Abstract
OBJECTIVE— β-Cell regeneration is a fundamental but elusive goal for type 1 diabetes research. Our objective is to review newer human and animal studies of β-cell destruction and regeneration and consider the implications for treatment of type 1 diabetes.
RESEARCH DESIGN AND METHODS— Recent human and animal studies of β-cell destruction and regeneration in type 1 diabetes are reviewed.
RESULTS— The loss of β-cells that characterizes type 1 diabetes reflects the net effects of destruction and regeneration. These processes have been examined in the nonobese diabetic (NOD) mouse; uncertainty remains about β-cell dynamics in humans. Islet inflammation stimulates β-cell replication that produces new insulin-positive cells. The regenerative process may tide the loss of overall β-cell function, but it also may enhance the autoimmune attack on β-cells by providing new epitopes. The highest rates of β-cell replication are at the time of diagnosis of diabetes in NOD mice, and if autoimmunity and islet inflammation are arrested, new β-cells are formed. However, the majority of β-cells after treatment with immune modulators such as anti-CD3 monoclonal antibody, and most likely during the “honeymoon” in human disease, are recovered β-cells that had been degranulated but present at the time of diagnosis of diabetes.
CONCLUSIONS— Residual β-cells play a significant role for the design of therapeutic trials: they not only may respond to combination therapies that include stimulants of metabolic function but are also the potential source of new β-cells.
Studies largely done in nonobese diabetic (NOD) mice have described a linear loss of β-cell mass from the time of initiation of insulitis through presentation with hyperglycemia and afterward until there is complete loss of β-cells (1,2). Results from the Diabetes Prevention Trial-1 (DPT-1) have described changes in insulin secretion from pre-diabetes to presentation with hyperglycemia, and other studies have depicted the progressive loss of insulin secretion after diagnosis (3–5). Whereas the data from animal studies and clinical investigations are largely consistent, other studies indicate that a simple linear loss of β-cell function may be an oversimplification of a process that involves an undulating downhill course. Variation in the rate of progression of β-cell loss may be due to waxing and waning of the inflammatory response because of exposure of new antigens; intercurrent insults to β-cells involving infectious agents, metabolic demands, or other factors; and a continuous attempt at β-cell regeneration that tides the progression. Inflammation itself appears to stimulate β-cell regeneration, but at the same time may expose new antigenic epitopes that become the drivers of a broadening autoimmune response (6). Ultimately, the failure to contain and counter this enlarging process results in presentation with clinical disease. Understanding the way in which the process develops and progresses has implications for treatment and reversal of the disease.
β-cell turnover is increased during progression of diabetes.
In NOD mice, β-cell proliferation increases with islet inflammation during progression of diabetes. By 4 weeks of age, the proportion of Ki67+insulin-positive cells in the islets of Langerhans in NOD mice is significantly increased compared with control NOD/scid mice that do not have insulitis (7). β-Cell proliferation continues to increase, with insulitis, during the development of disease; by 12 weeks of age, 3.03 ± 0.94% of insulin-positive cells are Ki67+. The highest levels of Ki67+insulin-positive cells are found at the time that the mice develop hyperglycemia. The increased rate of β-cell proliferation is found even before a significant decline in β-cell mass or an increase in glucose levels suggesting that metabolic demands per se are not responsible for the β-cell proliferation. Similar findings of heightened proliferation of β-cells were reported by Sreenan et al. (1) using bromodeoxyuridine (BrdU) labeling. These investigators also found an inverse change in β-cell mass and proliferation rates.
The β-cell proliferation is linked to islet inflammation (7). It increases in the recipients of diabetogenic spleen cells that are transferred to NOD/scid recipients. By 4 weeks after transfer of cells, the rates of β-cell replication increases more than 10-fold compared with NOD/scid mice (from 0.22 ± 0.05% to 3.14 ± 1.07%, P = 0.007) (7). Measures that modulate islet inflammation decrease β-cell proliferation. After treatment with anti-CD3 monoclonal antibody (mAb), β-cell proliferation fell from 3.09 ± 0.8% (at diagnosis) to 1.57 ± 0.28% 3 weeks after treatment (P < 0.05). Moreover, when diabetogenic spleen cells were transferred into NOD/scid recipients together with CD4+CD25+ regulatory T-cells, the rate of diabetes decreased from 79 to 17% (P < 0.01), but β-cell proliferation also decreased from 2.95 ± 0.27 to 1.98 ± 0.26%.
The increased rates of β-cell proliferation that are found in mice with insulitis result in an increase in the mass of “new” β-cells if autoimmunity is arrested. To demonstrate this directly, we treated hyperglycemic NOD mice with anti-CD3 mAb to reverse hyperglycemia, studied the changes in β-cell area after treatment, and enumerated the proportion of new β-cells after recovery of diabetes. We added BrdU to the drinking water of the mice after treatment with anti-CD3 mAb and enumerated the “new” β-cells in the islets after euglycemia was established. In the islets of these mice, 10.9 ± 1.38% of the β-cells were BrdU+ 3 weeks after mAb treatment compared with 4.22 ± 0.49% of the β-cells in untreated NOD/scid mice that were given BrdU over the same period of time (P < 0.001) (7).
These findings are consistent with the recent report by Nir et al. (8) that there may be spontaneous recovery of β-cell mass and function in the absence of autoimmunity. In these studies, the investigators acutely eliminated 70–80% of β-cells by the transgenic expression of diphtheria toxin. When the transgene expression was quenched, hyperglycemia resolved over a 4-week period and β-cell mass increased. Dor, Melton, and colleagues (9) have previously observed that β-cell mass expansion primarily occurs via insulin-positive precursors. Indeed, diphtheria-mediated β-cell mass reduction primarily occurs by expansion of insulin-positive progenitors (8). New data indicate specialized progenitors do not contribute to β-cell mass growth or regeneration (10,11). Taken together, these studies suggest that β-cell mass in young rodents is highly dynamic and that non–autoimmune-stimulated β-cell regeneration primarily occurs via simple β-cell replication.
Despite the consistent evidence for β-cell regeneration in rodents, it is not clear whether similar β-cell dynamics occur in humans. Butler et al. (12) did not find evidence for increased β-cell replication in patients with recent-onset diabetes who died of ketoacidosis compared with age-matched subjects. In’t Veld et al. (13) did identify Ki67+insulin-positive cells in islets with insulitis in anti-islet autoantibody–positive organ donors, but the percentage of islets in the pancreas with insulitis was small (3 and 9%). However, there are differences between the material sampled in these two reports and the pancreases of NOD mice that develop diabetes. The timing of the analysis is different in patients with diabetes compared with pre-diabetic NOD mice, and in the case of the organ donors, it is not clear whether the pathologic process that was studied would in fact lead to diabetes. Nonetheless, these observational studies identify a number of important differences between human disease and the NOD model. First, the proliferative responses of human islets may be different from those seen in rodents. Second, the inflammatory response in islets of patients with diabetes is clearly different from that seen in NOD mice in which inflammatory infiltrates that permeate and often obliterate islets are routinely seen (14). The insulitis that occurs in patients is reduced, involving 0–33% of islets and in only 52% of islets, and the modest cellular infiltrate is predominated by CD8+ T-cells (15,16).
The dynamic process of new β-cell formation and β-cell destruction creates an environment for expression and presentation of new antigenic targets.
The mechanisms involved in the destruction of β-cells have been the subject of intensive research and include direct lysis of β-cells by CD8+ T-cells or other lytic cells, the damaging effects of cytokines that are produced by effector CD4+ T-cells that recognize their antigenic targets on non–β-cells, or even by non–T-cells that release innate inflammatory mediators, such as interleukin (IL)-1β, which can damage β-cells and break tolerance through activation of adaptive responses (17–25). The turnover of β-cells may fuel this process through the expression of new antigens or possibly by post-translational modification of proteins, which has been described in other disease settings (6,26–28). Some experimental evidence suggests that β-cell death throughout the life of NOD mice during development primes autoreactive T-cells. The detection of dead β-cells in pancreatic lymph nodes by day 20 of age and the ability of isolated dendritic cells from these lymph nodes to stimulate the TCR transgenic diabetogenic T-cell, BDC2.5, provides an indication for the role of β-cell death and contraction during development in the priming of autoreactive T-cells (29). β-Cell injury and death may underlie the spreading of specificities of pathogenic autoimmune cells during progression of disease by exposing new epitopes (30). Epitope spreading allows for the activation of new T- and B-cell clones in response to novel antigens that are expressed in the target organ. Isolated T-cells from NOD mice showed a wider ability to respond to novel intramolecular determinants of GAD65, which increased with age (31). In fact, tolerized NOD mice showed reduced recognition of novel epitope by autoreactive T-cells and protection from disease. Not only intramolecular but also intermolecular epitope spreading is observed in the NOD mouse (32).
At least one protein, reg, is involved in β-cell proliferation and can serve as an autoantigen (33,34). In the NOD mouse, array studies have shown reg gene expression during progression of disease as one of the products to which cellular immune responses ultimately develop (26). The recognition of new antigens may be the consequence or the cause of progression. Ott et al. (35) have examined the reactivity of T-cells from type 1 diabetic patients, people at high risk of developing diabetes, and healthy control subjects who respond to GAD65 and proinsulin in vitro. The authors demonstrated increased T-cell reactivity to GAD65 antigens and to proinsulin in both type 1 diabetic patients and high-risk individuals. T-cell reactivity to whole protein and the variable memory response in different type 1 diabetic patients led the authors to conclude that cryptic epitopes are recognized in type 1 diabetes. They found that epitope spreading occurs in these individuals, whereas these cryptic epitopes do not activate T-cells from individuals who do not progress to disease.
The acquisition of antigenicity during progression of diabetes is more accurately described as instructive rather than stochastic. There is an orderly unfolding of antigenic responses over time: there is a hierarchy of antigens. Recent studies by Nakayama et al. and Krishnamurthy et al. support the notion that insulin is a “primary” antigen in NOD diabetes, whereas the islet-specific G6Pase catalytic subunit-related protein (IGRP), clearly an antigen recognized by diabetogenic T-cells later in the course of the disease, appears to be secondary. Insulin 1 and insulin 2 gene knockout mice with a mutated proinsulin transgene (in which residue 16 on the B-chain was changed to alanine) in NOD mice did not develop insulin autoantibodies, insulitis, or diabetes (36). NOD mice tolerized to proinsulin did not develop cytotoxic IGRP-reactive T-cells or diabetes, whereas mice tolerized to IGRP did not develop IGRP reactive T-cells but did develop diabetes (22). These results provide direct evidence that the response against IGRP is downstream of the response to proinsulin in this model. Human data are also consistent with this notion: autoantibody responses to insulin are highest in younger individuals and the risk of disease increases with the number of antigens that are recognized (37–39). Most information on the specificities of antigenic targets is cross-sectional rather than prospective, but the available evidence supports a sequential rather than simultaneous appearance of autoantigen reactivity (38).
Presentation with hyperglycemia reflects a transient shift in the dynamic process that may be precipitated by acute islet inflammation, increased insulin resistance, or other factors.
As β-cell mass declines, a critical level is reached at which small disturbances in either insulin requirements or insulin production can have a large effect on the clinical picture. Acute viral infections are one example. Coxsackie B4 virus has been isolated from the blood of patients with new-onset diabetes, and certain of these isolates have been shown to cause diabetes in mice (40). Histopathologic studies have shown expression of IFN-α in the islets of individuals who have died at the time of presentation of type 1 diabetes (A. Poulous, personal communication). A recent study found evidence for infection with Coxsackie B4 enterovirus in the pancreata of three of six patients with type 1 diabetes whose pancreas was examined up to 9 months after diagnosis (a pancreas graft was studied from one patient) (41). Interestingly, in two of the patients, the number of β-cells was similar to that from three age-matched nondiabetic control subjects, whereas in three patients, the islets with enterovirus showed reduced insulin secretion in response to secretagogues and the virus from the islets could infect islets from nondiabetic donors and cause viral inclusions and pyknosis. There was NK cell (CD94+) and to a lesser extent T-cell infiltration, occasional B-cell and CD68+ cell infiltration, and IFN-α expression in three of the patients, suggesting ongoing or previous islet viral infection.
In addition to reduced insulin production, insulin resistance has been suggested as an accelerator of diabetes at presentation. Insulin resistance has been thought to contribute to the frequent presentation of type 1 diabetes during adolescence when insulin sensitivity declines. Innate immune responses may account both for impaired insulin sensitivity as well as a decline in β-cell function (42). Koulmanda et al. (43) found that “triple therapy” with rapamycin, IL-2 Ig, and IL-15 Ig, which reversed hyperglycemia in NOD mice, dampened the expression of inflammatory genes including IL-1β, tumor necrosis factor-α, and granzyme in diabetic mice. Interestingly, these investigators found that the triple therapy restored insulin signaling in peripheral tissues suggesting that inflammatory mediators may affect responses as well as production of insulin. This is consistent with clinical findings of insulin resistance at the time of presentation of diabetes and the widely recognized role of inflammatory products such as tumor necrosis factor-α in mediating it (44,45). In addition, prospective human data from the Diabetes Prevention Trial have shown that factors other than a precipitous decline in insulin secretion account for clinical presentation. The comparison between the C-peptide responses to either a mixed meal or oral glucose before and after diagnosis showed only a modest difference, where the major effect is on the peak C-peptide response to the metabolic challenge. Basal insulin secretion is relatively well preserved at the time of diagnosis (3,5,46). In our studies of β-cell function before and after diagnosis, the C-peptide response to a mixed-meal test after diagnosis was 87 ± 7% (n = 31) of the prediagnosis response. It was on a third test, in a subset of the original subjects, that was done 6 months or more later, that a more dramatic decrease to 53 ± 13% (n = 10) of the response before diagnosis was seen. In an analysis of the C-peptide response to an oral glucose tolerance test, the total C-peptide area under the curve was reduced by 18% from 6 months before to after diagnosis. These studies suggest that in addition to the decline in C-peptide responses, additional factors are involved in the metabolic decompensation at presentation.
β-Cell area degranulation and regranulation?
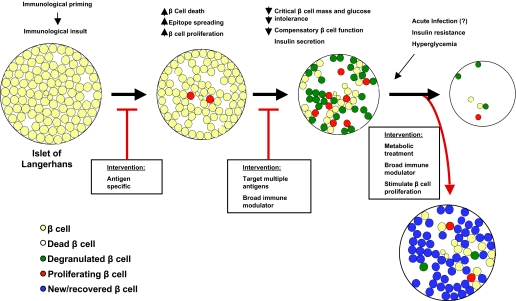
In NOD mice, there is a precipitous drop in the β-cell area at presentation with hyperglycemia (2). The effects of the acute precipitants of disease may not be permanent and may be partially reversible. Indeed, the rapid recovery of β-cell function after the diagnosis during the “honeymoon” period suggests a reversible component to the metabolic dysfunction at diagnosis. Our studies of β-cell mass in NOD mice at diagnosis of type 1 diabetes are consistent with this notion. As noted above, there was a significant increase in β-cell area after treatment with anti-CD3 mAb, but only ∼11% of the β-cells were new (i.e., BrdU+ and insulin-positive). Therefore, ∼90% of the β-cells had been present in the islets at the time of presentation with hyperglycemia. The difference between the total area after correction of hyperglycemia (0.367 ± 0.065 mm2) and the β-cell area found at diagnosis (0.086 ± 0.04 mm2) plus the 11% of the β-cells that had been newly formed (0.037 mm2) does not account for the entire β-cell mass. This suggests that there had been β-cells in the islets at the time of diagnosis that could not be identified by conventional staining with insulin and accounted for over half of the β-cells that were found after correction of hyperglycemia. When we stained islets from mice with new-onset diabetes with antibodies to GLUT2, we identified GLUT2+ insulin cells in the islets at diagnosis, but all of the GLUT2+ cells in the islets after treatment with anti-CD3 mAb were insulin-positive. The GLUT2+ insulin cells that were found in the islets at diagnosis may represent a functionally “exhausted” or degranulated pool of cells that have not been destroyed and may recover. Thus, the precipitation of disease onset may involve a rapidly accelerating cycle in which an initial loss of β-cell function, due to inflammatory mediators, coupled with a reduced β-cell mass after autoimmune destruction, results in modest hyperglycemia, which is met by increased demands on the residual β-cells to produce insulin (Fig. 1). If the expansion of β-cell mass or improvement in function does not keep pace with the demands, additional β-cells may become functionally exhausted and degranulated leading to worsening degrees of hyperglycemia (47,48). The immediate recovery after the initial treatment with immune modulators and/or even insulin involves the net effect of β-cell replication during islet inflammation, but even more significantly, the recovery of β-cell function that was reduced at the time of diagnosis. It should be noted, however, that although insulin treatment and correction of the decompensated metabolic conditions results in temporary improvement in insulin responses in humans, a similar “honeymoon” does not occur in NOD mice treated with insulin. The differences between mice and humans could reflect differences in the relative pace of the autoimmune process or the significant impact of the precipitants of human disease. It may also reflect our relatively poor ability to control the glucose level in NOD mice with new-onset diabetes. This resulting condition, however, is precarious because modest increases in insulin requirements, inflammatory insults to the islets, or progression of the anti-islet adaptive response may result in recurrence of the cycle.
FIG. 1.
Changes in β-cells during progression of type 1 diabetes and the selection of interventions: the initial event that primes the adaptive immune response is not known but is postulated to involve β-cell death. Antigen-specific immune interventions (red arrow) have the greatest chance of efficacy in this early stage. With progression of the response, there are increased rates of β-cell proliferation but also a loss of β-cells and expansion of the immune response. A greater proportion of replicating β-cells is seen in the islet as insulitis progresses, and some β-cells are unable to keep up with the metabolic demands. Most likely, a broader approach to immune intervention is needed at this point, since the autoreactive repertoire is broader than at the earlier stages. Left untreated, there is continued loss of β-cells. However, interventions such as immune modulators can prevent the ongoing adaptive immune response against β-cells and, with metabolic treatment, recovery of degranulated β-cells can occur.
Implications for therapy of diabetes.
These new concepts regarding the dynamics of β-cell mass in type 1 diabetes have implications for the design of clinical interventions that are targeted to arrest the adaptive autoimmune response. First, the total amount of residual β-cell mass is an important variable in the outcome of immune intervention. Metabolic interventions will only be effective if there is sufficient β-cell mass with which they can work. Furthermore, some studies indicate that new β-cells are derived by replication of existing cells and therefore growth of the total β-cell mass will be more substantial with greater starting material (9–11). The clinical data have supported the importance of residual β-cell function in promoting responses to interventions. In the studies of anti-CD3 mAb by Keymeulen et al. (49), the greatest responses to the immune intervention were in individuals with the highest C-peptide levels at the time of diagnosis. To improve the recovery of diabetes in NOD mice after anti-CD3 mAb, we tested the addition of the GLP-1 receptor agonist, exendin-4, to treatment with anti-CD3 mAb (50). Previous studies had indicated that exendin-4 increased replication of β-cells in rodents and had glucose-lowering effects in mice and humans (51–54). We found that exendin-4 enhanced the reversal of diabetes in mice in which the glucose level was <350 mg/dl at the time of diagnosis. These mice would be expected to have a residual β-cell mass that is greater than those with higher glucose levels at diagnosis in which the exendin-4 and anti-CD3 mAb were less effective. The total β-cell area and rate of β-cell proliferation was similar in islets from mice that did or did not receive exendin-4 with anti-CD3 mAb. The exendin-4 treatment did not have an effect on the immune response or the antigenicity of the islets. However, the glucose tolerance of the exendin-4–treated mice and the insulin content of their pancreases were improved compared with those treated with just the anti-CD3 mAb. Thus, it may be possible to enhance the efficacy of single immunologic agents by combining them with agents that have other mechanisms such as enhancing β-cell function. With this combination, the efficacy of the combination will depend on the residual β-cell mass that can be affected by the drug.
Finally, in our studies, immune therapy diminished the replicative response of β-cells that are found during development of diabetes. More than 6 weeks after treatment of NOD mice with anti-CD3 mAb, β-cell replication fell to levels that were less than in younger mice but were still higher than in control (NOD/scid) mice (1.11 ± 0.15 vs. 0.37 ± 0.07%, P < 0.001) (7), possibly reflecting some degree of ongoing inflammation or residual β-cell deficit. The “boost” of β-cell replication that occurs during inflammation may therefore decline, but the recent studies by Nir et al. (8) imply that there may be spontaneous recovery of β-cell mass if inflammation and autoimmunity are prevented.
In summary, clinical and preclinical studies suggest that there is a progressive loss of β-cell mass and function from the initiation of insulitis through presentation with hyperglycemia and afterward. However, the course is undulating and in mice, reflects the opposing factors of β-cell destruction by an expanding adaptive immune response, β-cell replication, and the effects of acute insults with infectious agents and other inflammatory mediators. The accelerated rates of β-cell turnover during progression may be an incendiary to the process by providing and presenting new antigenic targets. This model has largely been developed in mice; human studies have yet to confirm the β-cell replicative process or even the extent of insulitis that is found in the NOD mouse. At the time of diagnosis, a critical threshold of β-cell function has been crossed, but there is potentially recoverable β-cell mass that can restore β-cell function to a prediagnosis level. However, even with recovery, β-cell mass is significantly reduced. Therefore, increases in insulin demand or further progression of the immune process results in significant clinical consequences and further loss of β-cells. The time at which the progression of disease is identified and treated with immune modifiers is of practical importance, since the residual cells may represent the source of new β-cells and combination agents are more likely to be effective if there are β-cells that can respond.
Acknowledgments
This study was supported by grants DK57846 and DK068678 from the National Institutes of Health, grant 1-2007-234 from the Juvenile Diabetes Research Foundation, and a gift from the Brehm Foundation.
REFERENCES
- 1.Sreenan S, Pick AJ, Levisetti M, Baldwin AC, Pugh W, Polonsky KS: Increased beta-cell proliferation and reduced mass before diabetes onset in the nonobese diabetic mouse. Diabetes 48: 989–996, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Sherry NA, Tsai EB, Herold KC: Natural history of β-cell function in type 1 diabetes. Diabetes 54 (Suppl. 1): S25–S31, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Sosenko JM, Palmer JP, Greenbaum CJ, Mahon J, Cowie C, Krischer JP, Chase HP, White NH, Buckingham B, Herold KC, Cuthbertson D, Skyler JS: Patterns of metabolic progression to type 1 diabetes in the Diabetes Prevention Trial-Type 1. Diabetes Care 29: 643–649, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Sosenko JM, Palmer JP, Greenbaum CJ, Mahon J, Cowie C, Krischer JP, Chase HP, White NH, Buckingham B, Herold KC, Cuthbertson D, Skyler JS: Increasing the accuracy of oral glucose tolerance testing and extending its application to individuals with normal glucose tolerance for the prediction of type 1 diabetes: the Diabetes Prevention Trial-Type 1. Diabetes Care 30: 38–42, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Tsai EB, Sherry NA, Palmer JP, Herold KC: The rise and fall of insulin secretion in type 1 diabetes mellitus. Diabetologia 49: 261–270, 2006 [DOI] [PubMed] [Google Scholar]
- 6.von Herrath M, Sanda S, Herold K: Type 1 diabetes as a relapsing-remitting disease? Nat Rev Immunol 7: 988–994, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Sherry NA, Kushner JA, Glandt M, Kitamura T, Brillantes AM, Herold KC: Effects of autoimmunity and immune therapy on beta-cell turnover in type 1 diabetes. Diabetes 55: 3238–3245, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Nir T, Melton DA, Dor Y: Recovery from diabetes in mice by beta cell regeneration. J Clin Invest 117: 2553–2561, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dor Y, Brown J, Martinez OI, Melton DA: Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature 429: 41–46, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Brennand K, Huangfu D, Melton D: All beta cells contribute equally to islet growth and maintenance. PLoS Biol 5: e163, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teta M, Rankin MM, Long SY, Stein GM, Kushner JA: Growth and regeneration of adult beta cells does not involve specialized progenitors. Dev Cell 12: 817–826, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Butler AE, Galasso R, Meier JJ, Basu R, Rizza RA, Butler PC: Modestly increased beta cell apoptosis but no increased beta cell replication in recent-onset type 1 diabetic patients who died of diabetic ketoacidosis. Diabetologia 50: 2323–2331, 2007 [DOI] [PubMed] [Google Scholar]
- 13.In’t Veld P, Lievens D, De Grijse J, Ling Z, Van der Auwera B, Pipeleers-Marichal M, Gorus F, Pipeleers D: Screening for insulitis in adult autoantibody-positive organ donors. Diabetes 56: 2400–2404, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Delovitch TL, Singh B: The nonobese diabetic mouse as a model of autoimmune diabetes: immune dysregulation gets the NOD. Immunity 7: 727–738, 1997 [DOI] [PubMed] [Google Scholar]
- 15.Imagawa A, Hanafusa T, Tamura S, Moriwaki M, Itoh N, Yamamoto K, Iwahashi H, Yamagata K, Waguri M, Nanmo T, Uno S, Nakajima H, Namba M, Kawata S, Miyagawa JI, Matsuzawa Y: Pancreatic biopsy as a procedure for detecting in situ autoimmune phenomena in type 1 diabetes: close correlation between serological markers and histological evidence of cellular autoimmunity. Diabetes 50: 1269–1273, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Itoh N, Hanafusa T, Miyazaki A, Miyagawa J, Yamagata K, Yamamoto K, Waguri M, Imagawa A, Tamura S, Inada M, et al.: Mononuclear cell infiltration and its relation to the expression of major histocompatibility complex antigens and adhesion molecules in pancreas biopsy specimens from newly diagnosed insulin-dependent diabetes mellitus patients. J Clin Invest 92: 2313–2322, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amrani A, Verdaguer J, Serra P, Tafuro S, Tan R, Santamaria P: Progression of autoimmune diabetes driven by avidity maturation of a T-cell population. Nature 406: 739–742, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Katz JD, Benoist C, Mathis D: T helper cell subsets in insulin-dependent diabetes. Science 268: 1185–1188, 1995 [DOI] [PubMed] [Google Scholar]
- 19.Eizirik DL, Darville MI: Beta-cell apoptosis and defense mechanisms: lessons from type 1 diabetes. Diabetes 50 (Suppl. 1): S64–S69, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Cnop M, Welsh N, Jonas JC, Jorns A, Lenzen S, Eizirik DL: Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes 54 (Suppl. 2): S97–S107, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Campbell IL, Kay TW, Oxbrow L, Harrison LC: Essential role for interferon-gamma and interleukin-6 in autoimmune insulin-dependent diabetes in NOD/Wehi mice. J Clin Invest 87: 739–742, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krishnamurthy B, Dudek NL, McKenzie MD, Purcell AW, Brooks AG, Gellert S, Colman PG, Harrison LC, Lew AM, Thomas HE, Kay TW: Responses against islet antigens in NOD mice are prevented by tolerance to proinsulin but not IGRP. J Clin Invest 116: 3258–3265, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Sullivan BJ, Thomas HE, Pai S, Santamaria P, Iwakura Y, Steptoe RJ, Kay TW, Thomas R: IL-1 beta breaks tolerance through expansion of CD25+ effector T cells. J Immunol 176: 7278–7287, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Thomas HE, Irawaty W, Darwiche R, Brodnicki TC, Santamaria P, Allison J, Kay TW: IL-1 receptor deficiency slows progression to diabetes in the NOD mouse. Diabetes 53: 113–121, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Wang B, Andre I, Gonzalez A, Katz JD, Aguet M, Benoist C, Mathis D: Interferon-gamma impacts at multiple points during the progression of autoimmune diabetes. Proc Natl Acad Sci U S A 94: 13844–13849, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vukkadapu SS, Belli JM, Ishii K, Jegga AG, Hutton JJ, Aronow BJ, Katz JD: Dynamic interaction between T cell-mediated beta-cell damage and beta-cell repair in the run up to autoimmune diabetes of the NOD mouse. Physiol Genomics 21: 201–211, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Doyle HA, Zhou J, Wolff MJ, Harvey BP, Roman RM, Gee RJ, Koski RA, Mamula MJ: Isoaspartyl post-translational modification triggers anti-tumor T and B lymphocyte immunity. J Biol Chem 281: 32676–32683, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Doyle HA, Mamula MJ: Posttranslational modifications of self-antigens. Ann N Y Acad Sci 1050: 1–9, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Hoglund P, Mintern J, Waltzinger C, Heath W, Benoist C, Mathis D: Initiation of autoimmune diabetes by developmentally regulated presentation of islet cell antigens in the pancreatic lymph nodes. J Exp Med 189: 331–339, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turley S, Poirot L, Hattori M, Benoist C, Mathis D: Physiological beta cell death triggers priming of self-reactive T cells by dendritic cells in a type-1 diabetes model. J Exp Med 198: 1527–1537, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaufman DL, Clare-Salzler M, Tian J, Forsthuber T, Ting GS, Robinson P, Atkinson MA, Sercarz EE, Tobin AJ, Lehmann PV: Spontaneous loss of T-cell tolerance to glutamic acid decarboxylase in murine insulin-dependent diabetes. Nature 366: 69–72, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zechel MA, Krawetz MD, Singh B: Epitope dominance: evidence for reciprocal determinant spreading to glutamic acid decarboxylase in non-obese diabetic mice. Immunol Rev 164: 111–118, 1998 [DOI] [PubMed] [Google Scholar]
- 33.Gurr W, Yavari R, Wen L, Shaw M, Mora C, Christa L, Sherwin RS: A Reg family protein is overexpressed in islets from a patient with new-onset type 1 diabetes and acts as T-cell autoantigen in NOD mice. Diabetes 51: 339–346, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Gurr W, Shaw M, Li Y, Sherwin R: RegII is a beta-cell protein and autoantigen in diabetes of NOD mice. Diabetes 56: 34–40, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Ott PA, Dittrich MT, Herzog BA, Guerkov R, Gottlieb PA, Putnam AL, Durinovic-Bello I, Boehm BO, Tary-Lehmann M, Lehmann PV: T cells recognize multiple GAD65 and proinsulin epitopes in human type 1 diabetes, suggesting determinant spreading. J Clin Immunol 24: 327–339, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Nakayama M, Abiru N, Moriyama H, Babaya N, Liu E, Miao D, Yu L, Wegmann DR, Hutton JC, Elliott JF, Eisenbarth GS: Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice. Nature 435: 220–223, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verge CF, Gianani R, Kawasaki E, Yu L, Pietropaolo M, Jackson RA, Chase HP, Eisenbarth GS: Prediction of type I diabetes in first-degree relatives using a combination of insulin, GAD, and ICA512bdc/IA-2 autoantibodies. Diabetes 45: 926–933, 1996 [DOI] [PubMed] [Google Scholar]
- 38.Yu L, Rewers M, Gianani R, Kawasaki E, Zhang Y, Verge C, Chase P, Klingensmith G, Erlich H, Norris J, Eisenbarth GS: Antiislet autoantibodies usually develop sequentially rather than simultaneously. J Clin Endocrinol Metab 81: 4264–4267, 1996 [DOI] [PubMed] [Google Scholar]
- 39.Yu L, Robles DT, Abiru N, Kaur P, Rewers M, Kelemen K, Eisenbarth GS: Early expression of antiinsulin autoantibodies of humans and the NOD mouse: evidence for early determination of subsequent diabetes. Proc Natl Acad Sci U S A 97: 1701–1706, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoon JW, Austin M, Onodera T, Notkins AL: Isolation of a virus from the pancreas of a child with diabetic ketoacidosis. N Engl J Med 300: 1173–1179, 1979 [DOI] [PubMed] [Google Scholar]
- 41.Dotta F, Censini S, van Halteren AG, Marselli L, Masini M, Dionisi S, Mosca F, Boggi U, Muda AO, Prato SD, Elliott JF, Covacci A, Rappuoli R, Roep BO, Marchetti P: Coxsackie B4 virus infection of beta cells and natural killer cell insulitis in recent-onset type 1 diabetic patients. Proc Natl Acad Sci U S A 104: 5115–5120, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Caprio S, Plewe G, Diamond MP, Simonson DC, Boulware SD, Sherwin RS, Tamborlane WV: Increased insulin secretion in puberty: a compensatory response to reductions in insulin sensitivity. J Pediatr 114: 963–967, 1989 [DOI] [PubMed] [Google Scholar]
- 43.Koulmanda M, Budo E, Bonner-Weir S, Qipo A, Putheti P, Degauque N, Shi H, Fan Z, Flier JS, Auchincloss H Jr, Zheng XX, Strom TB: Modification of adverse inflammation is required to cure new-onset type 1 diabetic hosts. Proc Natl Acad Sci U S A 104: 13074–13079, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hotamisligil GS, Shargill NS, Spiegelman BM: Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 259: 87–91, 1993 [DOI] [PubMed] [Google Scholar]
- 45.Popa C, Netea MG, van Riel PL, van der Meer JW, Stalenhoef AF: The role of TNF-alpha in chronic inflammatory conditions, intermediary metabolism, and cardiovascular risk. J Lipid Res 48: 751–762, 2007 [DOI] [PubMed] [Google Scholar]
- 46.O'Meara NM, Sturis J, Herold KC, Ostrega DM, Polonsky KS: Alterations in the patterns of insulin secretion before and after diagnosis of IDDM. Diabetes Care 18: 568–571, 1995 [DOI] [PubMed] [Google Scholar]
- 47.Prentki M, Nolan CJ: Islet beta cell failure in type 2 diabetes. J Clin Invest 116: 1802–1812, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Steil GM, Trivedi N, Jonas JC, Hasenkamp WM, Sharma A, Bonner-Weir S, Weir GC: Adaptation of beta-cell mass to substrate oversupply: enhanced function with normal gene expression. Am J Physiol Endocrinol Metab 280: E788–E796, 2001 [DOI] [PubMed] [Google Scholar]
- 49.Keymeulen B, Vandemeulebroucke E, Ziegler AG, Mathieu C, Kaufman L, Hale G, Gorus F, Goldman M, Walter M, Candon S, Schandene L, Crenier L, De Block C, Seigneurin JM, De Pauw P, Pierard D, Weets I, Rebello P, Bird P, Berrie E, Frewin M, Waldmann H, Bach JF, Pipeleers D, Chatenoud L: Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med 352: 2598–2608, 2005 [DOI] [PubMed] [Google Scholar]
- 50.Sherry N, Chen W, Kushner JA, Glandt M, Tang Q, Tsai S, Santamaria P, Bluestone J, Brillantes AM, Herold K: Exendin-4 improves reversal of diabetes in NOD mice treated with anti-CD3 mAb by enhancing recovery of beta-cells. Endocrinology 148: 5136–5144, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Xu G, Stoffers DA, Habener JF, Bonner-Weir S: Exendin-4 stimulates both beta-cell replication and neogenesis, resulting in increased beta-cell mass and improved glucose tolerance in diabetic rats. Diabetes 48: 2270–2276, 1999 [DOI] [PubMed] [Google Scholar]
- 52.Nielsen LL, Baron AD: Pharmacology of exenatide (synthetic exendin-4) for the treatment of type 2 diabetes. Curr Opin Investig Drugs 4: 401–405, 2003 [PubMed] [Google Scholar]
- 53.De Leon DD, Deng S, Madani R, Ahima RS, Drucker DJ, Stoffers DA: Role of endogenous glucagon-like peptide-1 in islet regeneration after partial pancreatectomy. Diabetes 52: 365–371, 2003 [DOI] [PubMed] [Google Scholar]
- 54.Kolterman OG, Buse JB, Fineman MS, Gaines E, Heintz S, Bicsak TA, Taylor K, Kim D, Aisporna M, Wang Y, Baron AD: Synthetic exendin-4 (exenatide) significantly reduces postprandial and fasting plasma glucose in subjects with type 2 diabetes. J Clin Endocrinol Metab 88: 3082–3089, 2003 [DOI] [PubMed] [Google Scholar]