Abstract
The Plasmodium falciparum major merozoite surface protein gp195 is a candidate antigen for a vaccine against human malaria. The significance of allelism and polymorphism in vaccine-induced immunity to gp195 was investigated in this study. Rabbits were immunized with each of two allelic forms of gp195 that were affinity purified from the FUP and FVO parasite isolates. gp195-specific antibodies raised against one allelic form of gp195 cross-reacted extensively with the gp195 of the heterologous allele in enzyme-linked immunosorbent assays (ELISAs) and immunofluorescence assays. Competitive binding ELISAs with homologous and heterologous gp195s confirmed that a majority of the anti-gp195 antibodies produced against either allelic protein were cross-reactive. Moreover, the biological activities of the gp195 antibody responses were also highly cross-reactive, since anti-gp195 sera inhibited the in vitro growth of the homologous and heterologous parasites with equal efficiency. The degree of cross-reactivity with strain-specific and allele-specific determinants of gp195 in the ELISA was low. These results suggest that the immunological cross-reactivity between the two gp195 proteins is due to recognition of conserved determinants. They also suggest that a gp195-based vaccine may be effective against blood-stage infection with a diverse array of parasite isolates.
Full text
PDF


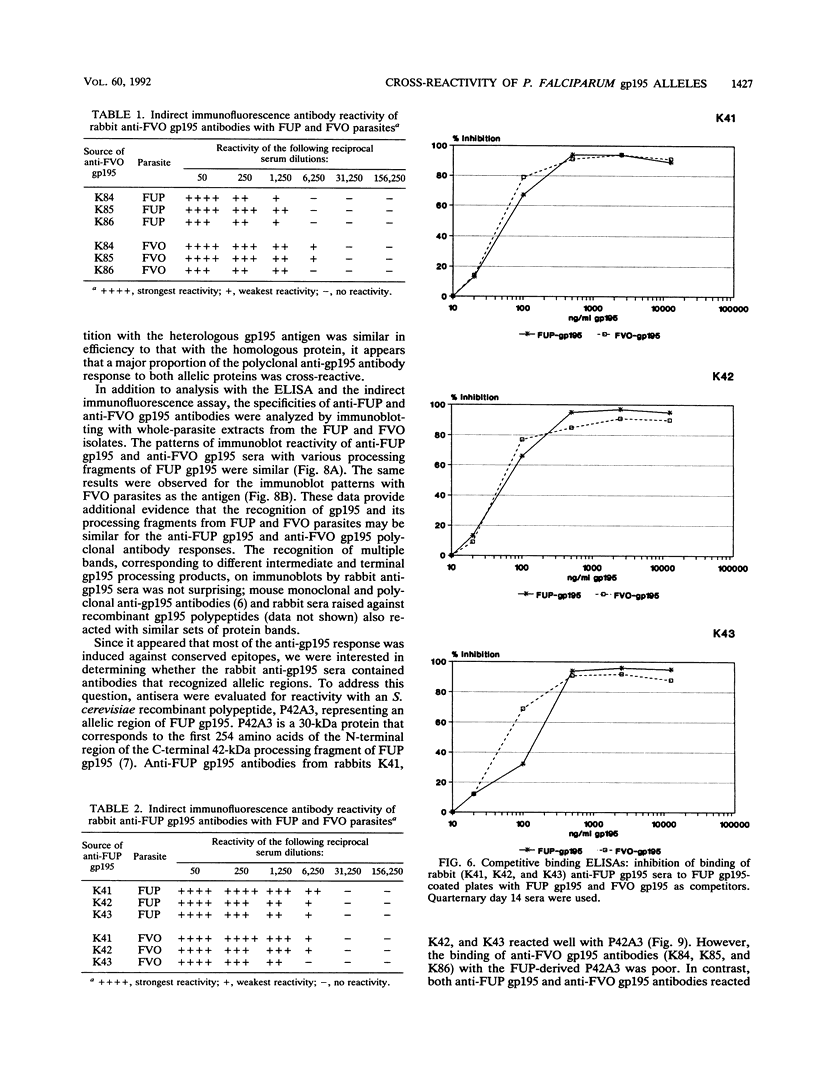
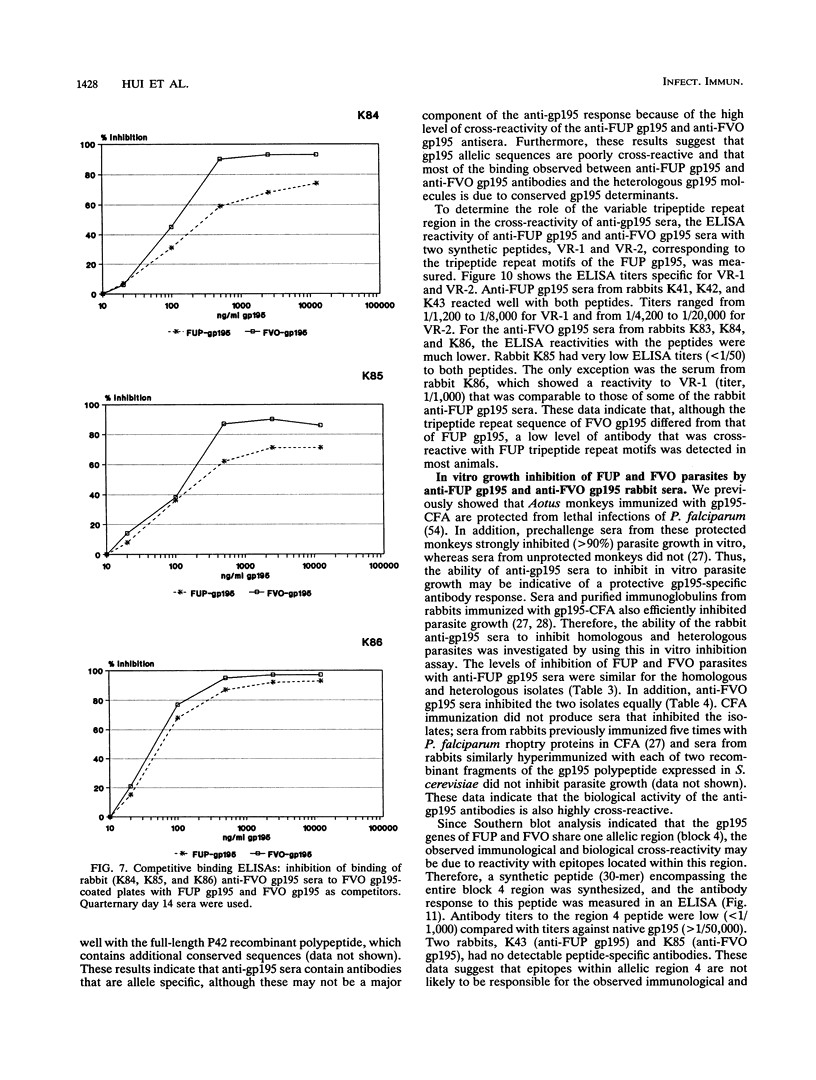
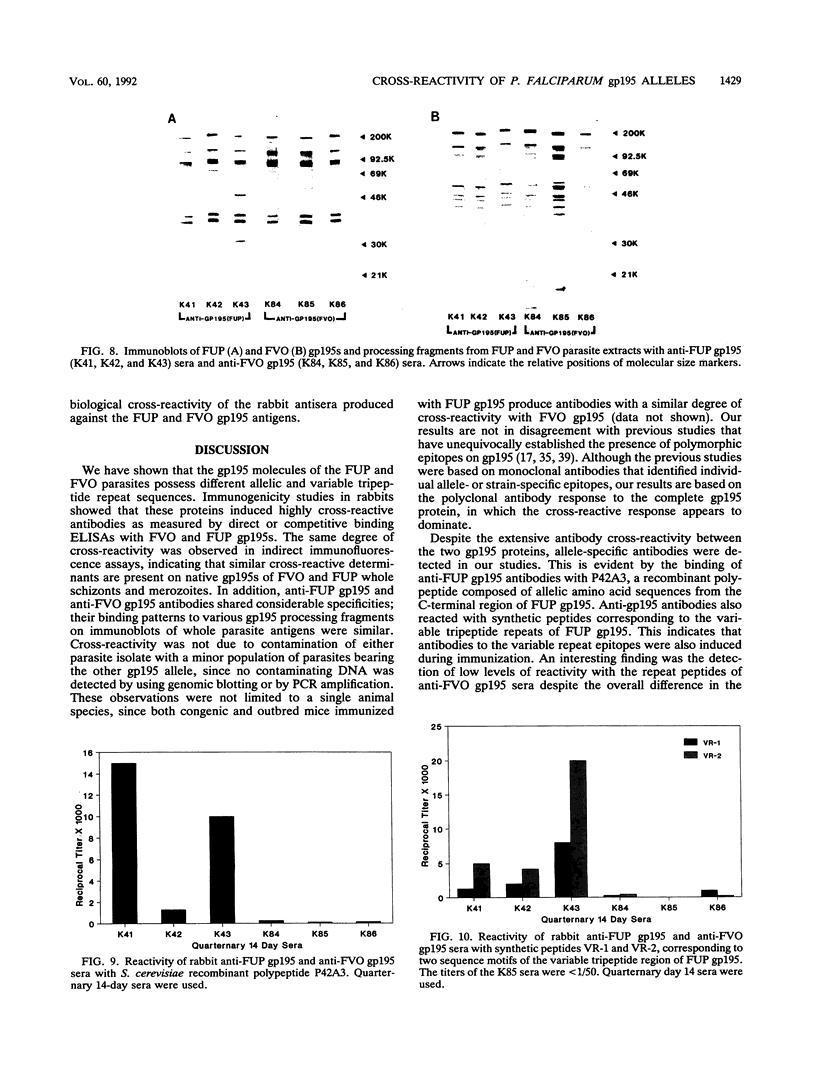
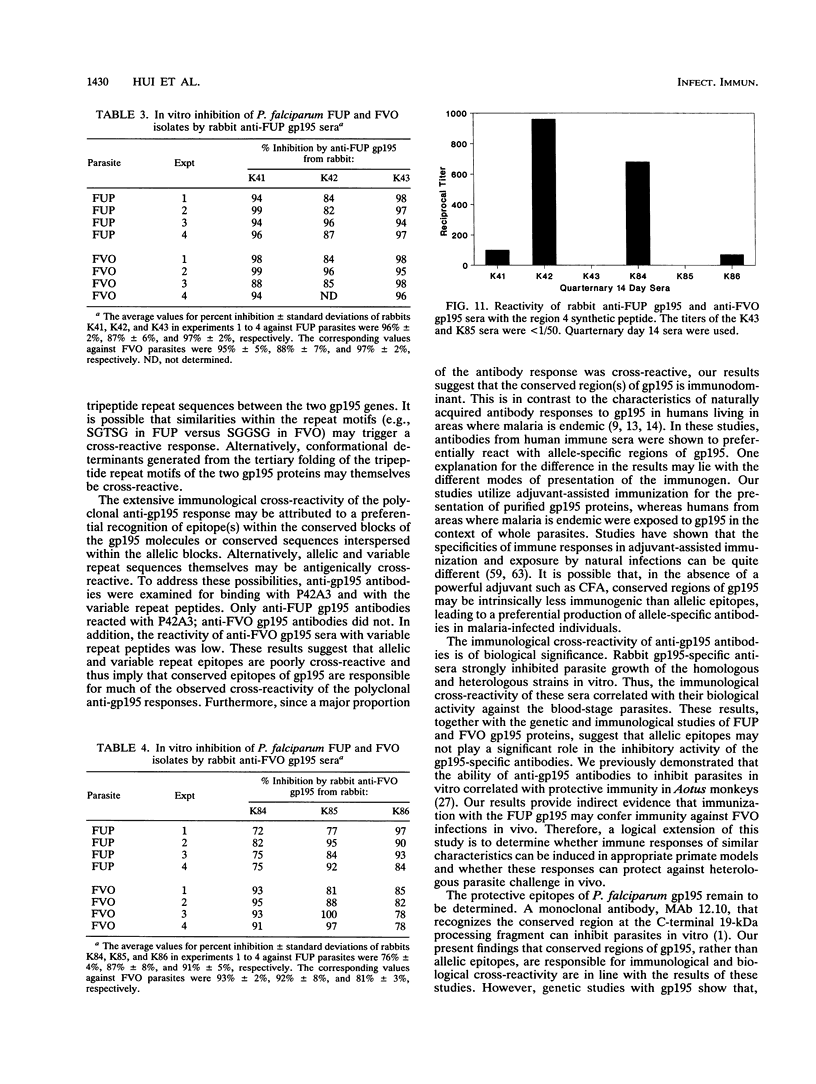



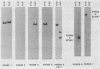
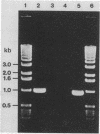
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blackman M. J., Heidrich H. G., Donachie S., McBride J. S., Holder A. A. A single fragment of a malaria merozoite surface protein remains on the parasite during red cell invasion and is the target of invasion-inhibiting antibodies. J Exp Med. 1990 Jul 1;172(1):379–382. doi: 10.1084/jem.172.1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns J. M., Jr, Daly T. M., Vaidya A. B., Long C. A. The 3' portion of the gene for a Plasmodium yoelii merozoite surface antigen encodes the epitope recognized by a protective monoclonal antibody. Proc Natl Acad Sci U S A. 1988 Jan;85(2):602–606. doi: 10.1073/pnas.85.2.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns J. M., Jr, Majarian W. R., Young J. F., Daly T. M., Long C. A. A protective monoclonal antibody recognizes an epitope in the carboxyl-terminal cysteine-rich domain in the precursor of the major merozoite surface antigen of the rodent malarial parasite, Plasmodium yoelii. J Immunol. 1989 Oct 15;143(8):2670–2676. [PubMed] [Google Scholar]
- Burns J. M., Jr, Parke L. A., Daly T. M., Cavacini L. A., Weidanz W. P., Long C. A. A protective monoclonal antibody recognizes a variant-specific epitope in the precursor of the major merozoite surface antigen of the rodent malarial parasite Plasmodium yoelii. J Immunol. 1989 Apr 15;142(8):2835–2840. [PubMed] [Google Scholar]
- Certa U., Rotmann D., Matile H., Reber-Liske R. A naturally occurring gene encoding the major surface antigen precursor p190 of Plasmodium falciparum lacks tripeptide repeats. EMBO J. 1987 Dec 20;6(13):4137–4142. doi: 10.1002/j.1460-2075.1987.tb02759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S. P., Hui G. S., Kato A., Siddiqui W. A. Generalized immunological recognition of the major merozoite surface antigen (gp195) of Plasmodium falciparum. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6343–6347. doi: 10.1073/pnas.86.16.6343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S. P., Kramer K. J., Yamaga K. M., Kato A., Case S. E., Siddiqui W. A. Plasmodium falciparum: gene structure and hydropathy profile of the major merozoite surface antigen (gp195) of the Uganda-Palo Alto isolate. Exp Parasitol. 1988 Oct;67(1):1–11. doi: 10.1016/0014-4894(88)90002-1. [DOI] [PubMed] [Google Scholar]
- Cheung A., Leban J., Shaw A. R., Merkli B., Stocker J., Chizzolini C., Sander C., Perrin L. H. Immunization with synthetic peptides of a Plasmodium falciparum surface antigen induces antimerozoite antibodies. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8328–8332. doi: 10.1073/pnas.83.21.8328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chizzolini C., Dupont A., Akue J. P., Kaufmann M. H., Verdini A. S., Pessi A., Del Giudice G. Natural antibodies against three distinct and defined antigens of Plasmodium falciparum in residents of a mesoendemic area in Gabon. Am J Trop Med Hyg. 1988 Aug;39(2):150–156. doi: 10.4269/ajtmh.1988.39.150. [DOI] [PubMed] [Google Scholar]
- Creasey A., Fenton B., Walker A., Thaithong S., Oliveira S., Mutambu S., Walliker D. Genetic diversity of Plasmodium falciparum shows geographical variation. Am J Trop Med Hyg. 1990 May;42(5):403–413. doi: 10.4269/ajtmh.1990.42.403. [DOI] [PubMed] [Google Scholar]
- Etlinger H. M., Caspers P., Matile H., Schoenfeld H. J., Stueber D., Takacs B. Ability of recombinant or native proteins to protect monkeys against heterologous challenge with Plasmodium falciparum. Infect Immun. 1991 Oct;59(10):3498–3503. doi: 10.1128/iai.59.10.3498-3503.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman R. R., Holder A. A. Surface antigens of malaria merozoites. A high molecular weight precursor is processed to an 83,000 mol wt form expressed on the surface of Plasmodium falciparum merozoites. J Exp Med. 1983 Nov 1;158(5):1647–1653. doi: 10.1084/jem.158.5.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Früh K., Doumbo O., Müller H. M., Koita O., McBride J., Crisanti A., Touré Y., Bujard H. Human antibody response to the major merozoite surface antigen of Plasmodium falciparum is strain specific and short-lived. Infect Immun. 1991 Apr;59(4):1319–1324. doi: 10.1128/iai.59.4.1319-1324.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabra M. S., Grossiord D., Perrin L. H., Shaw A., Cheung A., McGregor I. A. Defined Plasmodium falciparum antigens in malaria serology. Bull World Health Organ. 1986;64(6):889–896. [PMC free article] [PubMed] [Google Scholar]
- Geiman Q. M., Meagher M. J. Susceptibility of a New World monkey to Plasmodium falciparum from man. Nature. 1967 Jul 22;215(5099):437–439. doi: 10.1038/215437a0. [DOI] [PubMed] [Google Scholar]
- Hall R., Hyde J. E., Goman M., Simmons D. L., Hope I. A., Mackay M., Scaife J., Merkli B., Richle R., Stocker J. Major surface antigen gene of a human malaria parasite cloned and expressed in bacteria. 1984 Sep 27-Oct 3Nature. 311(5984):379–382. doi: 10.1038/311379a0. [DOI] [PubMed] [Google Scholar]
- Hall R., Osland A., Hyde J. E., Simmons D. L., Hope I. A., Scaife J. G. Processing, polymorphism, and biological significance of P190, a major surface antigen of the erythrocytic forms of Plasmodium falciparum. Mol Biochem Parasitol. 1984 Apr;11:61–80. doi: 10.1016/0166-6851(84)90055-0. [DOI] [PubMed] [Google Scholar]
- Haynes J. D., Vernes A., Lyon J. A. Serotypic antigens of Plasmodium falciparum recognized by serotype-restricted inhibitory human sera. Am J Trop Med Hyg. 1987 Mar;36(2):246–256. doi: 10.4269/ajtmh.1987.36.246. [DOI] [PubMed] [Google Scholar]
- Heidrich H. G., Miettinen-Baumann A., Eckerskorn C., Lottspeich F. The N-terminal amino acid sequences of the Plasmodium falciparum (FCB1) merozoite surface antigens of 42 and 36 kilodalton, both derived from the 185-195-kilodalton precursor. Mol Biochem Parasitol. 1989 May 1;34(2):147–154. doi: 10.1016/0166-6851(89)90006-6. [DOI] [PubMed] [Google Scholar]
- Herrera S., Herrera M. A., Perlaza B. L., Burki Y., Caspers P., Döbeli H., Rotmann D., Certa U. Immunization of Aotus monkeys with Plasmodium falciparum blood-stage recombinant proteins. Proc Natl Acad Sci U S A. 1990 May;87(10):4017–4021. doi: 10.1073/pnas.87.10.4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder A. A., Freeman R. R., Nicholls S. C. Immunization against Plasmodium falciparum with recombinant polypeptides produced in Escherichia coli. Parasite Immunol. 1988 Nov;10(6):607–617. doi: 10.1111/j.1365-3024.1988.tb00248.x. [DOI] [PubMed] [Google Scholar]
- Holder A. A., Freeman R. R. The three major antigens on the surface of Plasmodium falciparum merozoites are derived from a single high molecular weight precursor. J Exp Med. 1984 Aug 1;160(2):624–629. doi: 10.1084/jem.160.2.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard R. J., McBride J. S., Aley S. B., Marsh K. Antigenic diversity and size diversity of P. falciparum antigens in isolates from Gambian patients. II. the schizont surface glycoprotein of molecular weight approximately 200 000. Parasite Immunol. 1986 Jan;8(1):57–68. doi: 10.1111/j.1365-3024.1986.tb00833.x. [DOI] [PubMed] [Google Scholar]
- Hui G. S., Chang S. P., Gibson H., Hashimoto A., Hashiro C., Barr P. J., Kotani S. Influence of adjuvants on the antibody specificity to the Plasmodium falciparum major merozoite surface protein, gp195. J Immunol. 1991 Dec 1;147(11):3935–3941. [PubMed] [Google Scholar]
- Hui G. S., Palmer K. L., Siddiqui W. A. Use of human plasma for continuous in vitro cultivation of Plasmodium falciparum. Trans R Soc Trop Med Hyg. 1984;78(5):625–626. doi: 10.1016/0035-9203(84)90222-0. [DOI] [PubMed] [Google Scholar]
- Hui G. S., Siddiqui W. A. Serum from Pf195 protected Aotus monkeys inhibit Plasmodium falciparum growth in vitro. Exp Parasitol. 1987 Dec;64(3):519–522. doi: 10.1016/0014-4894(87)90068-3. [DOI] [PubMed] [Google Scholar]
- Hui G. S., Tam L. Q., Chang S. P., Case S. E., Hashiro C., Siddiqui W. A., Shiba T., Kusumoto S., Kotani S. Synthetic low-toxicity muramyl dipeptide and monophosphoryl lipid A replace Freund complete adjuvant in inducing growth-inhibitory antibodies to the Plasmodium falciparum major merozoite surface protein, gp195. Infect Immun. 1991 May;59(5):1585–1591. doi: 10.1128/iai.59.5.1585-1591.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery G. M. Epidemiological significance of repeated infections with homologous and heterologous strains and species of Plasmodium. Bull World Health Organ. 1966;35(6):873–882. [PMC free article] [PubMed] [Google Scholar]
- Jongwutiwes S., Tanabe K., Nakazawa S., Uemura H., Kanbara H. Coexistence of GP195 alleles of Plasmodium falciparum in a small endemic area. Am J Trop Med Hyg. 1991 Mar;44(3):299–305. doi: 10.4269/ajtmh.1991.44.299. [DOI] [PubMed] [Google Scholar]
- Kimura E., Mattei D., di Santi S. M., Scherf A. Genetic diversity in the major merozoite surface antigen of Plasmodium falciparum: high prevalence of a third polymorphic form detected in strains derived from malaria patients. Gene. 1990 Jul 2;91(1):57–62. doi: 10.1016/0378-1119(90)90162-k. [DOI] [PubMed] [Google Scholar]
- Kramer K. J., Kan S. C., Siddiqui W. A. Concentration of Plasmodium falciparum-infected erythrocytes by density gradient centrifugation in Percoll. J Parasitol. 1982 Apr;68(2):336–337. [PubMed] [Google Scholar]
- Lambros C., Vanderberg J. P. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979 Jun;65(3):418–420. [PubMed] [Google Scholar]
- Lew A. M., Beck D. J. The epitope of a protective monoclonal antibody occurs in a region of microheterogeneity in Plasmodium chabaudi. Mol Biochem Parasitol. 1990 Aug;42(1):153–154. doi: 10.1016/0166-6851(90)90123-4. [DOI] [PubMed] [Google Scholar]
- Lyon J. A., Haynes J. D., Diggs C. L., Chulay J. D., Haidaris C. G., Pratt-Rossiter J. Monoclonal antibody characterization of the 195-kilodalton major surface glycoprotein of Plasmodium falciparum malaria schizonts and merozoites: identification of additional processed products and a serotype-restricted repetitive epitope. J Immunol. 1987 Feb 1;138(3):895–901. [PubMed] [Google Scholar]
- Mackay M., Goman M., Bone N., Hyde J. E., Scaife J., Certa U., Stunnenberg H., Bujard H. Polymorphism of the precursor for the major surface antigens of Plasmodium falciparum merozoites: studies at the genetic level. EMBO J. 1985 Dec 30;4(13B):3823–3829. doi: 10.1002/j.1460-2075.1985.tb04154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majarian W. R., Daly T. M., Weidanz W. P., Long C. A. Passive immunization against murine malaria with an IgG3 monoclonal antibody. J Immunol. 1984 Jun;132(6):3131–3137. [PubMed] [Google Scholar]
- McBride J. S., Newbold C. I., Anand R. Polymorphism of a high molecular weight schizont antigen of the human malaria parasite Plasmodium falciparum. J Exp Med. 1985 Jan 1;161(1):160–180. doi: 10.1084/jem.161.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrifield R. B. Solid-phase peptide synthesis. Adv Enzymol Relat Areas Mol Biol. 1969;32:221–296. doi: 10.1002/9780470122778.ch6. [DOI] [PubMed] [Google Scholar]
- Patarroyo M. E., Romero P., Torres M. L., Clavijo P., Moreno A., Martínez A., Rodríguez R., Guzman F., Cabezas E. Induction of protective immunity against experimental infection with malaria using synthetic peptides. Nature. 1987 Aug 13;328(6131):629–632. doi: 10.1038/328629a0. [DOI] [PubMed] [Google Scholar]
- Perrin L. H., Merkli B., Loche M., Chizzolini C., Smart J., Richle R. Antimalarial immunity in Saimiri monkeys. Immunization with surface components of asexual blood stages. J Exp Med. 1984 Aug 1;160(2):441–451. doi: 10.1084/jem.160.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson M. G., Coppel R. L., McIntyre P., Langford C. J., Woodrow G., Brown G. V., Anders R. F., Kemp D. J. Variation in the precursor to the major merozoite surface antigens of Plasmodium falciparum. Mol Biochem Parasitol. 1988 Jan 15;27(2-3):291–301. doi: 10.1016/0166-6851(88)90049-7. [DOI] [PubMed] [Google Scholar]
- Peterson M. G., Coppel R. L., Moloney M. B., Kemp D. J. Third form of the precursor to the major merozoite surface antigens of Plasmodium falciparum. Mol Cell Biol. 1988 Jun;8(6):2664–2667. doi: 10.1128/mcb.8.6.2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirson P. J., Perkins M. E. Characterization with monoclonal antibodies of a surface antigen of Plasmodium falciparum merozoites. J Immunol. 1985 Mar;134(3):1946–1951. [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Scherf A., Barbot P., Langsley G. Sequence and length polymorphism of a major malaria vaccine candidate analysed following DNA amplification. Nucleic Acids Res. 1989 Feb 25;17(4):1774–1774. doi: 10.1093/nar/17.4.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherf A., Mattei D., Sarthou J. L. Multiple infections and unusual distribution of block 2 of the MSA1 gene of Plasmodium falciparum detected in west African clinical isolates by polymerase chain reaction analysis. Mol Biochem Parasitol. 1991 Feb;44(2):297–299. doi: 10.1016/0166-6851(91)90016-y. [DOI] [PubMed] [Google Scholar]
- Schwarz R. T., Riveros-Moreno V., Lockyer M. J., Nicholls S. C., Davey L. S., Hillman Y., Sandhu J. S., Freeman R. R., Holder A. A. Structural diversity of the major surface antigen of Plasmodium falciparum merozoites. Mol Cell Biol. 1986 Mar;6(3):964–968. doi: 10.1128/mcb.6.3.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui W. A., Schnell J. V., Geiman Q. M. A model in vitro system to test the susceptibility of human malarial parasites to antimalarial drugs. Am J Trop Med Hyg. 1972 Jul;21(4):393–399. [PubMed] [Google Scholar]
- Siddiqui W. A., Tam L. Q., Kramer K. J., Hui G. S., Case S. E., Yamaga K. M., Chang S. P., Chan E. B., Kan S. C. Merozoite surface coat precursor protein completely protects Aotus monkeys against Plasmodium falciparum malaria. Proc Natl Acad Sci U S A. 1987 May;84(9):3014–3018. doi: 10.1073/pnas.84.9.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sy N. E., Oberst R. B., Macalagay P. S., Fallarme V. D., Cruzada S. F., Laughlin L. W. In vitro growth inhibition of Plasmodium falciparum by sera from different regions of the Philippines. Am J Trop Med Hyg. 1990 Sep;43(3):243–247. doi: 10.4269/ajtmh.1990.43.243. [DOI] [PubMed] [Google Scholar]
- Tanabe K., Mackay M., Goman M., Scaife J. G. Allelic dimorphism in a surface antigen gene of the malaria parasite Plasmodium falciparum. J Mol Biol. 1987 May 20;195(2):273–287. doi: 10.1016/0022-2836(87)90649-8. [DOI] [PubMed] [Google Scholar]
- Tanabe K., Murakami K., Doi S. Plasmodium falciparum: dimorphism of the p190 alleles. Exp Parasitol. 1989 May;68(4):470–473. doi: 10.1016/0014-4894(89)90132-x. [DOI] [PubMed] [Google Scholar]
- Taylor D. W., Siddiqui W. A. Susceptibility of owl monkeys to Plasmodium falciparum infection in relation to location of origin, phenotype, and karyotype. J Parasitol. 1979 Apr;65(2):267–271. [PubMed] [Google Scholar]
- Tomlinson L. A., Christie J. F., Fraser E. M., McLaughlin D., McIntosh A. E., Kennedy M. W. MHC restriction of the antibody repertoire to secretory antigens, and a major allergen, of the nematode parasite Ascaris. J Immunol. 1989 Oct 1;143(7):2349–2356. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Vernes A., Haynes J. D., Tapchaisri P., Williams J. L., Dutoit E., Diggs C. L. Plasmodium falciparum strain-specific human antibody inhibits merozoite invasion of erythrocytes. Am J Trop Med Hyg. 1984 Mar;33(2):197–203. doi: 10.4269/ajtmh.1984.33.197. [DOI] [PubMed] [Google Scholar]
- Yamashita K., Heber-Katz E. Lack of immunodominance in the T cell response to herpes simplex virus glycoprotein D after administration of infectious virus. J Exp Med. 1989 Sep 1;170(3):997–1002. doi: 10.1084/jem.170.3.997. [DOI] [PMC free article] [PubMed] [Google Scholar]