Abstract
The present study aimed to define the ability of erythropoietin (EPO) to mobilize hematopoietic stem cells (c-kit+/sca-1+/lin-1−; KSL cells) and hematopoietic progenitor cells (CD34+ cells), including vascular endothelial growth factor receptor 2 expressing hematopoietic progenitor cells (CD34+/Flk-1+ cells). We also sought to determine the role of endothelial nitric oxide synthase (eNOS) in EPO-induced mobilization. Wild type (WT) and eNOS−/− mice were injected biweekly with recombinant erythropoietin (EPO, 1000 U/kg, s.c.) for 14 days. EPO increased the number of KSL, CD34+, CD34+/Flk-1+ cells in circulating blood of wild type mice. These effects of EPO were abolished in eNOS−/− mice. Our results demonstrate that, EPO stimulates mobilization of hematopoietic stem and progenitor cells. This effect of EPO is critically dependent on activation of eNOS.
1. Introduction
In 1997, Asahara and colleagues demonstrated that so-called circulating “endothelial progenitor cells (EPCs)” are mobilized from bone marrow to the site of neovascularization, and participate in the formation of new blood vessels in situ [4]. Currently, there is no consensus in the literature on the phenotypic characteristics of endothelial progenitors. However, prior studies demonstrate that the number of circulating endothelial progenitor cells is inversely correlated with cardiovascular risk factors such as diabetes, hypertension, hyperlipidemia and smoking [17,23,26–28], underscoring the importance of these cells in pathogenesis of vascular disease.
In peripheral blood, hematopoietic progenitor cells (CD34-positive cells) expressing receptor for vascular endothelial growth factor (VEGF), VEGFR2 or Flk-1, have been considered to represent the population of cells enriched with EPCs [1,12,26]. Although the number of CD34+/Flk-1+ cells has been inversely correlated with cardiovascular risk, Case and colleagues recently demonstrated that culturing human CD34+AC133+VEGFR2+ cells or CD34+VEGFR2+ cells do not yield functional endothelial cells [7,8], arguing against their endothelial progenitor nature. Nevertheless, it has been suggested that cells expressing CD34+/Flk-1+ participate in therapeutic neovascularization [20]. It is also important to note that, hematopoietic stem cells, characterized by co-expression of stem cell antigen-1 (Sca-1) and receptor for stem cell factor, c-kit [21,22,24], posses the ability to differentiate into non-hematopoietic tissues, including cardiac myocytes [19].
Erythropoietin (EPO) is a hematopoietic cytokine that stimulates proliferation, survival and differentiation of erythroid precursor cells, as well as maturation of erythroid cells [15]. In addition to erythropoiesis, EPO stimulates proliferation of endothelial cells in vitro [3]. The importance of EPO for repair of injured endothelium and revascularization of ischemic tissues appears to depend on EPO-induced mobilization of circulating progenitor cells [5,12]. However, the exact mechanism underlying the effect of EPO on mobilization of circulating progenitors is unknown.
In the present study, we hypothesized that mobilization of stem and progenitor cells by EPO is critically dependent on activation of eNOS. To test this hypothesis, we enumerated circulating stem/progenitor cells in peripheral blood by flow cytometry and investigated the role of endothelial nitric oxide synthase (eNOS) in EPO-induced mobilization of hematopoietic stem/progenitor cells in wild type and eNOS−/− mice.
2. Methods
Male C57BL/6J (wild-type) and eNOS-deficient mice (C57BL/6J-Nos3tm1Unc) (Jackson Laboratory, Bar Harbor, ME) were distributed into two groups: no treatment (n = 6 mice) and EPO treatment (n = 6 mice, recombinant human EPO 1000 U/kg body weight subcutaneous, bi-weekly, Amgen Thousand Oaks, CA)[10,12]. After 14 days, mice were anesthetized and euthanized by an intraperitoneal injection of pentobarbital (Nembutal Sodium, 60 mg/kg body weight). All the experimental protocols were approved by the Institutional Animal Care and Use Committee of the Mayo Clinic.
To enumerate circulating stem (c-kit+/sca-1+/lin-1−, KSL) cells after EPO treatment, anticoagulated blood was collected by cardiac puncture in anesthetized mice. After removal of red blood cells by Ficoll Paque centrifugation, mononuclear cells in Hank’s Balanced Salt Solution (HBSS, pH 7.4, Invitrogen) containing 0.5% bovine serum albumin and EDTA (1 mmol/l) were incubated at 4°C with rat anti-mouse antibodies (BD Biosciences) against FITC-conjugated Lin-1, PE-conjugated Sca-1, APC-conjugated c-kit (CD117) for 1 hour. IgG antibodies conjugated with the respective fluorochromes were incubated with mononuclear cells in parallel and served as controls. After incubation, cells were washed and fixed in 2% paraformaldehyde and cells were acquired using a FACS Calibur flow cytometer and data analyzed by Cell Quest Software (BD Biosciences, San Jose, CA).
The number of CD34-positive (CD34+) cells in mice was measured by BD Procount Progenitor Cell Enumeration Kit (BD Biosciences, San Jose, CA) according to manufacturer’s instructions. Briefly, 100 µl of mouse blood was divided into two BD Trucount™ tubes containing the Control reagent (IgG1) and CD34 reagent (mouse monoclonal CD34) respectively, and incubated in dark for 15 minutes at room temperature. Subsequently, 450 µl FACS lysing solution was added to each tube, and the cells were acquired using a FACS Calibur flow cytometer and analyzed by Cell Quest Software (BD Biosciences, San Jose, CA).
Percentage of cells positive for both CD34+ and Flk-1+ in the mononuclear fraction was measured by incubating mononuclear cells with rat anti-mouse antibodies (BD Biosciences) against CD34 and Flk-1 for 1 hour in dark and analyzed as described for KSL cells.
The profile of blood cells in wild type and eNOS-deficient mice treated with EPO were performed with ABAXIS VetScan HMII Hematology System (Union City, CA), as reported in our previous study [10].
Results are expressed as means ± SEM and “n” indicates the number of animals. For comparisons between two groups, an un-paired Student's t-test was used where appropriate. Multiple comparisons were performed by one way ANOVA followed by Bonferroni post hoc test. A value of P<0.05 was considered significant.
3. Results
Bi-weekly treatment with EPO for 14 days significantly increased the number of reticulocytes (3 fold), erythrocytes (1.2 fold), hematocrit (1.3 fold) and hemoglobin levels (1.2 fold), while circulating white blood cells and platelets remained unchanged (Table 1). The representative dot plots for quantification of KSL-cells are presented in Supplemental Figure S1. The percentage of KSL-cells was significantly elevated in blood after EPO treatment in wild type mice (Figure 1a), while absence of eNOS abolished the EPO-induced mobilization of KSL-cells. Treatment with EPO produced five-fold increase in circulating levels of CD34+ cells in wild type mice (Supplemental Figure S2 and Figure 1b). The elevation in CD34+ levels by EPO treatment was significantly attenuated in eNOS-deficient mice (Figure 1b). The representative dot plots for quantification of CD34+/Flk-1+ cells are presented in Supplemental Figure S3.The number of cells expressing both CD34 and Flk-1 (VEGFR2), were also significantly increased on EPO-treatment (Figure 1c). In eNOS-deficient mice, EPO failed to elevate the number of circulating CD34+/Flk-1+ cells (Figure 1c).
Table 1.
Profile of Blood Cells in wild type and eNOS-deficient mice after EPO treatment for 14 days
| Parameters | Wild type mice | eNOS−/− mice | ||
|---|---|---|---|---|
| Control | EPO | Control | EPO | |
| White blood cells, 103/mm3 | 11.44 ± 0.62 | 12.18 ± 0.90 | 11.01 ± 1.23 | 10.63 ± 1.17 |
| Lymphocytes, 103/mm3 | 10.47 ± 0.57 | 11.13 ± 0.81 | 9.27 ± 0.89 | 9.74 ± 0.91 |
| Granulocytes, 103/mm3 | 0.72 ± 0.10 | 0.44 ± 0.09 | 1.41 ± 0.58 | 0.38 ± 0.25 |
| Monocytes, 103/mm3 | 0.31 ± 0.07 | 0.82 ± 0.25 | 0.32 ± 0.09 | 0.54 ± 0.31 |
| Red blood cells, 106/mm3 | 10.47 ± 0.20 | 12.96 ± 0.33*** | 10.60 ± 0.22 | 11.56 ± 0.12 |
| Hematocrit, % | 44.04 ± 0.71 | 55.47 ± 0.93*** | 44.76 ± 0.59 | 51.68 ± 0.29*** |
| Hemoglobin, g/dL | 15.11 ± 0.17 | 18.96 ± 0.34*** | 15.23 ± 0.33 | 20.17 ± 0.61*** |
| Platelet, 103/mm3 | 859.4 ± 35.1 | 764.8 ± 31.1 | 808.0 ± 47.9 | 681.0 ± 41.3 |
Data are represented as mean ± SEM of 4–8 mice.
P<0.001 in EPO-treated mice as compared to wild type controls.
Figure 1.
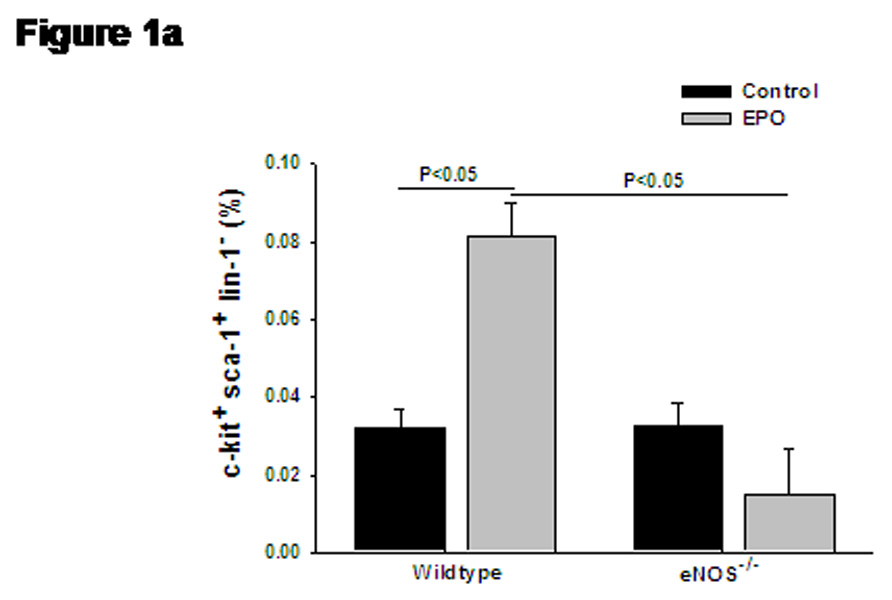
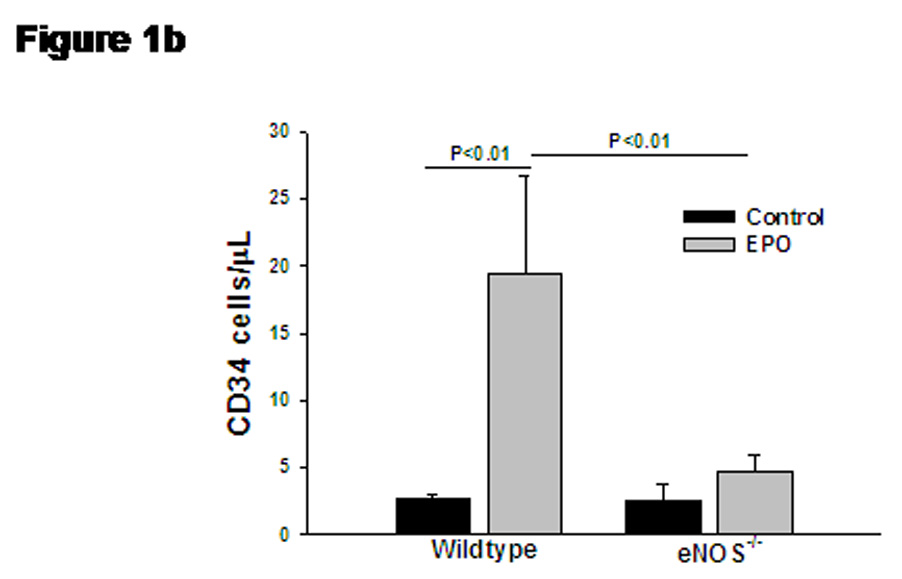
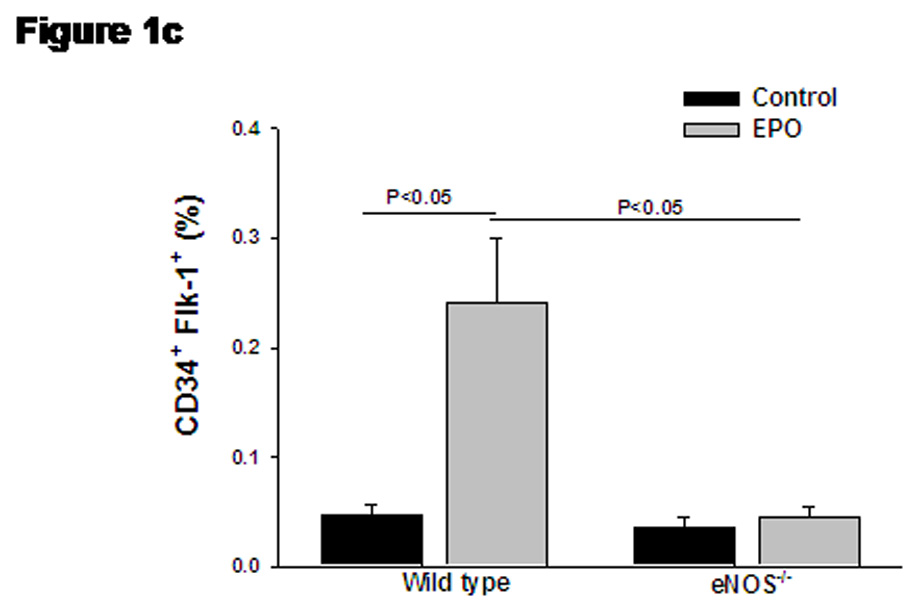
(a) Bar diagram representing c-kit+/sca-1+/lin-1− (KSL) cell population in wild type and eNOS−/− mice after treatment with EPO. Gated mononuclear cells that stained negative for lin-1-FITC were further analyzed for co-expression of sca-1-PE (FL2) and c-kit-APC (FL4), and KSL cell population were represented as percentage of gated events. (b) Bar diagram representing enumeration of CD34+ cells by Procount progenitor cell enumeration kit (BD Biosciences). Events gated in region R3 are cells expressing CD34. (c) Bar diagram representing cells co-expressing CD34+ (FITC, FL-1) and Flk-1 (PE, FL-2), in wild type and eNOS−/− in the presence of EPO. EPO-mediated increase in KSL cells, CD34+ cells and CD34+/Flk-1+ cells were abolished in eNOS−/− mice. Data are represented as mean ± SEM of six mice in each group.
4. Discussion
Our findings demonstrate that activation of eNOS is a critical mechanism responsible for the stimulatory effect of EPO on elevation of KSL, CD34+ and CD34+/Flk-1+ cells in circulating blood. These observations offer important mechanistic insights into the vascular and tissue protective effects of EPO.
In addition to representing hematopoietic stem cells, c-Kit+/Sca-1+/Lin-1− (KSL) cells in circulating blood have also been considered vascular progenitor cells derived from bone marrow [21,22,24]. CD34+ cells (hematopoietic progenitors) are population of cells enriched with EPCs [4,18], and CD34+/Flk-1+ cells participate in therapeutic neovascularization [20]. Recombinant human EPO mobilizes CD34+/Flk-1+ cells from the bone marrow in animals as well as in humans [5,12]. Darbepoetin, an analogue of EPO, also elevated the circulating levels of CD34+ cells in patients with renal anemia [6]. Consistent with these reports, we demonstrated that bi-weekly treatment with EPO significantly mobilized hematopoietic progenitor cells, including VEGFR2-expressing progenitor cells.
Endothelial nitric oxide synthase (eNOS), the enzyme responsible for nitric oxide (NO) production in endothelium, plays an essential role in stem and progenitor cell mobilization in response to VEGF, statins and estrogen [1,13,16]. Impaired VEGF-induced mobilization of EPCs was observed in eNOS-deficient mice [1,11]. Based on the ability of EPO to activate PI3K/Akt pathway (like VEGF and estrogen), it was speculated that mobilization of progenitor cells by EPO was likely to be mediated by eNOS [2,26]. We present evidence demonstrating that indeed, EPO-induced mobilization of progenitor cells is dependent on activation of eNOS. Pro-angiogenic population of cells has now been identified in almost all hematopoietic cell lineages [15]. The ability of EPO to stimulate mobilization of both hematopoietic stem and progenitor cells, irrespective of their phenotypic character, is indicative of the unique ability of EPO to participate in angiogenesis as well as repair of injured endothelium.
Under physiologic conditions, only low numbers of progenitor cells circulate, and mobilization of progenitor cells is assumed to be stimulated by ischemic insults [9]. In accordance, EPO stimulated ischemia-induced angiogenesis and repair of injured arterial wall by mobilization of progenitor cells [5,12,25]. In the present study, we demonstrate that EPO stimulates mobilization of hematopoietic progenitor cells through an Enos-dependent mechanism even in the absence of ischemia. The observed effects of EPO are similar to the effects of VEGF and estrogen. The ability to activate PI3K/Akt pathway provides a plausible signaling mechanism underlying EPO-induced mobilization of progenitor cells [2]. In conclusion, the present study presents evidence that EPO stimulates mobilization of cells known to have beneficial effect on angiogenesis and endothelial repair, and this effect is critically dependent on activation of eNOS.
Supplementary Material
Acknowledgements
This work was supported in part by National Heart, Lung, and Blood Institute Grant HL-53524 (ZSK), the American Heart Association (AHA) Scientist Development Grant (LVD), AHA Postdoctoral fellowship (AVRS), Roche Foundation for Anemia Research and the Mayo Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Aicher A, Heeschen C, Mildner-Rihm C, Urbich C, Ihling C, Technau-Ihling K, Zeiher AM, Dimmeler S. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nature Med. 2003;9:1370–1376. doi: 10.1038/nm948. [DOI] [PubMed] [Google Scholar]
- 2.Aicher A, Zeiher AM, Dimmeler S. Mobilizing endothelial progenitor cells. Hypertension. 2005;45:321–325. doi: 10.1161/01.HYP.0000154789.28695.ea. [DOI] [PubMed] [Google Scholar]
- 3.Anagnostou A, Lee ES, Kessimian N, Levinson R, Steiner M. Erythropoietin has a mitogenic and positive chemotactic effect on endothelial cells. Proc Natl Acad Sci USA. 1990;87:5978–5982. doi: 10.1073/pnas.87.15.5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 5.Bahlmann FH, De Groot K, Spandau JM, Landry AL, Hertel B, Duckert T, Boehm SM, Menne J, Haller H, Fliser D. Erythropoietin regulates endothelial progenitor cells. Blood. 2004;103:921–926. doi: 10.1182/blood-2003-04-1284. [DOI] [PubMed] [Google Scholar]
- 6.Bahlmann FH, DeGroot K, Duckert T, Niemczyk E, Bahlmann E, Boehm SM, Haller H, Fliser D. Endothelial progenitor cell proliferation and differentiation is regulated by erythropoietin. Kidney Int. 2003;64:1648–1652. doi: 10.1046/j.1523-1755.2003.00279.x. [DOI] [PubMed] [Google Scholar]
- 7.Case J, Mead LE, Bessler WK, Prater D, White HA, Saadatzadeh MR, Bhavsar JR, Yoder MC, Haneline LS, Ingram DA. Human CD34+AC133+VEGFR-2+ cells are not endothelial progenitor cells but distinct, primitive hematopoietic progenitors. Exp Hematol. 2007;35:1109–1118. doi: 10.1016/j.exphem.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 8.Case J, Haneline LS, Yoder MC, Ingram DA. Reply to Fadini et al: Critical assessment of putative endothelial progenitor phenotypes. Exp Hematol. 2007;35:1481–1482. doi: 10.1016/j.exphem.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ceradini DJ, Gurtner GC. Homing to hypoxia: HIF-1 as a mediator of progenitor cell recruitment to injured tissue. Trends Cardiovasc Med. 2005;15:57–63. doi: 10.1016/j.tcm.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 10.d'Uscio LV, Smith LA, Santhanam AV, Richardson D, Nath KA, Katusic ZS. Essential role of endothelial nitric oxide synthase in vascular effects of erythropoietin. Hypertension. 2007;49:1142–1148. doi: 10.1161/HYPERTENSIONAHA.106.085704. [DOI] [PubMed] [Google Scholar]
- 11.Fukumura D, Gohongi T, Kadambi A, Izumi Y, Ang J, Yun CO, Buerk DG, Huang PL, Jain RK. Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability. Proc Natl Acad Sci USA. 2001;98:2604–2609. doi: 10.1073/pnas.041359198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heeschen C, Aicher A, Lehmann R, Fichtlscherer S, Vasa M, Urbich C, Mildner-Rihm C, Martin H, Zeiher AM, Dimmeler S. Erythropoietin is a potent physiologic stimulus for endothelial progenitor cell mobilization. Blood. 2003;102:1340–1346. doi: 10.1182/blood-2003-01-0223. [DOI] [PubMed] [Google Scholar]
- 13.Iwakura A, Luedemann C, Shastry S, Hanley A, Kearney M, Aikawa R, Isner JM, Asahara T, Losordo DW. Estrogen-mediated, endothelial nitric oxide synthase-dependent mobilization of bone marrow-derived endothelial progenitor cells contributes to reendothelialization after arterial injury. Circulation. 2003;108:3115–3121. doi: 10.1161/01.CIR.0000106906.56972.83. [DOI] [PubMed] [Google Scholar]
- 14.Kopp HG, Ramos CA, Rafii S. Contribution of endothelial progenitors and proangiogenic hematopoietic cells to vascularization of tumor and ischemic tissue. Curr Opin Hematol. 2006;13:175–181. doi: 10.1097/01.moh.0000219664.26528.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krantz SB. Erythropoietin. Blood. 1991;77:419–434. [PubMed] [Google Scholar]
- 16.Landmesser U, Engberding N, Bahlmann FH, Schaefer A, Wiencke A, Heineke A, Spiekermann S, Hilfiker-Kleiner D, Templin D, Kotlarz D, Mueller M, Fuchs M, Hornig B, Haller H, Drexler H. Statin-induced improvement of endothelial progenitor cell mobilization, myocardial neovascularization, left ventricular function, and survival after experimental myocardial infarction requires endothelial nitric oxide synthase. Circulation. 2004;110:1933–1939. doi: 10.1161/01.CIR.0000143232.67642.7A. [DOI] [PubMed] [Google Scholar]
- 17.Loomans CJ, de Koning EJ, Staal FJ, Rookmaaker MB, Verseyden C, de Boer HC, Verhaar MC, Braam B, Rabelink TJ, van Zonneveld AJ. Endothelial progenitor cell dysfunction: a novel concept in the pathogenesis of vascular complications of type I diabetics. Diabetes. 2004;53:195–199. doi: 10.2337/diabetes.53.1.195. [DOI] [PubMed] [Google Scholar]
- 18.Majka M, Janowska-Wieczorek A, Ratajczak J, Ehrenman K, Pietrzkowski Z, Kowalska MA, Gewirtz AM, Emerson SG, Ratajczak MZ. Numerous growth factors, cytokines, and chemokines are secreted by human CD34+ cells, myeloblasts, erythroblasts, megakaryoblasts and regulate hematopoiesis in an autocrine/paracrine manner. Blood. 2001;97:3075–3085. doi: 10.1182/blood.v97.10.3075. [DOI] [PubMed] [Google Scholar]
- 19.Matsuura K, Nagai T, Nishigaki N, Oyama T, Nishi J, Wada H, Sano M, Toko H, Akazawa H, Sata T, Nakaya H, Kasanuki H, Komuro I. Adult cardiac sca-1-positive cells differentiate into beating cardiomyocytes. J Biol Chem. 2004;279:11384–11391. doi: 10.1074/jbc.M310822200. [DOI] [PubMed] [Google Scholar]
- 20.Masuda H, Asahara T. Post-natal endothelial progenitor cells for neovascularization in tissue regeneration. Cardiovasc Res. 2003;58:390–398. doi: 10.1016/s0008-6363(02)00785-x. [DOI] [PubMed] [Google Scholar]
- 21.Sakihama H, Masunaga T, Yamaashita K, Hashimoto T, Inobe M, Todo S, Uede T. Stromal cell-derived factor-1 and CXCR4 interaction is critical for development of transplant arteriosclerosis. Circulation. 2004;110:2924–2930. doi: 10.1161/01.CIR.0000146890.93172.6C. [DOI] [PubMed] [Google Scholar]
- 22.Sata M. Circulating vascular progenitor cells contribute to vascular repair, remodeling, and lesion formation. Trends Cardiovasc Med. 2003;13:249–253. doi: 10.1016/s1050-1738(03)00106-3. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt-Lucke C, Rossig L, Fichtlscherer S, Vasa M, Britten M, Kamper U, Dimmeler S, Zeiher AM. Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: proof of concept for the clinical importance of endogenous vascular repair. Circulation. 2005;111:2981–2987. doi: 10.1161/CIRCULATIONAHA.104.504340. [DOI] [PubMed] [Google Scholar]
- 24.Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- 25.Urao N, Okigaki M, Yamada H, Aadachi Y, Matsuno K, Matsui A, Matsunaga S, Tateishi K, Nomura T, Takahashi T, Tatsumi T, Matsubara H. Erythropoietin-mobilized endothelial progenitors enhance reendothelialization via Akt-endothelial nitric oxide synthase activation and prevent neointimal hyperplasia. Circ Res. 2006;98:1405–1413. doi: 10.1161/01.RES.0000224117.59417.f3. [DOI] [PubMed] [Google Scholar]
- 26.Urbich C, Dimmeler S. Endothelial Progenitor Cells. Characterization and role in vascular biology. Circ Res. 2004;95:343–353. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 27.Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:e1–e7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 28.Werner N, Koisol S, Schiegl T, Ahlers P, Walenta K, Link A, Bohm M, Nickenig G. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353:999–1007. doi: 10.1056/NEJMoa043814. 2005. [DOI] [PubMed] [Google Scholar]
- 29.Werner N, Nickenig G. Influence of cardiovascular risk factors on endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2006;26:257–266. doi: 10.1161/01.ATV.0000198239.41189.5d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


