Abstract
OBJECTIVE—Diabetes is associated with an increased risk of death in women. Oxidative stress due to chronic hyperglycemia leads to the generation of reactive oxygen species and loss of chromosomal integrity. To clarify whether diabetes is a premature aging syndrome, we determined telomere erosion dynamics and occurrence of structural chromosomal aberrations in women of the Ludwigshafen Risk and Cardiovascular Health (LURIC) Study.
RESEARCH DESIGN AND METHODS—Telomere lengths and karyotypes were examined in peripheral blood mononuclear cells. Regarding these parameters, surviving and deceased type 2 diabetic women of the LURIC study were compared with nondiabetic LURIC women with or without coronary heart disease and with healthy female control subjects.
RESULTS—Significantly enhanced telomere attrition was seen in all LURIC subjects compared with healthy control subjects. Although the average telomere-length loss is equivalent to well >10 years of healthy aging, telomere erosion was not associated with outcome within the LURIC cohort. However, strikingly high numbers of stable chromosomal aberrations were found in type 2 diabetic women but not in LURIC disease control subjects or in healthy individuals. Furthermore, within the younger age- groups, deceased type 2 diabetes patients had significantly more marker chromosomes than the surviving type 2 diabetic patients.
CONCLUSIONS—All women at high risk for cardiovascular death have accelerated telomere erosion, not caused by type 2 diabetes per se but likely linked to other risk factors, including dyslipidemia. By contrast, the occurrence of marker chromosomes is associated with type 2 diabetes and is a novel risk factor for type 2 diabetes–related early death.
Type 2 diabetes is characterized by increased morbidity and all-cause mortality (1,2). The combination of excess caloric intake and reduced physical activity leading to obesity, dyslipidemia, and hypertension increases the risk for diabetes and coronary heart disease (CHD). Recent data show that among diabetic men, the mortality rate has decreased significantly, whereas in diabetic women, no such trend was found (3). The all-cause mortality rate difference between diabetic and nondiabetic women is considerable. Therefore, the combination of diabetes with multiple risk factors identifies women at particularly high risk (2,4).
The relative risk for morbidity and mortality in women with diabetes is increased compared with nondiabetic control subjects (2,5). Diabetes may therefore be regarded as a premature aging syndrome in which the overall metabolic shift leads to genotoxic stress that results in loss of chromosomal integrity (rev. in 6). Oxidative stress plays a crucial role in the pathogenesis of type 2 diabetes and in diabetes-associated complications. The generation of reactive oxygen species (ROS) is a common downstream mechanism whereby multiple by-products of glucose and (pro)inflammatory molecules exert adverse effects (7–11). DNA damage and telomere attrition can serve as markers of these processes and, consequently, mirror the pace of biological aging (rev. in 12–14).
Hypothesizing along these lines, we studied telomere erosion dynamics and/or the occurrence of structural chromosomal aberrations in women with type 2 diabetes who were participants of the Ludwigshafen Risk and Cardiovascular Health (LURIC) prospective cohort study (15). Life expectancy within the LURIC female cohort falls short by ∼10 years compared with the general female population in Germany.
Telomeric erosion was much further advanced in all LURIC women, irrespective of type 2 diabetes, compared with age-matched control subjects, the difference amounting to >10 life-years. We further found a strikingly enhanced number of structural chromosomal aberrations in the peripheral lymphocytes of women with type 2 diabetes that was diabetes specific and, within the younger age-groups, associated with mortality.
RESEARCH DESIGN AND METHODS
The LURIC study is an ongoing prospective cohort study investigating risk factors for cardiovascular death in Caucasian individuals (15). Between June 1997 and January 2000, 3,266 individuals (2,266 men and 1,002 women) who had undergone coronary angiography were included. All LURIC participants had elective coronary angiography and left ventriculography. CHD was assessed by angiography using the maximum luminal narrowing estimated by visual analysis. Clinically relevant CHD was defined as the occurrence of at least one stenosis ≥20% in at least 1 of 15 coronary segments. Individuals with stenosis <20% were considered as control subjects (16). The institutional review board at the Ärztekammer Rheinland-Pfalz approved the study. Informed written consent was obtained from each participant. All participants were profiled in detail with regard to established risk factors for cardiovascular disease. The cohort is followed for morbidity and mortality. Information on vital status was obtained from local registries. No patient was lost to follow-up. Death certificates were obtained in 97% of dead participants. Two modes of death were classified: cardiovascular death and others. Cardiovascular death included the following categories: sudden death, fatal myocardial infarction, death due to congestive heart failure, death immediately after intervention to treat CHD, fatal stroke, and other causes of death due to CHD. Other modes of death covered all kinds of noncardiovascular deaths; those succumbing to this mode of death were censored.
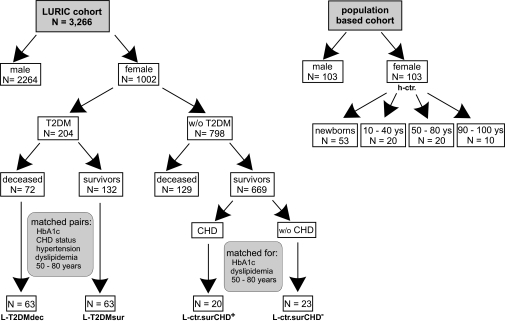
Because the chance to find a novel risk factor in diabetes mortality appeared more promising locking into a high-risk cohort, the present analysis is restricted to women (Fig. 1). Among the 1,002 women who entered the LURIC study, 204 had type 2 diabetes, and 798 had no signs of diabetes at the time of enrollment. Median time of follow-up was 5.45 years. Up to the fixed day (20 January 2000), of the 204 type 2 diabetes subjects, 72 had died (referred to as L-T2DMdec) and 132 had survived (referred to as L-T2DMsur). From the nondiabetic subgroup 129 died, and 669 were still alive. Clinical data of these patients are described in detail in Table 1.
FIG. 1.
Participants and selection criteria. T2DM, type 2 diabetes.
TABLE 1.
List of epidemiological, clinical, and biochemical parameters of the female LURIC participants on whom this study is based
| Parameter | Type 2 diabetes, survivors | Type 2 diabetes, deceased | CAD, no diabetes, survivors | No CAD, no diabetes, survivors | P values (Kruskal-Walis/ANOVA) |
|---|---|---|---|---|---|
| n | 63 | 63 | 20 | 23 | |
| Age (years) | 65.74 ± 8.94 | 66.33 ± 8.99 | 68.33 ± 8.21 | 65.97 ± 7.97 | Matching criteria |
| Type 2 diabetes* | 63 (100) | 63 (100) | 0 (0) | 0 (0) | Selection criteria |
| Diabetes diet | 63 (100) | 63 (100) | 0 (0) | 0 (0) | |
| Death | 0 (0) | 63 (100) | 0 (0) | 0 (0) | Selection criteria |
| Active smoker | 8 (12.7) | 10 (15.9) | 2 (10) | 3 (13) | |
| Hypertension† | 63 (100) | 63 (100) | 12 (60) | 23 (100) | Selection criterion |
| BMI (kg/m2) | 27.26 ± 4.15 | 26.85 ± 4.38 | 27.31 ± 4.24 | 28.63 ± 4.90 | 0.42122 |
| CHD | 43 (68.3) | 50 (79.4) | 0 (0) | 23 (100) | Selection criterion |
| MI | 14 (22.2) | 31 (49.2) | 0 (0) | 14 (60.8) | |
| Stroke | 5 (7.9) | 7 (11.1) | 1 (5) | 1 (4.3) | |
| Dyslipidemia‡ | 63 (100) | 63 (100) | 20 (95) | 23 (100) | Selection criterion |
| A1C (%) | 6.43 ± 1.03 | 6.70 ± 1.70 | 5.74 ± 0.60 | 5.63 ± 0.53 | 0.02320 |
| OAD | 6 (9.5) | 9 (14.3) | 0 (0) | 0 (0) | Selection criterion |
| Insulin treatment | 4 (6.3) | 10 (15.9) | 0 (0) | 0 (0) | Selection criterion |
| Creatinine (μmol/l) | 82 ± 20 | 89 ± 57 | 69 ± 8.8 | 72 ± 11 | 0.00020 |
| Creatinine clearance (Cockroft-Gault formula) | 81.54 ± 24.81 | 77.83 ± 29.22 | 79.03 ± 17.17 | 83.41 ± 25.31 | 0.59448 |
| Creatinine clearance (MDRD-short formula) | 78.22 ± 17.84 | 76.51 ± 22.20 | 79.98 ± 12.09 | 77.22 ± 14.58 | 0.93046 |
| Clearance below 60 ml/min | 5 (7.9) | 10 (15.9) | 1 (5) | 3 (13) | |
| Total cholesterol (mg/dl) | 209.27 ± 45.63 | 206.90 ± 46.05 | 244.30 ± 57.65 | 224.54 ± 48.62 | 0.01976 |
| Triglycerides (mg/dl) | 165.78 ± 78.42 | 182.48 ± 132.40 | 170.95 ± 115.87 | 151.42 ± 46.02 | 0.90922 |
| Fibrinogen (mg/dl) | 407.43 ± 116.5 | 442.67 ± 114.57 | 379.7 ± 113.56 | 351.46 ± 63.15 | 0.00007 |
| Interleukin-6 | 15.96 ± 8.84 | 14.21 ± 14.21 | ND | ND | |
| hsCRP | 10.75 ± 23.08 | 12.37 ± 23.79 | 7.44 ± 15.54 | 3.84 ± 4.39 | 0.00763 |
Data are means ± SD or n (%) of patients.
Diabetes was defined according to World Health Organization/American Diabetes Association criteria; in all probands without known history of diabetes, diabetes was ruled out by an oral glucose tolerance test.
Hypertension defined by the use of antihypertensive drugs and/or systolic blood pressure >140 and/or diastolic blood pressure >90 mmHg.
Dyslipidemia defined by use of lipoid lowering drugs and/or HDL <35 mg/dl, triglycerides >170 mg/dl, and total cholesterol >240 mg/dl. CAD, angiographically proven coronary artery disease; MI, myocardial infarction; ND, not done; OAD, oral antidiabetic drug.
The study combines a cross-sectional analysis and a follow-up approach. From the L-T2DMdec and L-T2DMsur, 63 matching pairs were found on the basis of CHD status, presence versus absence of dyslipidemia and/or hypertension, and age. To test the impact of type 2 diabetes against this high-risk profile, two LURIC control groups were drawn from the surviving nondiabetic patients. Of those, 23 and 20 matched with individuals of the type 2 diabetes cohort for presence of dyslipidemia and age, respectively, solely differing in the presence versus absence of angiographically proven CHD. These LURIC control cohorts are in the following referred to as L-ctr.surCHD+ and L-ctr.surCHD−, respectively (Fig. 1).
As fundamental healthy control subjects, we included and reexamined the large cohort of normal women published by Perner et al. (17) and Mayer et al. (18), comprising newborns to centenarians. To our knowledge and to their own account, these individuals were healthy. The analysis of healthy control subjects had been done using the same probes and the same microscope and software. Blood samples from the LURIC cohort were drawn at baseline between 1997 and 2000. Blood samples from the control subjects were drawn between 1997 and 2002.
Clinical chemistry.
Standard laboratory procedures were used as described previously (15). Specifically, diabetes was diagnosed if 2 h after the oral glucose load, plasma glucose was >1.25 g/l in the fasting state or >2 g/l, respectively, or if individuals were receiving oral antidiabetics or insulin. Aliquots of the 0-h sample were taken for determination of total serum cholesterol and HDL and LDL fractions. A1C (normal range 3.4–6.1%) was measured for all patients using an immunoassay (hemoglobin A1c UNIMATE 5; Hoffmann-LaRoche, Grenzach-Whylen, Germany).
Cell culture and metaphase preparation.
Peripheral blood mononuclear cells were isolated on density gradients (Lymphoprep; Nycomed Pharma, Oslo, Norway) and washed in RPMI-1640 (Life Technologies, Paisley, U.K.) twice before use. Peripheral lymphocytes were cultured and phytohemagglutinin stimulated as described previously to obtain metaphase cells (17). Fifteen metaphases from each individual were karyotyped according to standard criteria (19) and analyzed under an Axioscope microscope (Zeiss, Jena, Germany) equipped with a charged-coupled device camera, linked to the Isis and telomere software (MetaSystems, Altlussheim, Germany). Abnormal chromosomes were detected and further characterized by inverted gray scales using the software.
Peptide nucleic acid probes and fluorescence in situ hybridization.
The peptide nucleic acid (PNA) probe for telomeric sequences is a ready-to-use probe included in the Telomere PNA FISH Kit/Cy3 (Dako, Glostrup, Denmark). The PNA centromeric probe for chromosome 2 was generated by Dako and is available on request. The hybridization was performed according to the manufacturer's instructions and as detailed by Perner et al. (17). Reliability and reproducibility of the telomere/centromere fluorescence in situ hybridization (T/C-FISH) method had been tested by Perner et al. (17). The examiner of the T/C-FISH and cytogenetic analyses (see below) was blinded to the clinical status of the subjects analyzed.
Cytogenetic analysis.
Stable chromosomal aberrations (translocations, insertions, interstitial deletions, and inversions) are easily detectable while performing T/C-FISH and were further characterized by inverted gray scales of the original DAPI counterstaining. The frequencies of marker chromosomes were quantified in all metaphases within each age-group (15 metaphases per person) and expressed in percent. The same procedure was done with healthy control subjects.
Statistical analysis of T/C data.
Normalized data were derived by calculating the ratio of absolute telomere intensities and the centromeric reference signal intensities of each chromosome 2 (T/C value). For each individual, 15 metaphases were analyzed. The primary data were compiled using the telomere software program, and telomere intensities of the p- and q-arms of each chromosome were obtained. From each patient, ∼1,300 single values were analyzed, resulting in an individual median. These individual medians were then used for further statistical analysis, which was performed with SPSS 11.0 and SAS 9.1 for Windows. Individually matched samples were compared using the Wilcoxon's signed-rank test (metric and ordinal variables) and conditional logistic regression to adjust for covariates. The Kruskal-Wallis and the Mann-Whitney U test were used for nonmatched comparisons. Survival distributions were drawn by means of the Kaplan-Meier method and compared using the log-rank test. Regression curves were generated using linear regression or with generalized linear models. Two-sided P values are given; the criterion for statistical significance was P < 0.05. The sample size for the two additional LURIC control groups, L-ctr.sur CHD+ and L-ctr.surCHD−, in relation to the type 2 diabetes LURIC cohort (i.e., L-T2DMdec plus L-T2DMsur) was planned based on estimated means and SDs of telomere lengths in the LURIC cohort and the healthy control group. Here, normal distribution of the data was assumed.
RESULTS
Telomere-length analysis.
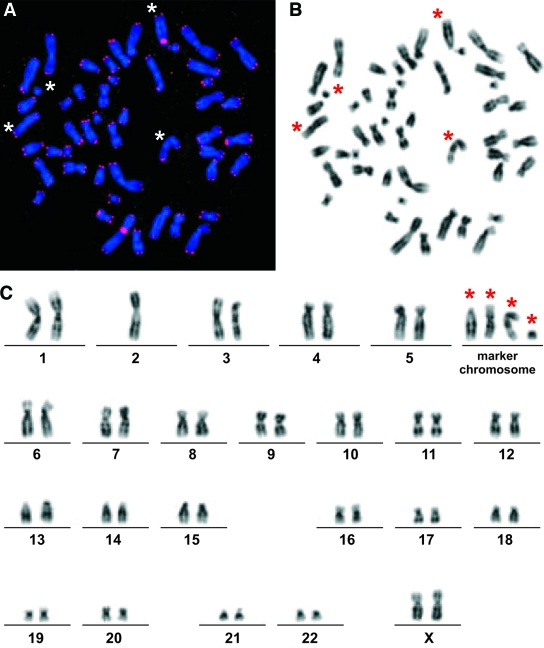
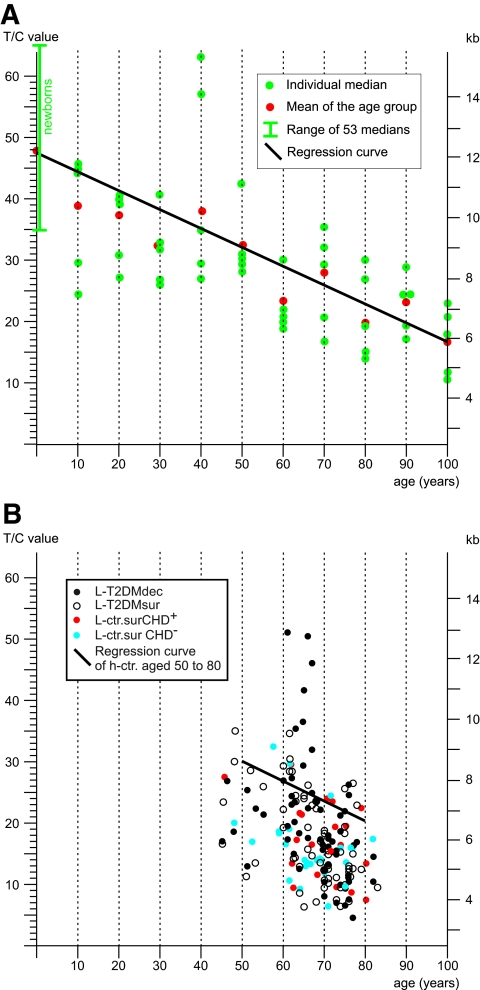
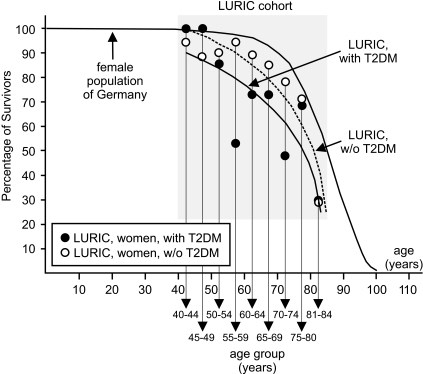
Metaphase chromosomes from mononuclear cells taken at the baseline examination of the type 2 diabetes cohort were subjected to T/C-FISH telomere-length assessment and compared with age-matched healthy control subjects. Figure 2A gives an example of telomeric signals in a single metaphase, Fig. 2B shows the same metaphase in inverted gray scales for karyotyping, and Fig. 2C gives the resulting karyogram. Telomere length was significantly shorter in the entire type 2 diabetes cohort (P = 0.0001) compared with healthy control subjects (see telomere lengths of healthy control subjects in Fig. 3A and telomere lengths of all LURIC subgroups in Fig. 3B). Focusing on outcome, a difference in telomere length between the L-T2DMsur and the L-T2DMdec subjects was not demonstrated using conditional logistic regression (Fig. 3B, black and white dots) (P = 0.104 without adjustment and 0.070 after adjustment for C-reactive protein and the number of marker chromosomes). Overall, the loss in telomere length in type 2 diabetic patients corresponded to a remarkable telomere attrition of ∼1 kb corresponding to >10 years compared with normal age-matched control subjects (Fig. 3B). This number is compatible with the reduced life expectancy of all female LURIC patients. Figure 4 shows the mortality curves of LURIC type 2 diabetic patients, LURIC patients without type 2 diabetes, and for comparison, the mortality curve of the general female population in Germany as issued by the Statistische Bundesamt (20). To verify these results, two further nondiabetic LURIC control groups were defined for comparison with the type 2 diabetes cohort, L-ctr.surCHD+ and L-ctr.surCHD− (Fig. 1). The sample sizes were estimated based on the observed average telomere lengths and SDs of the type 2 diabetes cohort (18.4 ± 9.1) and of the healthy control subjects (26.3 ± 7.5). Under conservative assumption of a difference between means of 7 and a common SD of 10, the power of the comparison is >80% for a control group of a size n = 20. Although L-ctr.surCHD+ (n = 20) and L-ctr.surCHD− (n = 23) showed mean T/C values of 16.3 ± 6 and 17.4, respectively, which are lower than the mean value in type 2 diabetes (18.4 ± 9.1), the differences were not statistically significant (P > 0.57 and 0.94, respectively) (Fig. 3B, red and blue dots). Thus, all LURIC women had accelerated telomere erosion and reduced life expectancy irrespective of type 2 diabetes and/or CHD.
FIG. 2.
A representative metaphase and the corresponding karyogram. A: Metaphase hybridized with PNA probes and counterstained with DAPI. Blue, DAPI; red, FISH with telomere- and chromosome 2-specific probes. B: The corresponding gray scale image (black and white) reveals the chromosome bands that allowed marker chromosomes to be identified (*). C: Corresponding karyogram with the following karyotype: 49, XX,−2,+4mar. (Please see http://dx.doi.org/10.2337/db08-0274 for a high-quality digital representation of this image.)
FIG. 3.
Telomere shortening between healthy control subjects and the LURIC cohort. Telomere shortening of control subjects (h-ctr) (A) and the LURIC cohort (B) is shown by their individual medians (dots) and resulting regression curves. The x-axis gives the age of the donor, and the y-axis gives the T/C ratios of fluorescence intensities on the left and the scale of kilobase pairs (kb) on the right. T2DM, type 2 diabetes.
FIG. 4.
Proportion of survivors of LURIC patients with (n = 204) and without (n = 798) type 2 diabetes according to Kaplan-Meier. The x-axis gives the age or the age-group, and the y-axis gives the percentage of survivors in this age group. The mean survival time in the cohort of patients without type 2 diabetes is 78.5 years and 75.1 years in patients with type 2 diabetes. Survival time between these two groups is different according to the log-rank test (P < 0.0001). Additionally, for comparison only, the corresponding curve of the female population of Germany (20) is shown in which the life expectancy is ∼82 years. Because the 95% CIs for the mean survival time in the patient groups do not cover 82 years, we argue that in both patient groups, life expectancy is lower than in the general population. T2DM, type 2 diabetes.
Chromosomal aberrations.
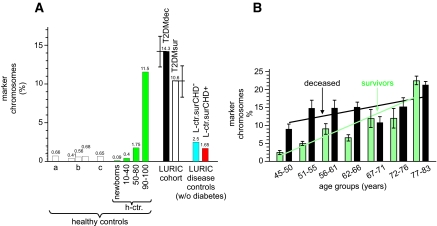
The T/C-FISH technique further allows structural chromosomal aberrations to be identified. The results are based on the number of marker chromosomes (Fig. 2, asterisk) per metaphase, where the proband is the statistical unit. Although the prevalence of marker chromosomes was negligible in newborns and low in the age-matched healthy control subjects, the number of marker chromosomes found in L-T2DMdec and L-T2DMsur was significantly increased (P < 0.0001) and even exceeded that in the advanced-age healthy control subcohort (Fig. 5A).
FIG. 5.
A: Number of marker chromosomes in the LURIC cohort and cohorts of healthy control subjects denotes data taken from Tawn and Cartmell (38) (a); data taken from Kleinerman et al. (39) (b); and prevalence rates of marker chromosomes from Bender et al. (40) (c). A group of newborns (n = 53), young women (n = 20; mean age 25.6 ± 9.8 years), the age-matched cohort to the LURIC cohort (65.3 ± 4.3 years), and a group of women of advanced age (n = 10; mean age 95.2 ± 5.1 years) were recruited into this study. Percentage of marker chromosomes for the LURIC cohort is also given. B: Number of marker chromosomes, in percent, of different age-groups of the LURIC cohort. Each age-group spans 5 years: black, deceased individuals; and white, survivors. The regression line for the deceased patients is indicated by a black line, and that of the survivors is shown in green. The equations for the regression lines and their correlation coefficients are given in the text.
Specifically, we verified the statistically significant difference between the type 2 diabetes cohort and healthy control subjects by comparing type 2 diabetes with L-ctr.surCHD+ and L-ctr.surCHD−. Although in type 2 diabetes, a mean frequency of 12.45% per metaphase was observed, the corresponding values in the 50–80 years subgroup of healthy control subjects were 1.75% (n = 20), 1.65% in L-ctr.surCHD+ (n = 20), and 2.5% in L-ctr.surCHD− (n = 23). No differences could be shown between the three control groups (P > 0.3 in each pairwise comparison); however, each of the control groups differed from type 2 diabetes (P < 0.0001 in each comparison). Therefore, the high frequency of marker chromosomes can be attributed to type 2 diabetes.
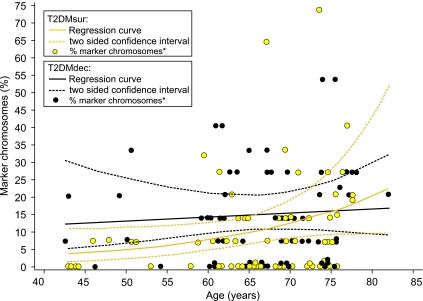
Regarding survival, the number of stable chromosomal aberrations was higher in L-T2DMdec when compared with L-T2DMsur (P = 0.09) (Figs. 5 and 6). Regression analysis (Fig. 5B) yielded an age-dependent increase in the number of marker chromosomes in L-T2DMsur (y = 2.7543x − 0.1629; r2 = 0.8326). However, in L-T2DMdec, only a weak association with age was found (y = 1.17x + 9.7086; r2 = 0.4208). In Fig. 6, the corresponding regression curves fitting the proportion of marker chromosomes per person are given together with 95% CIs for the curves (Fig. 6). They were determined using a generalized linear model with log link function and negative binomial distribution. For L-T2DMsur but not for L-T2DMdec, an association with age was found (P = 0.042 and 0.65, respectively) (Fig. 5B). Furthermore, Fig. 5B suggests a difference in the proportion of marker chromosomes between L-T2DMdec and L-T2DMsur and a rising risk of dying with increase in marker chromosomes for the younger women. Therefore, the 63 case-control pairs (which were all matched for age in yearly intervals) were split into two groups: those younger than the median age (being 69 years) and those older. In the younger group (32 pairs) L-T2DMdec had, in median, one marker chromosome more than the matched L-T2DMsur (P = 0.034, Wilcoxon's signed-rank test), whereas in the older group (31 pairs), no such difference was found (P = 0.86).
FIG. 6.
Regression curves and two-sided CIs of marker chromosomes in L-T2DMdec and L-T2DMsur. They were determined under a generalized linear model with log link function and negative binomial probability distribution. The x-axis gives the age of the donor, and the y-axis gives the number of marker chromosomes in percent. Each dot represents the number of marker chromosomes of one individual patient.
DISCUSSION
In LURIC women, pan-genomic telomere length was significantly shorter compared with healthy control subjects. The accelerated telomere attrition amounts to well >10 years of healthy aging (6,12,18). Although telomere length was not associated with outcome within this cohort, the cohort itself, however, has an increased risk of death, reducing life expectancy by ∼10 years compared with the general female population in Germany (Fig. 4). This study was designed to find chromosomal abnormalities associated with diabetes-related mortality. We intentionally restricted our analysis to women, who are known to carry the greater burden (21,22). Obviously, our core finding that stable marker chromosomes in peripheral lymphocytes are associated with an elevated risk of diabetes-related death in younger women will have to be reevaluated in a similar male setting to gain information on the overall power of this novel risk factor. Our data are in line with recent studies showing that enhanced telomere attrition is associated with atherosclerosis (23,24). Accelerated telomere erosion was found in diabetic patients (25–27). This is confirmed here; however, as evidenced by our disease control subjects, acceleration of telomere attrition is independent of type 2 diabetes. Alternatively, our data strongly suggest that accelerated erosion is caused by dyslipidemia (hypercholesterolemia and hypertriglyceridemia), a state that is frequently associated with type 2 diabetes.
Dyslipidemia leads to an increased production of aldehydes, including methylglyoxal. A direct role for methylglyoxal in telomeric attrition has not been observed so far. However, methylglyoxal induces an increase of free radicals (ROS) (28), and ROS might, albeit indirectly, accelerate telomere attrition (29). Furthermore, methylglyoxal causes stable modification of DNA bases, which in turn induces chromosomal aberrations, sister chromatid exchanges, and micronuclei in human lymphocytes treated in vitro (30). Although there is an enzymatic defense against methylglyoxal-induced DNA mutation, e.g., the enzymes glyoxalase I and aldehyde reductase (31), both enzymes are highly dependent on the concentration of glutathione, which is severely reduced in diabetes (32). Hence, it is conceivable that methylglyoxal-induced chromosomal breaks are incorrectly repaired in diabetes patients.
The rate of chromosome aberrations in human peripheral lymphocytes of healthy individuals has been known to increase with advancing age (rev. in 33), possibly caused by the permanent attack of ROS (14,34), diminished DNA repair (35), altered DNA repair mechanisms (36), and modified T-cell repertoire (37).
The most important finding of our study is the high load of stable chromosomal aberrations in type 2 diabetes. These stable chromosomal aberrations in type 2 diabetic women outnumbered by far the marker chromosomes in our control populations, i.e., healthy control subjects and both LURIC control subgroups, and can be regarded as dramatic also against the background of published data (Fig. 5A). Tawn and Cartmell (38) found a prevalence of 0.66% of stable aberrations in healthy control subjects aged 28–50 years. Kleinerman et al. (39) found a frequency of 0.41 (6 months of age), 0.56 (age range 6–7 years), and 0.68% (age range 18–68 years) in three independent control groups. Likewise, Bender et al. (40) reported a frequency of 0.65% in a cohort of 18- to 68-year-old individuals. These prevalence rates compare well with the rates of marker chromosomes in our healthy control subjects.
The similar proportion of marker chromosomes in the age-groups of deceased individuals suggests the presence of a threshold (∼15%) that when reached, heralds death within the mean follow-up time, i.e., ∼5 years.
At present, a conceptional gap exists between the biomarker “load of marker chromosomes in lymphocytes” and the mechanism of death in type 2 diabetic patients. It is even unclear whether lymphocytes are in this aspect representative of somatic cells or of other tissues or cell systems with their well-known differences in cellular turnover rates. Theoretically, the key players in CHD, i.e., blood monocytes, lymphocytes, endothelial cells, vascular fibroblasts, and smooth muscle cells, while engaged in the injury and repair struggle eventually giving rise to fatal plaque formation and rupture (41), might acquire fatal dysfunctions caused by diabetes-induced DNA damage. There is a wealth of detailed data on diabetes-related malfunctioning of these cells and platelets and on oxidative stress on endothelial cells in atherosclerosis (42). In addition, chromosomal alterations have been found in atherosclerotic plaques (43). Further research aiming at a potentially causal link between DNA damage and cellular malfunction is warranted in the light of our finding that the load of chromosomal markers in lymphocytes of diabetic women is a senescence marker prognosticating premature death.
In summary, the current study provides the largest dataset currently available to address the relationship between stable chromosomal aberrations and telomere length and an association with survival outcome in women affected by type 2 diabetes. We show that telomere shortening is associated with classical cardiovascular disease risk factors other than type 2 diabetes, whereas, in younger women, the occurrence of chromosomal aberrations is associated with type 2 diabetes and directly correlated with the risk of death. Our observation that most women with an increased load of marker chromosomes died during the observation period suggests the presence of a threshold value (6). This assumption might be supported by the fact that the state-of-the-art polypharmacy used to treat the LURIC cohort did not alter the survival outcome (15).
In conclusion, future prospective studies in high-risk populations should take into account telomere dynamics and stable chromosomal alterations as global biomarkers for outcome.
Acknowledgments
B.O.B. has received support from the Institute of Pathology at Ulm University and Deutsche Forschungsgemeinschaft Grants SFB 518 and GRK 1041.
We thank T. Boehm for helpful suggestions. We also thank the German registration offices and local public departments for their assistance. We extend our appreciation to the participants of the LURIC study, because without their collaboration, this study could not have been conducted. We thank the LURIC study team temporarily or permanently involved in patient recruitment, sample, and data handling and the laboratory staff at the Ludwigshafen General Hospital and the Universities of Freiburg and Ulm.
Published ahead of print at http://diabetes.diabetesjournals.org on 23 July 2008.
B.O.B. and P.M. contributed equally to this work.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.International Diabetes Federation: Diabetes Atlas. 2nd ed. Brussels, International Diabetes Federation, 2003
- 2.Hu FB, Stampfer MJ, Solomon CG, et al.: The impact of diabetes mellitus on mortality from all causes and coronary heart disease in women: 20 years of follow-up. Arch Intern Med 161: 1717–1723, 2001 [DOI] [PubMed] [Google Scholar]
- 3.He J, Gu D, Wu X, et al.: Major causes of death among men and women in China. N Engl J Med 353: 1124–1134, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Anand SS, Islam S, Rosengren A, et al.: Risk factors for myocardial infarction in women and men: insights from the INTERHEART study. Eur Heart J 29: 932–940, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Juutilainen A, Lehto S, Ronnemaa T, et al.: Type 2 diabetes as a “coronary heart disease equivalent”: an 18-year prospective population-based study in Finnish subjects. Diabetes Care 28: 2901–2907, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Lombard DB, Chua KF, Mostoslavsky R, et al.: DNA repair, genome stability, and aging. Cell 120: 497–512, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Brownlee M: Biochemistry and molecular cell biology of diabetic complications. Nature 414: 813–820, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Nishikawa T, Edelstein D, Du XL, et al.: Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 404: 787–790, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Yang RZ, Lee MJ, Hu H, et al.: Acute-phase serum amyloid A: an inflammatory adipokine and potential link between obesity and its metabolic complications. PLoS Med 3: e287, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lehrke M, Reilly MP, Millington SC, et al.: An inflammatory cascade leading to hyperresistinemia in humans. PLoS Med 1: e45, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dröge W: Free radicals in the physiological control of cell function. Physiol Rev 82: 47–95, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Cawthon RM, Smith KR, O’Brien E, et al.: Association between telomere length in blood and mortality in people aged 60 years or older. Lancet 361: 393–395, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Valdes AM, Andrew T, Gardner JP, et al.: Obesity, cigarette smoking, and telomere length in women. Lancet 366: 662–664, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Burkle A: DNA repair and PARP in aging. Free Radic Res 40: 1295–1302, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Winkelmann BR, März W, Boehm BO, et al.: Rationale and design of the LURIC study: a resource for functional genomics, pharmacogenomics and long-term prognosis of cardiovascular disease. Pharmacogenomics 2: S1–S73, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Friesinger GC, Page EE, Ross RS: Prognostic significance of coronary arteriography. Trans Assoc Am Physicians 83: 78–92, 1970 [PubMed] [Google Scholar]
- 17.Perner S, Brüderlein S, Hasel C, et al.: Quantifying telomere lengths of human individual chromosome arms by centromere-calibrated fluorescence in situ hybridization and digital imaging. Am J Pathol 163: 1751–1756, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mayer S, Brüderlein S, Perner S, et al.: Sex-specific telomere length profiles and age-dependent erosion dynamics of individual chromosome arms in humans. Cytogenet Genome Res 112: 194–201, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Shaffer LG, Tommerup N (Eds.): ISCN 2005: An International System for Human Cytogenetic Nomenclature. Basel, Karger, 2004
- 20.Genesis-online: Das statistische Informationssystem. Statistisches Bundesamt Deutschland [article online], 2007, Wiesbaden, Germany, Statistisches Bundesamt. Available from https://www-geresis.destatsis.de/genesis/online/. Accessed 24 September 2008
- 21.Juutilainen A, Kortelainen S, Lehto S, et al.: Gender difference in the impact of type 2 diabetes on coronary heart disease risk. Diabetes Care 27: 2898–2904, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Natarajan S, Liao Y, Sinha D, et al.: Sex differences in the effect of diabetes duration on coronary heart disease mortality. Arch Intern Med 165: 430–435, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Aviv A: Telomeres, sex, reactive oxygen species, and human cardiovascular aging. J Mol Med 80: 689–695, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Brouilette SW, Moore JS, McMahon AD, et al.: Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: a nested case-control study. Lancet 369: 107–114, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Sampson MJ, Winterbone MS, Hughes JC, et al.: Monocyte telomere shortening and oxidative DNA damage in type 2 diabetes. Diabetes Care 29: 283–289, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Adaikalakoteswari A, Balasubramanyam M, Mohan V: Telomere shortening occurs in Asian Indian type 2 diabetic patients. Diabet Med 22: 1151–1156, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Obana N, Takagi S, Kinouchi Y, et al.: Telomere shortening of peripheral blood mononuclear cells in coronary disease patients with metabolic disorders. Intern Med 42: 150–153, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Bourajjaj M, Stehouwer CD, van Hinsbergh VW, et al.: Role of methylglyoxal adducts in the development of vascular complications in diabetes mellitus. Biochem Soc Trans 31: 1400–1402, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Liu L, Trimarchi JR, Smith PJ, et al.: Mitochondrial dysfunction leads to telomere attrition and genomic instability. Aging Cell 1: 40–46, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Migliore L, Barale R, Bosco E, et al.: Genotoxicity of methylglyoxal: cytogenetic damage in human lymphocytes in vitro and in intestinal cells of mice. Carcinogenesis 11: 1503–1507, 1990 [DOI] [PubMed] [Google Scholar]
- 31.Thornalley PJ: The enzymatic defence against glycation in health, disease and therapeutics: a symposium to examine the concept. Biochem Soc Trans 31: 1341–1342, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Barati MT, Merchant ML, Kain AB, et al.: Proteomic analysis defines altered cellular redox pathways and advanced glycation end product (AGE) metabolism in glomeruli of db/db diabetic mice. Am J Physiol Renal Physiol 293: F1157–F1165, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Wojda A, Witt M: Manifestations of ageing at the cytogenetic level. J Appl Genet 44: 383–399, 2003 [PubMed] [Google Scholar]
- 34.Sohal RS, Mockett RJ, Orr WC: Mechanisms of aging: an appraisal of the oxidative stress hypothesis. Free Radic Biol Med 33: 575–586, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Lou Z, Chen J: Cellular senescence and DNA repair. Exp Cell Res 312: 2641–2646, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Engels WR, Johnson-Schlitz D, Flores C, et al.: A third link connecting aging with double strand break repair. Cell Cycle 6: 131–135, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Vallejo AN: Immune remodeling: lessons from repertoire alterations during chronological aging and in immune-mediated disease. Trends Mol Med 13: 94–102, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Tawn EJ, Cartmell CL: The effect of smoking on the frequencies of asymmetrical and symmetrical chromosome exchanges in human lymphocytes. Mutat Res 224: 151–156, 1989 [DOI] [PubMed] [Google Scholar]
- 39.Kleinerman RA, Littlefield LG, Tarone RE, et al.: : Chromosome aberrations in relation to radiation dose following partial-body exposures in three populations. Radiat Res 123: 93–101, 1990 [PubMed] [Google Scholar]
- 40.Bender MA, Preston RJ, Leonard RC, et al.: Chromosomal aberration and sister-chromatid exchange frequencies in peripheral blood lymphocytes of a large human population sample. Mutat Res 204: 421–433, 1988 [DOI] [PubMed] [Google Scholar]
- 41.Lusis AJ: Atherosclerosis. Nature 407: 233–241, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng J, Cui R, Chen CH, et al.: Oxidized low-density lipoprotein stimulates p53-dependent activation of proapoptotic Bax leading to apoptosis of differentiated endothelial progenitor cells. Endocrinology 148: 2085–2094, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Matturri L, Cazzullo A, Turconi P, et al.: Chromosomal alterations in atherosclerotic plaques. Atherosclerosis 154: 755–761, 2001 [DOI] [PubMed] [Google Scholar]