Abstract
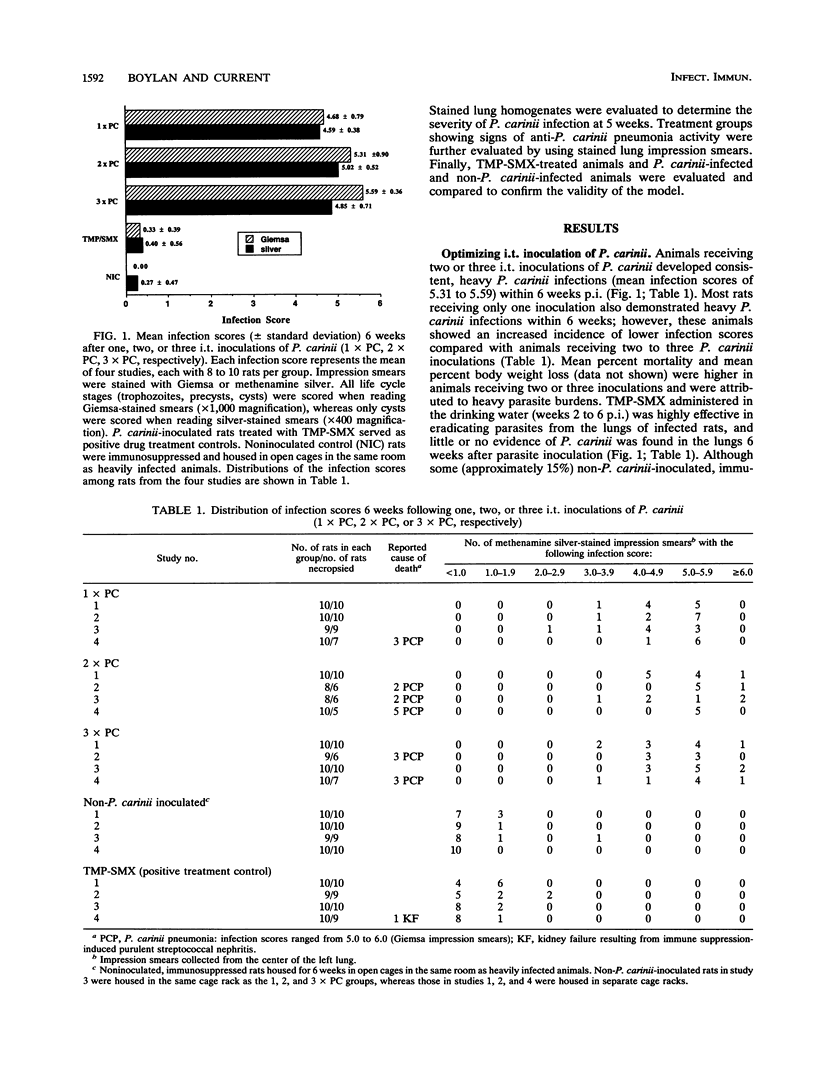
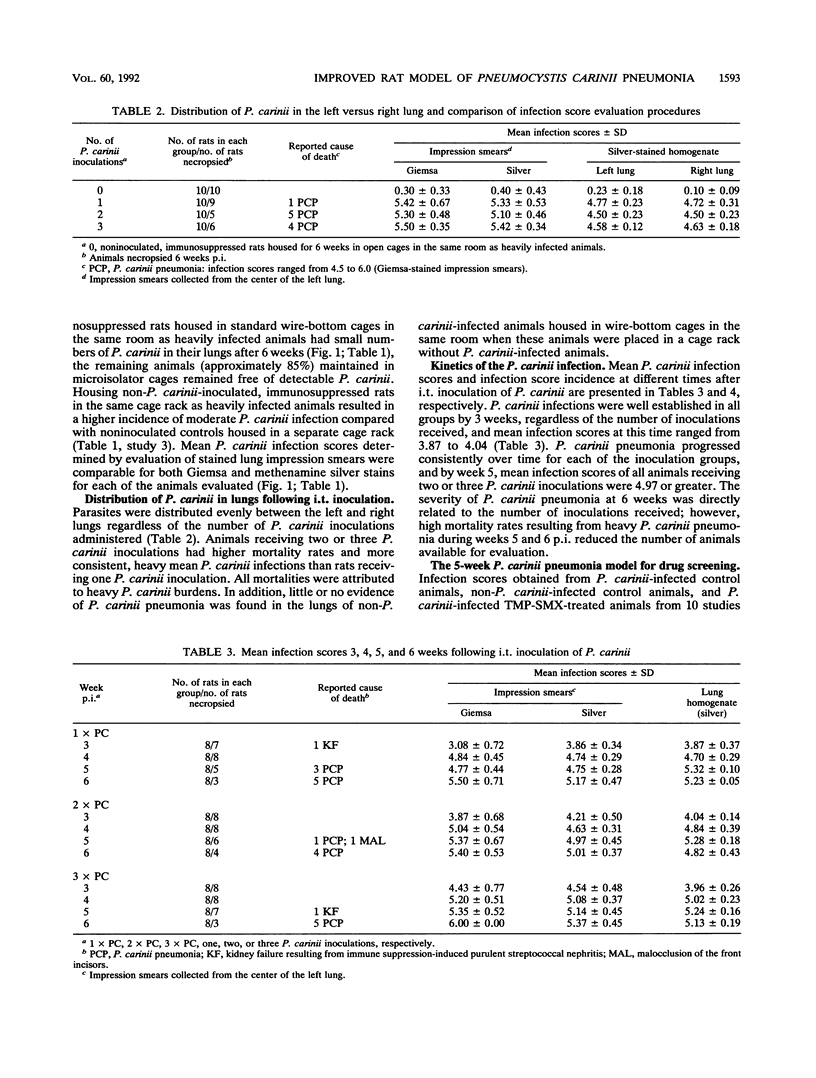
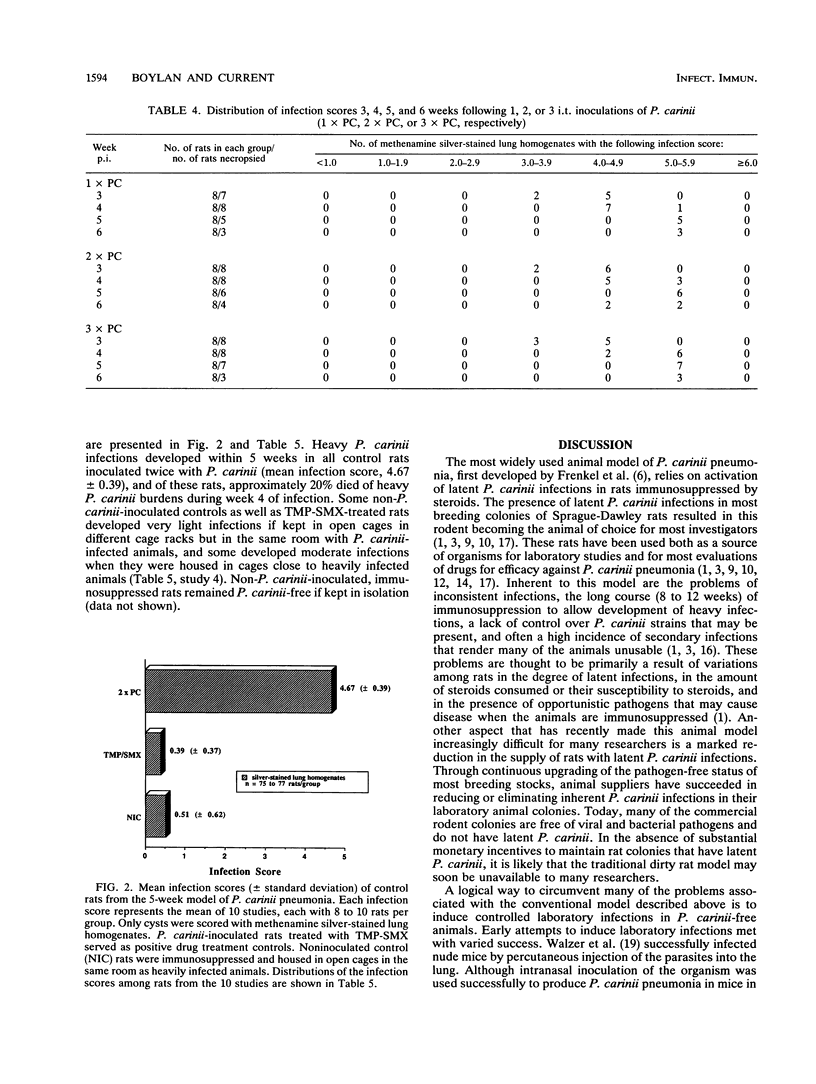
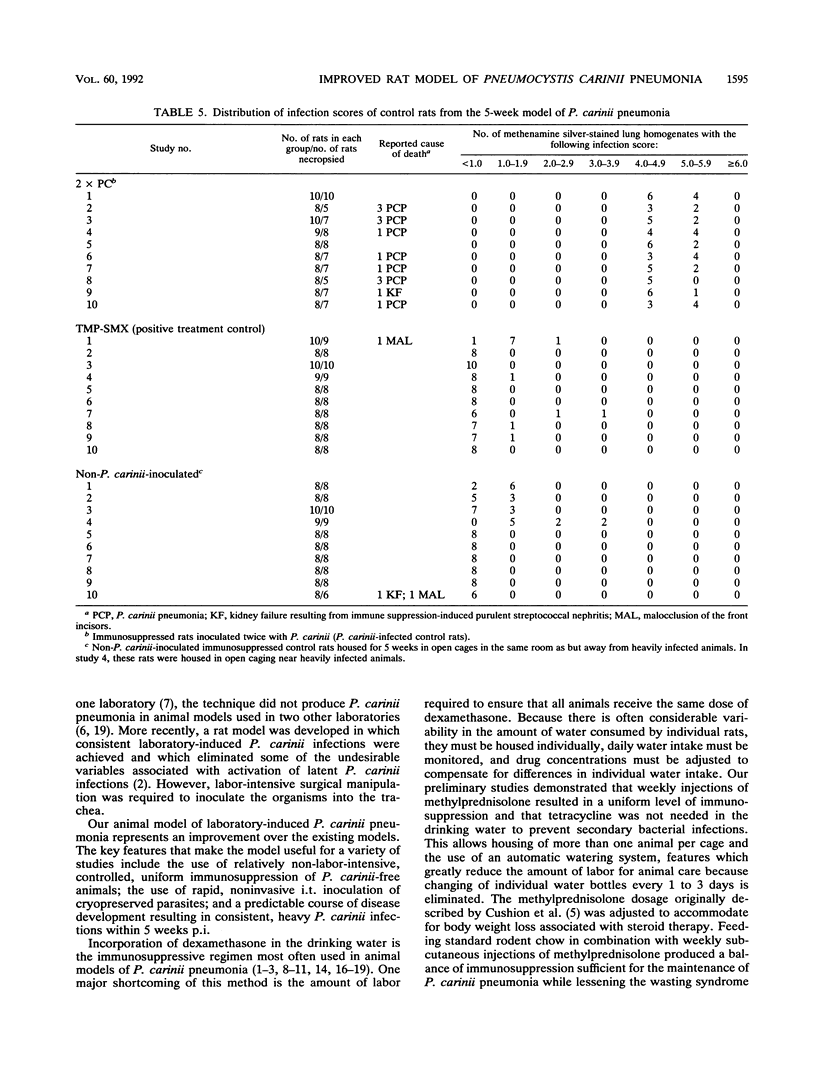
An immunosuppressed rat model of Pneumocystis carinii pneumonia is described that utilizes simple, noninvasive intratracheal (i.t.) inoculation of cryopreserved parasites and results in development of severe P. carinii pneumonia within 5 weeks. This is an improvement over the most commonly used models of P. carinii pneumonia that rely on immune suppression to activate latent P. carinii infections and that often require 8 to 12 weeks to produce heavy infections of P. carinii. It is also less labor intensive than more recent models requiring surgical instillation of parasites. Our report describes a series of preliminary studies to select an appropriate strain of rat; to determine suitable methods for inducing uniform immunosuppression, P. carinii inoculation, and laboratory maintenance of P. carinii; and to determine effective animal husbandry methods for maintaining animals free from serious secondary infections. Results of our more detailed studies demonstrate that animals receiving two or three i.t. inoculations of approximately 10(6) cryopreserved P. carinii organisms have a predictable course of disease progression which includes moderate P. carinii infections within 3 weeks, severe P. carinii pneumonia in 5 weeks, and a high percentage of mortality due to P. carinii pneumonia in 6 weeks. Parasites were distributed evenly between the right and left lungs, regardless of the number of P. carinii inoculations administered. Non-P. carinii-inoculated immunosuppressed control rats maintained in microisolator cages remained free of P. carinii, thus providing an important control that is missing from many P. carinii pneumonia models. Most non-P. carinii-inoculated control animals and P. carinii-inoculated rats treated with trimethoprim-sulfamethoxazole that were housed in open caging in the same room containing heavily infected animals had no detectable infections after 5 to 6 weeks of immunosuppression; however, some had a small number of P. carinii in their lungs. Because heavy, reproducible infections are achieved 5 weeks after i.t. inoculation, because few animals are lost to secondary infections, and because animals can be maintained as noninfected contemporaneous controls, this animal model is useful for the maintenance of P. carinii strains, for studies of the transmission and natural history of P. carinii, for the production of large numbers of organisms for laboratory studies, and for the evaluation of potential anti-P. carinii drugs.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartlett M. S., Fishman J. A., Durkin M. M., Queener S. F., Smith J. W. Pneumocystis carinii: improved models to study efficacy of drugs for treatment or prophylaxis of Pneumocystis pneumonia in the rat (Rattus spp.). Exp Parasitol. 1990 Jan;70(1):100–106. doi: 10.1016/0014-4894(90)90089-u. [DOI] [PubMed] [Google Scholar]
- Bartlett M. S., Fishman J. A., Queener S. F., Durkin M. M., Jay M. A., Smith J. W. New rat model of Pneumocystis carinii infection. J Clin Microbiol. 1988 Jun;26(6):1100–1102. doi: 10.1128/jcm.26.6.1100-1102.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett M. S., Queener S. F., Jay M. A., Durkin M. M., Smith J. W. Improved rat model for studying Pneumocystis carinii pneumonia. J Clin Microbiol. 1987 Mar;25(3):480–484. doi: 10.1128/jcm.25.3.480-484.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushion M. T., DeStefano J. A., Walzer P. D. Pneumocystis carinii: surface reactive carbohydrates detected by lectin probes. Exp Parasitol. 1988 Dec;67(2):137–147. doi: 10.1016/0014-4894(88)90061-6. [DOI] [PubMed] [Google Scholar]
- Frenkel J. K., Good J. T., Shultz J. A. Latent Pneumocystis infection of rats, relapse, and chemotherapy. Lab Invest. 1966 Oct;15(10):1559–1577. [PubMed] [Google Scholar]
- Furuta T., Ueda K., Fujiwara K., Yamanouchi K. Cellular and humoral immune responses of mice subclinically infected with Pneumocystis carinii. Infect Immun. 1985 Feb;47(2):544–548. doi: 10.1128/iai.47.2.544-548.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes W. T., Gray V. L., Gutteridge W. E., Latter V. S., Pudney M. Efficacy of a hydroxynaphthoquinone, 566C80, in experimental Pneumocystis carinii pneumonitis. Antimicrob Agents Chemother. 1990 Feb;34(2):225–228. doi: 10.1128/aac.34.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes W. T., McNabb P. C., Makres T. D., Feldman S. Efficacy of trimethoprim and sulfamethoxazole in the prevention and treatment of Pneumocystis carinii pneumonitis. Antimicrob Agents Chemother. 1974 Mar;5(3):289–293. doi: 10.1128/aac.5.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. K., Hall J. E., Allen M. A., Morrison S. D., Ohemeng K. A., Reddy V. V., Geratz J. D., Tidwell R. R. Novel pentamidine analogs in the treatment of experimental Pneumocystis carinii pneumonia. Antimicrob Agents Chemother. 1990 Jun;34(6):1026–1030. doi: 10.1128/aac.34.6.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs J. A., Halpern J. L., Lundgren B., Swan J. C., Parrillo J. E., Masur H. Monoclonal antibodies to Pneumocystis carinii: identification of specific antigens and characterization of antigenic differences between rat and human isolates. J Infect Dis. 1989 Jan;159(1):60–70. doi: 10.1093/infdis/159.1.60. [DOI] [PubMed] [Google Scholar]
- Kovacs J. A., Masur H. Prophylaxis of Pneumocystis carinii pneumonia: an update. J Infect Dis. 1989 Nov;160(5):882–886. doi: 10.1093/infdis/160.5.882. [DOI] [PubMed] [Google Scholar]
- Pesanti E. L., Cox C. Metabolic and synthetic activities of Pneumocystis carinii in vitro. Infect Immun. 1981 Dec;34(3):908–914. doi: 10.1128/iai.34.3.908-914.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter W. P., Bitar Y. S., Strandberg J. D., Charache P. C. Absence of therapeutic blood concentrations of tetracycline in rats after administration in drinking water. Lab Anim Sci. 1985 Feb;35(1):71–75. [PubMed] [Google Scholar]
- Walzer P. D., Kim C. K., Foy J., Linke M. J., Cushion M. T. Cationic antitrypanosomal and other antimicrobial agents in the therapy of experimental Pneumocystis carinii pneumonia. Antimicrob Agents Chemother. 1988 Jun;32(6):896–905. doi: 10.1128/aac.32.6.896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walzer P. D., Rutledge M. E., Yoneda K. Experimental Pneumocystis carinii pneumonia in C3H/HeJ and C3HeB/FeJ mice. J Reticuloendothel Soc. 1983 Jan;33(1):1–9. [PubMed] [Google Scholar]
- Walzer P. D., Schnelle V., Armstrong D., Rosen P. P. Nude mouse: a new experimental model for Pneumocystis carinii infection. Science. 1977 Jul 8;197(4299):177–179. doi: 10.1126/science.301657. [DOI] [PubMed] [Google Scholar]


