Abstract
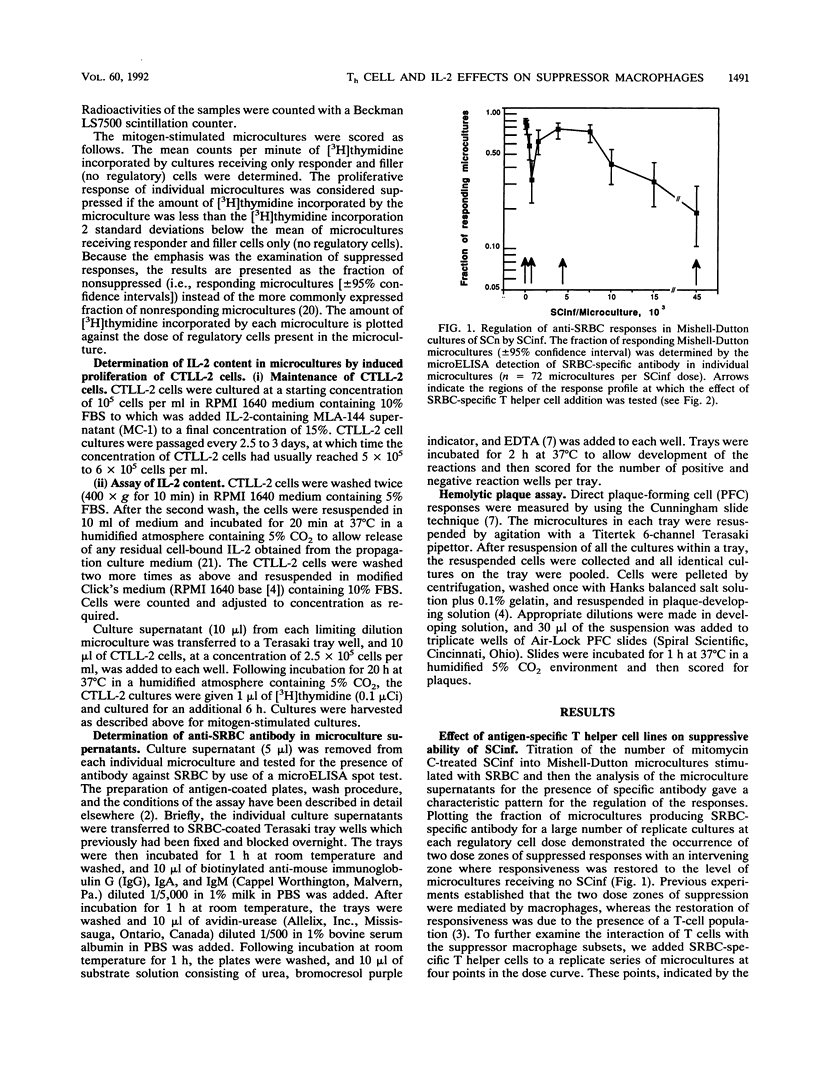
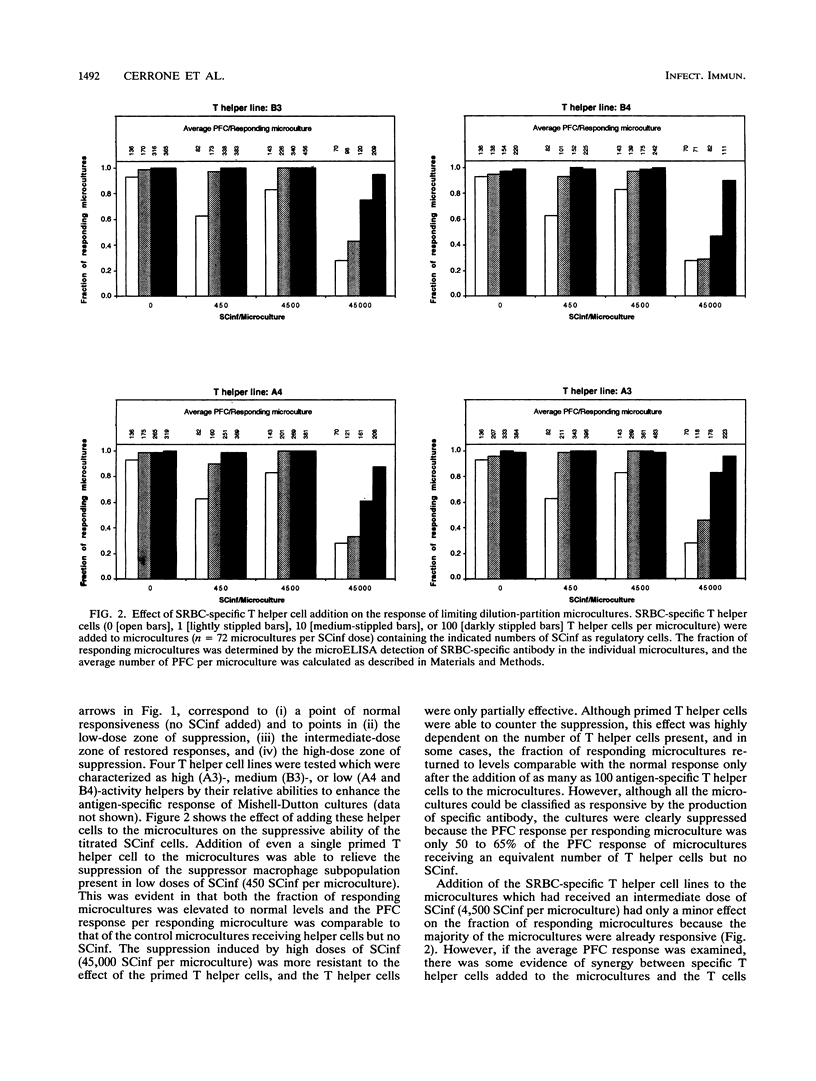
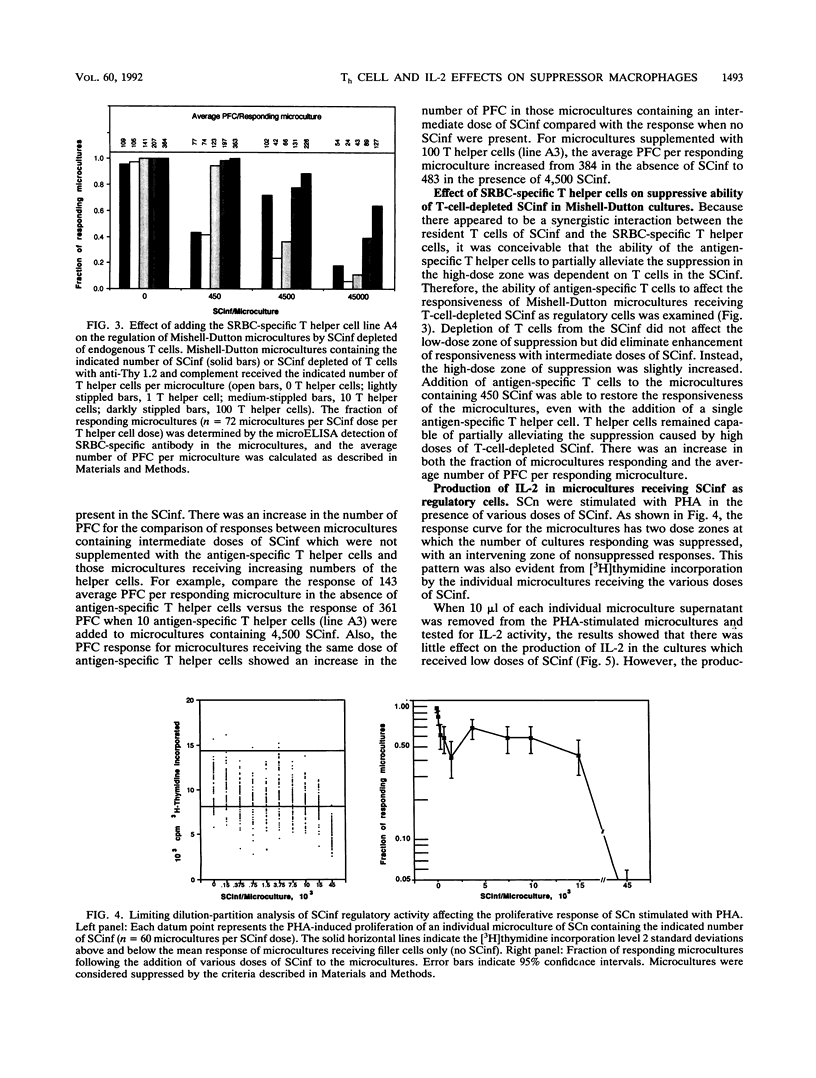
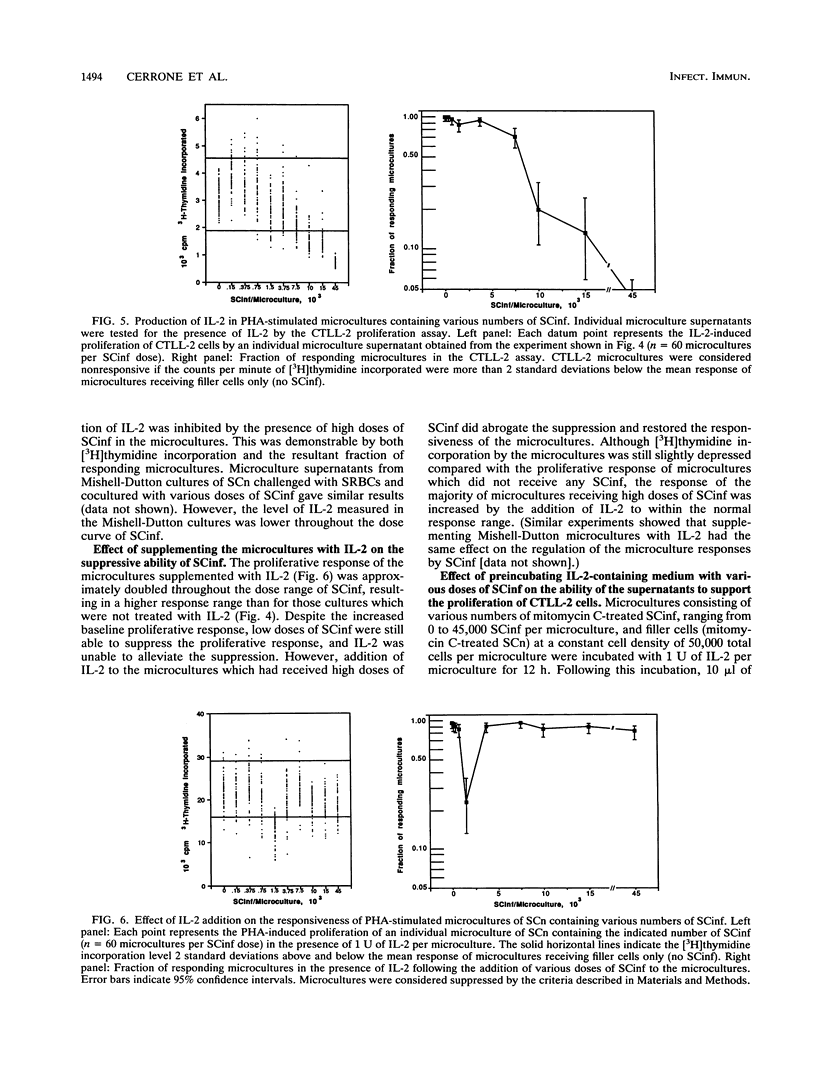
Trypanosoma cruzi, a protozoan parasite and the causative agent of Chagas' disease, induces a state of lymphocyte hyporesponsiveness to both mitogenic and antigenic stimuli in mice during the acute phase of infection. Addition of spleen cells from T. cruzi-infected mice (SCinf) to microcultures of spleen cells from noninfected mice (SCn) suppresses the responsiveness of such cultures to antigenic challenge and to mitogenic stimulation. We analyzed the regulatory cell populations in SCinf by limiting dilution-partition analysis and found a complex regulatory circuit in T. cruzi-infected mice consisting of two suppressive macrophage subsets and an enhancing T-cell population. This T-cell population was able to abrogate or escape the suppressive ability of one suppressor macrophage subset, yet was suppressed by the other macrophage subset. To further study the cellular interactions of this regulatory circuit and analyze the suppressive abilities of the two suppressor macrophage subsets, we examined the effect of adding either primed T helper cells of known specificity or interleukin-2 to the limiting dilution-partition analysis microcultures. The results of these experiments suggest that one suppressor macrophage subset, which is abundant and, therefore, detected with low doses of SCinf, is able to suppress both mitogen- and primary antigen-specific responses but is unable to inhibit cells once they are already activated or primed. The other macrophage subset, which is presumably a less abundant or less active population (since high doses of SCinf are required to detect it), is able to suppress the response of activated or primed T cells by the inhibition of interleukin-2 production.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burgess D. E., Kuhn R. E., Carlson K. S. Induction of parasite-specific helper T lymphocytes during Trypanosoma cruzi infections in mice. J Immunol. 1981 Nov;127(5):2092–2095. [PubMed] [Google Scholar]
- Cerrone M. C., Kuhn R. E. Description of a urease-based microELISA for the analysis of limiting dilution microcultures. J Immunol Methods. 1991 Apr 8;138(1):65–75. doi: 10.1016/0022-1759(91)90065-n. [DOI] [PubMed] [Google Scholar]
- Cerrone M. C., Kuhn R. E. Macrophage regulation of immune responses of spleen cells from mice infected with Trypanosoma cruzi. Cell Immunol. 1991 Dec;138(2):423–436. doi: 10.1016/0008-8749(91)90166-9. [DOI] [PubMed] [Google Scholar]
- Chandler H. M., Cox J. C., Healey K., MacGregor A., Premier R. R., Hurrell J. G. An investigation of the use of urease-antibody conjugates in enzyme immunoassays. J Immunol Methods. 1982 Sep 17;53(2):187–194. doi: 10.1016/0022-1759(82)90140-5. [DOI] [PubMed] [Google Scholar]
- Choromanski L., Kuhn R. E. Repeated antigenic stimulation overcomes immunosuppression in experimental Chagas' disease. Immunology. 1986 Oct;59(2):289–294. [PMC free article] [PubMed] [Google Scholar]
- Choromanski L., Kuhn R. E. Use of parasite antigens and interleukin-2 to enhance suppressed immune responses during Trypanosoma cruzi infection in mice. Infect Immun. 1987 Feb;55(2):403–408. doi: 10.1128/iai.55.2.403-408.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham A. J., Szenberg A. Further improvements in the plaque technique for detecting single antibody-forming cells. Immunology. 1968 Apr;14(4):599–600. [PMC free article] [PubMed] [Google Scholar]
- Cunningham D. S., Benavides G. R., Kuhn R. E. Differences in the regulation of humoral responses between mice infected with Trypanosoma cruzi and mice administered T. cruzi-induced suppressor substance. J Immunol. 1980 Nov;125(5):2317–2321. [PubMed] [Google Scholar]
- Cunningham D. S., Grogl M., Kuhn R. E. Suppression of antibody responses in humans infected with Trypanosoma cruzi. Infect Immun. 1980 Nov;30(2):496–499. doi: 10.1128/iai.30.2.496-499.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham D. S., Kuhn R. E. Lymphoblast transformation as a measure of immune competence during experimental Chagas' disease. J Parasitol. 1980 Jun;66(3):390–398. [PubMed] [Google Scholar]
- Cunningham D. S., Kuhn R. E., Rowland E. C. Suppression of humoral responses during Trypanosoma cruzi infections in mice. Infect Immun. 1978 Oct;22(1):155–160. doi: 10.1128/iai.22.1.155-160.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham D. S., Kuhn R. E., Tarleton R. L., Dunn R. S. Trypanosoma cruzi: effect on B-cell-responsive and -responding clones. Exp Parasitol. 1981 Apr;51(2):257–268. doi: 10.1016/0014-4894(81)90114-4. [DOI] [PubMed] [Google Scholar]
- Cunningham D. S., Kuhn R. E. Trypanosoma cruzi-induced suppression of the primary immune response in murine cell cultures to T-cell-dependent and -independent antigens. J Parasitol. 1980 Feb;66(1):16–27. [PubMed] [Google Scholar]
- Harel-Bellan A., Joskowicz M., Fradelizi D., Eisen H. Modification of T-cell proliferation and interleukin 2 production in mice infected with Trypanosoma cruzi. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3466–3469. doi: 10.1073/pnas.80.11.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson L. E., Hewetson J. F., Hopkins R. F., 3rd, Sowder R. C., Neubauer R. H., Rabin H. A rapid, large scale purification procedure for gibbon interleukin 2. J Immunol. 1983 Aug;131(2):810–815. [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kierszenbaum F., Hayes M. M. Evaluation of lymphocyte responsiveness to polyclonal activators during acute and chronic experimental Trypanosoma cruzi infection. Am J Trop Med Hyg. 1980 Jul;29(4):708–710. doi: 10.4269/ajtmh.1980.29.708. [DOI] [PubMed] [Google Scholar]
- Kuhn R. E., Murnane J. E. Trypanosoma cruzi: immune destruction of parasitized mouse fibroblasts in vitro. Exp Parasitol. 1977 Feb;41(1):66–73. doi: 10.1016/0014-4894(77)90130-8. [DOI] [PubMed] [Google Scholar]
- Michalski J. P., McCombs C. C. Reduction of excessive background counts in an IL-2 microassay by incubation of indicator cells at 37 degrees C. J Immunol Methods. 1985 Apr 8;78(1):159–160. doi: 10.1016/0022-1759(85)90339-4. [DOI] [PubMed] [Google Scholar]
- O'Daly J. A., Simonis S., de Rolo N., Caballero H. Suppression of humoral immunity and lymphocyte responsiveness during experimental Trypanosoma cruzi infections. Rev Inst Med Trop Sao Paulo. 1984 Mar-Apr;26(2):67–77. doi: 10.1590/s0036-46651984000200001. [DOI] [PubMed] [Google Scholar]
- Rabin H., Hopkins R. F., 3rd, Ruscetti F. W., Neubauer R. H., Brown R. L., Kawakami T. G. Spontaneous release of a factor with properties of T cell growth factor from a continuous line of primate tumor T cells. J Immunol. 1981 Nov;127(5):1852–1856. [PubMed] [Google Scholar]
- Ramos C., Lamoyi E., Feoli M., Rodriguez M., Perez M., Ortiz-Ortiz L. Trypanosoma cruzi: immunosuppressed response to different antigens in the infected mouse. Exp Parasitol. 1978 Aug;45(2):190–199. doi: 10.1016/0014-4894(78)90059-0. [DOI] [PubMed] [Google Scholar]
- Ramos C., Schädtler-Siwon I., Ortiz-Ortiz L. Suppressor cells present in the spleens of Trypanosoma cruzi-infected mice. J Immunol. 1979 Apr;122(4):1243–1247. [PubMed] [Google Scholar]
- Reed S. G., Inverso J. A., Roters S. B. Heterologous antibody responses in mice with chronic T. cruzi infection: depressed T helper function restored with supernatants containing interleukin 2. J Immunol. 1984 Sep;133(3):1558–1563. [PubMed] [Google Scholar]
- Reed S. G., Inverso J. A., Roters S. B. Suppressed antibody responses to sheep erythrocytes in mice with chronic Trypanosoma cruzi infections are restored with interleukin 2. J Immunol. 1984 Dec;133(6):3333–3337. [PubMed] [Google Scholar]
- Reed S. G., Pihl D. L., Grabstein K. H. Immune deficiency in chronic Trypanosoma cruzi infection. Recombinant IL-1 restores Th function for antibody production. J Immunol. 1989 Mar 15;142(6):2067–2071. [PubMed] [Google Scholar]
- Rowland E. C., Kuhn R. E. Suppression of cellular responses in mice during Trypanosoma cruzi infections. Infect Immun. 1978 May;20(2):393–397. doi: 10.1128/iai.20.2.393-397.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sette A., Adorini L., Marubini E., Doria G. A microcomputer program for probit analysis of interleukin-2 (IL-2) titration data. J Immunol Methods. 1986 Feb 12;86(2):265–277. doi: 10.1016/0022-1759(86)90463-1. [DOI] [PubMed] [Google Scholar]
- Tarleton R. L., Kuhn R. E. Changes in cell populations and immunoglobulin-producing cells in the spleens of mice infected with Trypanosoma cruzi: correlations with parasite-specific antibody response. Cell Immunol. 1983 Sep;80(2):392–404. doi: 10.1016/0008-8749(83)90126-0. [DOI] [PubMed] [Google Scholar]
- Tarleton R. L., Kuhn R. E. Measurement of parasite-specific immune responses in vitro: evidence for suppression of the antibody response to Trypanosoma cruzi. Eur J Immunol. 1985 Aug;15(8):845–850. doi: 10.1002/eji.1830150820. [DOI] [PubMed] [Google Scholar]
- Tarleton R. L., Kuhn R. E. Restoration of in vitro immune responses of spleen cells from mice infected with Trypanosoma cruzi by supernatants containing interleukin 2. J Immunol. 1984 Sep;133(3):1570–1575. [PubMed] [Google Scholar]
- Waldmann H., Lefkovits I., Quintáns J. Limiting dilution analysis of helper T-cell function. Immunology. 1975 Jun;28(6):1135–1148. [PMC free article] [PubMed] [Google Scholar]


