 1995;270:23667–23671. doi: 10.1074/jbc.270.40.23667. [DOI] [PubMed] [Google Scholar]
- Clift-O’Grady L, Desnos C, Lichtenstein Y, Faundez V, Horng J-T, Kelly RB. Reconstitution of synaptic vesicle biogenesis from PC12 cell membranes. Methods. 1998;16:150–159. doi: 10.1006/meth.1998.0662. [DOI] [PubMed] [Google Scholar]
- Clift-O’Grady L, Linstedt AD, Lowe AW, Grote E, Kelly RB. Biogenesis of synaptic vesicles-like structures in a pheochromocytoma cell line PC12. J Cell Biol. 1990;110:1693–1703. doi: 10.1083/jcb.110.5.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremona O, De Camilli P. Synaptic vesicle endocytosis. Curr Opin Neurobiol. 1997;7:323–330. doi: 10.1016/s0959-4388(97)80059-1. [DOI] [PubMed] [Google Scholar]
- Cutler DF, Cramer LP. Sorting during transport to the surface of PC12 cells: divergence of synaptic vesicles and secretory granule proteins. J Cell Biol. 1990;110:721–730. doi: 10.1083/jcb.110.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davletov BA, Sontag JM, Hata Y, Petrenko AG, Fykse EM, Jahn R, Südhof TC. Phosphorylation of synaptotagmin-I by casein kinase-II. J Biol Chem. 1993;268:6816–6822. [PubMed] [Google Scholar]
- Davletov BA, Südhof TC. A single C2 domain from synaptotagmin I is sufficient for high affinity Ca2+/phospholipid binding. J Biol Chem. 1993;268:26386–26390. [PubMed] [Google Scholar]
- Diment S, Eidelman M, Rodriguez GM, Orlow SJ. Lysosomal hydrolases are present in melanosomes and are elevated in melanizing cells. J Biol Chem. 1995;270:4213–4215. doi: 10.1074/jbc.270.9.4213. [DOI] [PubMed] [Google Scholar]
- Faundez V, Horng J-T, Kelly RB. ARF1 is required for synaptic vesicles budding in PC12 cells. J Cell Biol. 1997;138:505–515. doi: 10.1083/jcb.138.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faundez V, Horng J-T, Kelly RB. A function for the AP3 coat complex in synaptic vesicle formation from endosomes. Cell. 1998;93:423–432. doi: 10.1016/s0092-8674(00)81170-8. [DOI] [PubMed] [Google Scholar]
- Ferguson SS, Downey WE, III, Colapietro AM, Barak LS, Menard L, Caron MG. Role of β-arrestin in mediating agonist-promoted G protein-coupled receptor internalization. Science. 1996;271:363–366. doi: 10.1126/science.271.5247.363. [DOI] [PubMed] [Google Scholar]
- Fischer von Mollard G, Stahl B, Walch-Solimena C, Takei K, Daniels A, Khoklatchev A, De Camilli P, Südhof TC, Jahn R. Localization of Rab5 to synaptic vesicles identifies endosomal intermediate in synaptic vesicle recycling pathway. Eur J Cell Biol. 1994;65:319–326. [PubMed] [Google Scholar]
- Futter CE, Gibson A, Allchin EH, Maxwell S, Ruddock LJ, Odorizzi G, Domingo D, Trowbridge IS, Hopkins CR. In polarized MDCK cells basolateral vesicles arise from clathrin-γ-adaptin-coated domains on endosomal tubules. J Cell Biol. 1998;141:611–623. doi: 10.1083/jcb.141.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman OBJ, Krupnick JG, Santinin F, Gurevich VV, Penn RB, Gagnon AW, Keen JH, Benovic JL. β-Arrestin acts as a clathrin adaptor in endocytosis of the β2-adrenergic receptor. Nature. 1996;383:447–450. doi: 10.1038/383447a0. [DOI] [PubMed] [Google Scholar]
- Grote E, Hao JC, Bennet MK, Kelly RB. A targeting signal in VAMP regulating transport to synaptic vesicles. Cell. 1995;81:581–589. doi: 10.1016/0092-8674(95)90079-9. [DOI] [PubMed] [Google Scholar]
- Grote E, Kelly RB. Endocytosis of VAMPII is facilitated by a synaptic vesicle targeting signal. J Cell Biol. 1996;132:537–547. doi: 10.1083/jcb.132.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearing V, Tsukamoto K. Enzymatic control of pigmentation in mammals. FASEB J. 1991;5:2902–2909. [PubMed] [Google Scholar]
- Höning S, Sandoval IV, von Figura K. A di-leucine-based motif in the cytoplasmic tail of LIMP-II and tyrosinase mediates selective binding of AP3. EMBO J. 1998;17:1304–1314. doi: 10.1093/emboj/17.5.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunziker W, Fumey C. A di-leucine motif mediates endocytosis and basolateral sorting of macrophage IgG Fc receptors in MDCK cells. EMBO J. 1994;13:2963–2967. doi: 10.1002/j.1460-2075.1994.tb06594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantheti P, et al. Mutation in AP3 delta in the mocha mouse links endosomal transport to storage deficiency in platelets, melanosomes, and synaptic vesicles. Neuron. 1998;21:111–122. doi: 10.1016/s0896-6273(00)80519-x. [DOI] [PubMed] [Google Scholar]
- Kee Y, Scheller RH. Localization of synaptotagmin-binding domains of syntaxin. J Neurosci. 1996;16:1975–1981. doi: 10.1523/JNEUROSCI.16-06-01975.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhausen T, Bonifacino JS, Riezman H. Linking cargo to vesicle formation: receptor tail interactions with coat proteins. Curr Opin Cell Biol. 1997;9:488–495. doi: 10.1016/s0955-0674(97)80024-5. [DOI] [PubMed] [Google Scholar]
- Kwon BS, Haq AK, Pomerantz SH, Halaban R. Isolation and sequence of a cDNA clone for human tyrosinase that maps at the mouse c-albino locus. Proc Natl Acad Sci USA. 1987;84:7437–7477. doi: 10.1073/pnas.84.21.7473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letourner F, Klausner RD. A novel di-leucine motif and a tyrosine-based motif independently mediate lysosomal targeting and endocytosis of CD3 chains. Cell. 1992;69:1143–1157. doi: 10.1016/0092-8674(92)90636-q. [DOI] [PubMed] [Google Scholar]
- Li C, Ullrich B, Zhang JZ, Anderson RG, Brose N, Südhof TC. Ca2+-dependent and -independent activities of neural and nonneural synaptotagmins. Nature. 1995;375:594–599. doi: 10.1038/375594a0. [DOI] [PubMed] [Google Scholar]
- Lichtenstein Y, Desnos C, Faundez V, Kelly RB, Clift-O’Grady L. Vesiculation and sorting from PC12-derived endosomes in vitro. Proc Natl Acad Sci USA. 1998;95:11223–11228. doi: 10.1073/pnas.95.19.11223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh M, Pelchen-Matthews A. Endocytic and exocytic regulation of CD4-expression and function. Curr Top Microbiol Immunol. 1996;205:107–135. doi: 10.1007/978-3-642-79798-9_6. [DOI] [PubMed] [Google Scholar]
- Mauxion F, Le Borgne R, Munier-Lehmann H, Hoflack B. A casein kinase II phosphorylation site in the cytoplasmic domain of the cation-dependent mannose 6-phosphate receptor determines the high affinity interaction of the AP1 Golgi assembly proteins with membranes. J Biol Chem. 1996;271:2171–2178. doi: 10.1074/jbc.271.4.2171. [DOI] [PubMed] [Google Scholar]
- Mundigl O, De Camilli P. Formation of synaptic vesicles. Curr Opin Cell Biol. 1994;6:561–567. doi: 10.1016/0955-0674(94)90077-9. [DOI] [PubMed] [Google Scholar]
- Murthy VN, Stevens CF. Synaptic vesicles retain their identity through the endocytic cycle. Nature. 1998;392:497–501. doi: 10.1038/33152. [DOI] [PubMed] [Google Scholar]
- Norcott JP, Solari R, Cutler DF. Targeting of P-selectin to two regulated secretory organelles in PC12 cells. J Cell Biol. 1996;134:1229–1240. doi: 10.1083/jcb.134.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odorizzi G, Cowles CR, Emr SD. The AP3 complex: a coat of many colors. Trends Cell Biol. 1998;8:282–288. doi: 10.1016/s0962-8924(98)01295-1. [DOI] [PubMed] [Google Scholar]
- Odorizzi G, Trowbridge IS. Structural requirements for major histocompatibility complex class II invariant chain trafficking in polarized Madin-Darby canine kidney cells. J Biol Chem. 1997;272:11757–11762. doi: 10.1074/jbc.272.18.11757. [DOI] [PubMed] [Google Scholar]
- Ooi CE, Dell’Angelica EC, Bonifacino JS. ADP-ribosylation factor 1 (ARF1) regulates recruitment of the AP3 adaptor complex to membranes. J Cell Biol. 1998;142:391–402. doi: 10.1083/jcb.142.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooi CE, Moreira JE, Dell’Angelica EC, Poy G, Wassarman DA, Bonifacino JS. Altered expression of a novel adaptin leads to defective pigment granule biogenesis in the Drosophila eye color mutant garnet. EMBO J. 1997;16:4508–4518. doi: 10.1093/emboj/16.15.4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlow SJ. Melanosomes are specialized members of the lysosomal lineage of organelles. J Invest Dermatol. 1995;105:3–7. doi: 10.1111/1523-1747.ep12312291. [DOI] [PubMed] [Google Scholar]
- Pelchen-Matthews A, Armes JE, Griffiths G, Marsh M. Differential endocytosis of CD4 in lymphocytic and nonlymphocytic cells. J Exp Med. 1991;173:575–587. doi: 10.1084/jem.173.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelchen-Matthews A, DaSilva R, Bijmakers M-J, Signoret N, Gordon S, Marsh M. Lack of p56lck expression correlates with CD4 endocytosis in primary lymphoid and myeloid cells. Eur J Immunol. 1998;28:3639–3647. doi: 10.1002/(SICI)1521-4141(199811)28:11<3639::AID-IMMU3639>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Perin MS, Fried VA, Mignery GA, Jahn R, Südhof TC. Phospholipid binding by a synaptic vesicle protein homologous to the regulatory region of protein kinase C. Nature. 1990;345:260–263. doi: 10.1038/345260a0. [DOI] [PubMed] [Google Scholar]
- Pitcher C, Höning S, Fingerhut A, Bowers K, Marsh M. Cluster of differentiation antigen 4 (CD4) endocytosis and adaptor complex binding require activation of the CD4 endocytosis signal by serine phosphorylation. Mol Biol Cell. 1999;10:677–691. doi: 10.1091/mbc.10.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pond L, Kuhn L, Teyton L, Schutze M-P, Tainer JA, Jackson MR, Peterson PA. A role for acidic residues in di-leucine motif-based targeting to the endocytic pathway. J Biol Chem. 1995;270:19989–19997. doi: 10.1074/jbc.270.34.19989. [DOI] [PubMed] [Google Scholar]
- Salem N, Faundez V, Horng J-T, Kelly RB. A v-SNARE participates in synaptic vesicle formation mediated by the AP3 adaptor complex. Nat Neurosci. 1998;1:551–556. doi: 10.1038/2787. [DOI] [PubMed] [Google Scholar]
- Sandoval IV, Bakke O. Targeting of membrane proteins to endosomes and lysosomes. Trends Cell Biol. 1994;4:292–297. doi: 10.1016/0962-8924(94)90220-8. [DOI] [PubMed] [Google Scholar]
- Schafer W, Stroh A, Berghofer S, Seiler J, Vey M, Kruse ML, Kern HF, Klenk HD, Garten W. Two independent targeting signals in the cytoplasmic domain determine trans-Golgi network localization and endosomal trafficking of the proprotein convertase furin. EMBO J. 1995;14:2424–2435. doi: 10.1002/j.1460-2075.1995.tb07240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavo G, Gmachi MJS, Stenbeck G, Sollner TH, Rothman JE. A possible docking and fusion particle for synaptic transmission. Nature. 1995;378:733–736. doi: 10.1038/378733a0. [DOI] [PubMed] [Google Scholar]
- Schiavo G, Gu QM, Prestwich GD, Sollner TH, Rothman JE. Calcium-dependent switching of the specificity of phosphoinositide binding to synaptotagmin. Proc Natl Acad Sci USA. 1996;93:13327–13332. doi: 10.1073/pnas.93.23.13327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A, Hannah MJ, Huttner WB. Synaptic-like microvesicles of neuroendocrine cells originate from a novel compartment that is continuous with the plasma membrane and devoid of transferrin receptor. J Cell Biol. 1997;137:445–458. doi: 10.1083/jcb.137.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi G, Faundez V, Roos J, Dell’Angelica EC, Kelly RB. Neuroendocrine synaptic vesicles are formed in vitro by both clathrin-dependent and clathrin-independent pathways. J Cell Biol. 1998;143:947–955. doi: 10.1083/jcb.143.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibahara S, Tomita Y, Tagami H, Muller RM, Cohen T. Molecular basis for the heterogeneity of human tyrosinase. Tohoku J Exp Med. 1988;156:403–414. doi: 10.1620/tjem.156.403. [DOI] [PubMed] [Google Scholar]
- Shupliakov O, Low P, Grabs D, Gad H, Chen H, David C, Takei K, De Camilli P, Brodin L. Synaptic vesicles endocytosis impaired by disruption of dynamin-SH3 domain interactions. Science. 1997;276:259–263. doi: 10.1126/science.276.5310.259. [DOI] [PubMed] [Google Scholar]
- Simmen T, Schmidt A, Hunziker W, Beerman F. The tyrosinase tail mediates sorting to the lysosomal compartment in MDCK cells via a di-leucine and tyrosine-based signal. J Cell Sci. 1999;112:45–53. doi: 10.1242/jcs.112.1.45. [DOI] [PubMed] [Google Scholar]
- Simpson F, Peden AA, Christopolou L, Robinson MS. Characterization of the adaptor-related protein complex, AP3. J Cell Biol. 1997;137:835–845. doi: 10.1083/jcb.137.4.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser JE, Arribas M, Blagoveshchenskaya A, Cutler DF. Secretagogue-triggered transfer of membrane proteins from neuroendocrine secretory granules to synaptic-like microvesicles. Mol Biol Cell. 1999;10:2619–2630. doi: 10.1091/mbc.10.8.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subtil A, Delepierre M, Dautry-Varsat A. An alpha-helical signal in the cytosolic domain of the interleukin 2 receptor beta chain mediates sorting toward degradation after endocytosis. J Cell Biol. 1997;136:583–595. doi: 10.1083/jcb.136.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita S, Hata Y, Südhof TC. Distinct Ca2+-dependent properties of the first and second C2-domains of synaptotagmin I. J Biol Chem. 1996;271:1262–1265. doi: 10.1074/jbc.271.3.1262. [DOI] [PubMed] [Google Scholar]
- Sutton RB, Davletov BA, Berghuis AM, Südhof TC, Sprang SR. Structure of the first C2 domain of synaptotagmin I: a novel Ca2+/phospholipid-binding fold. Cell. 1995;80:929–938. doi: 10.1016/0092-8674(95)90296-1. [DOI] [PubMed] [Google Scholar]
- Takei K, Mundigl O, Daniell L, De Camilli P. The synaptic vesicle cycle: a single vesicle budding step involving clathrin and dynamin. J Cell Biol. 1996;133:1237–1250. doi: 10.1083/jcb.133.6.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowbridge IS, Collawn JF, Hopkins CR. Signal-dependent membrane protein trafficking in the endocytic pathway. Annu Rev Cell Biol. 1993;9:129–161. doi: 10.1146/annurev.cb.09.110193.001021. [DOI] [PubMed] [Google Scholar]
- Vijayasaradhi S, Xu Y, Bouchard B, Houghton AN. Intracellular sorting and targeting of melanosomal membrane proteins: identification of signals for sorting of the human brown locus protein, gp. J Cell Biol. 1995;130:807–820. doi: 10.1083/jcb.130.4.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorhees P, Deignan E, van Donselaar E, Humphrey J, Marks MS, Peters PJ, Bonifacino JS. An acidic sequence within the cytoplasmic domain of furin functions as a determinant of trans-Golgi network localization and internalization from the cell surface. EMBO J. 1995;14:4961–4975. doi: 10.1002/j.1460-2075.1995.tb00179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AE, Neve RL, Buckley KM. Targeting of the synaptic vesicle protein synaptobrevin in the axon of cultured hippocampal neurons: evidence for two distinct sorting steps. J Cell Biol. 1997;139:917–927. doi: 10.1083/jcb.139.4.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JZ, Davletov BA, Südhof TC, Anderson RG. Synaptotagmin I is a high affinity receptor for clathrin AP2: implications for membrane recycling. Cell. 1994;78:751–760. doi: 10.1016/s0092-8674(94)90442-1. [DOI] [PubMed] [Google Scholar]