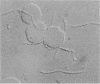
Abstract
Hemagglutination by different Treponema denticola strains was observed for erythrocytes of human, horse, bovine, and rabbit origin. The growth of T. denticola ATCC 33520 in serum-free medium in continuous culture enabled us to study the hemagglutinating activity of freshly harvested spirochetes of a defined physiological status. The hemagglutinating activity was cell bound and not related to motility or appendages, such as fimbriae. The activity was destroyed by proteolytic enzymes, heat, and alkylation, indicating that the agglutinin is of a proteinaceous nature. In addition, periodate oxidation of the spirochetes indicated the involvement of carbohydrate groups. Microscopic inspection of the hemagglutination mixtures at the titration endpoints revealed that only a part of the spirochete population was involved in the hemagglutination process. The hemagglutinating activity was found to be growth phase related. The activity was blocked by serum, while of all tested amino acids and carbohydrates, only sialic acid blocked the activity at low concentrations. In conclusion, we found a hemagglutinating activity in T. denticola which was cell bound and growth phase related. The agglutinin may be a glycoprotein, like lectin, that recognizes sialic acid as a receptor.
Full text
PDF
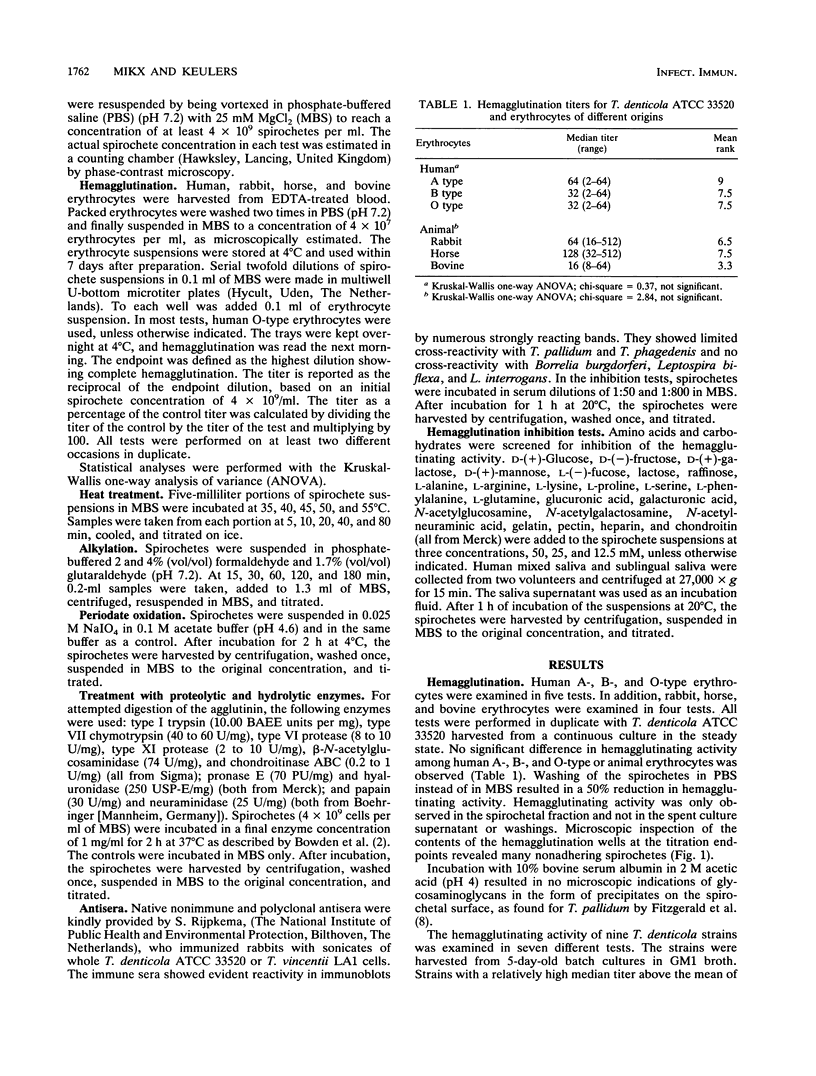
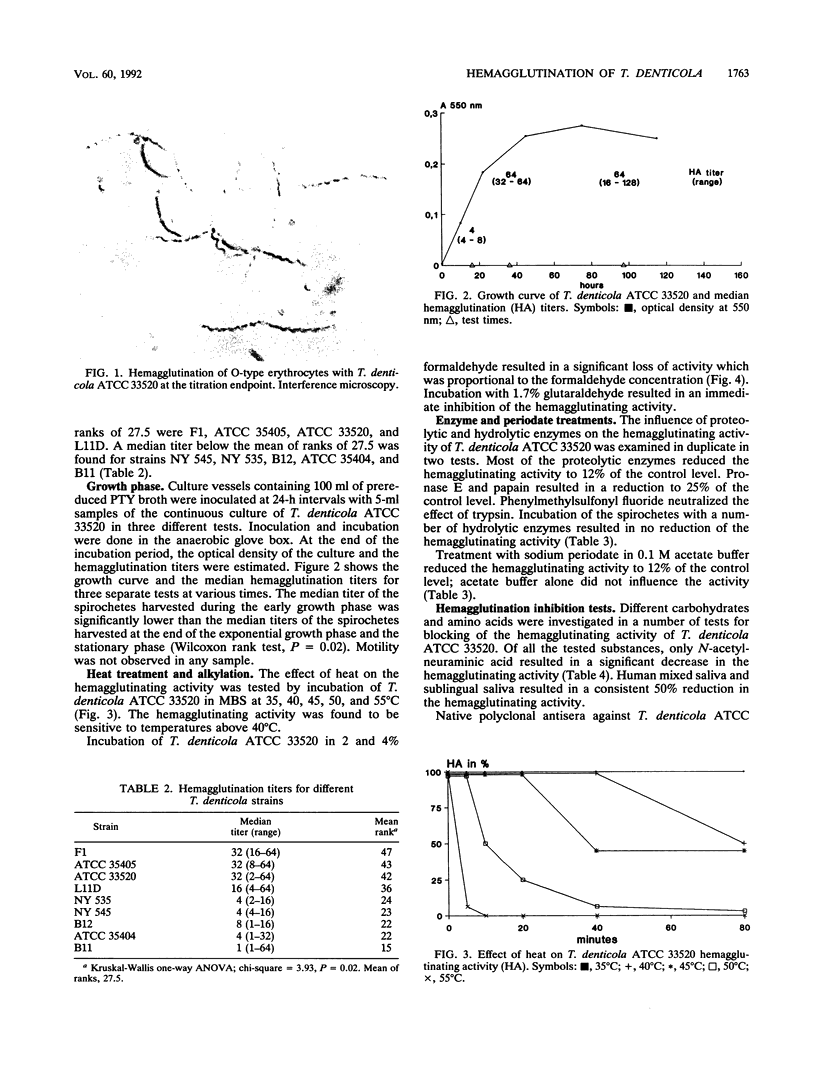
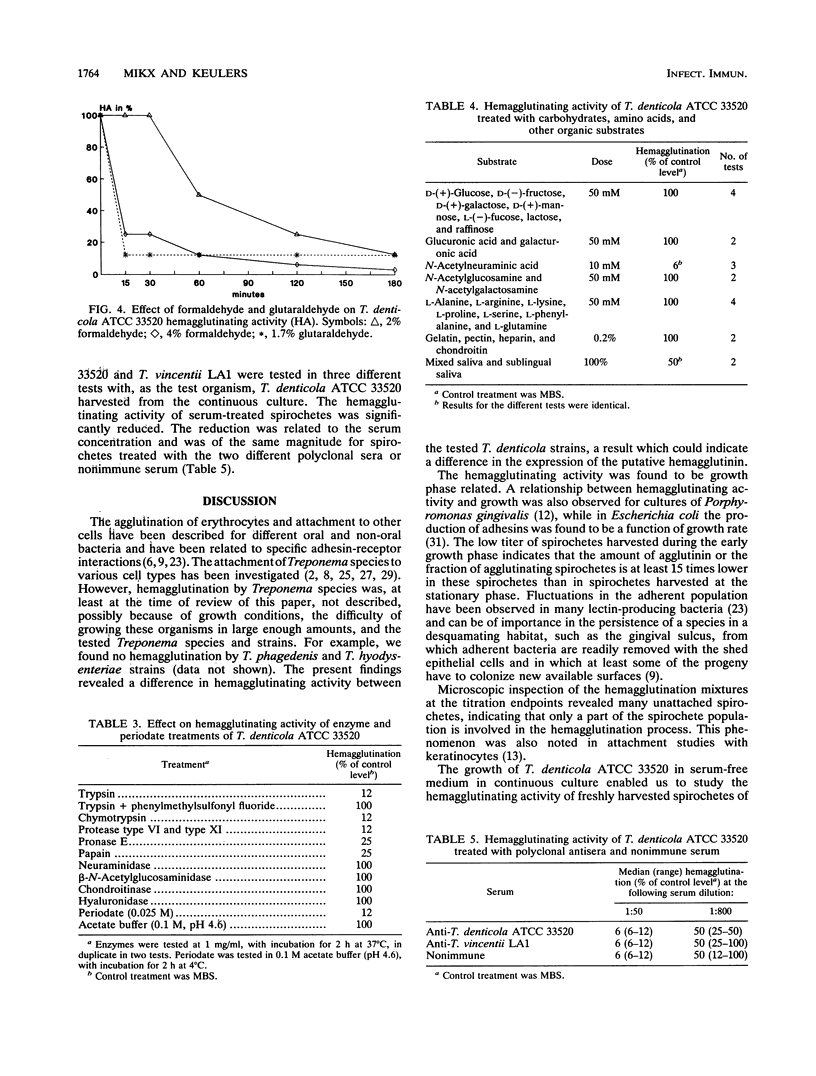


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blakemore R. P., Canale-Parola E. Arginine catabolism by Treponema denticola. J Bacteriol. 1976 Nov;128(2):616–622. doi: 10.1128/jb.128.2.616-622.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden C. A., Joens L. A., Kelley L. M. Characterization of the attachment of Treponema hyodysenteriae to Henle intestinal epithelial cells in vitro. Am J Vet Res. 1989 Sep;50(9):1481–1485. [PubMed] [Google Scholar]
- Cimasoni G., McBride B. C. Adherence of Treponema denticola to modified hydroxyapatite. J Dent Res. 1987 Dec;66(12):1727–1729. doi: 10.1177/00220345870660120601. [DOI] [PubMed] [Google Scholar]
- Dawson J. R., Ellen R. P. Tip-oriented adherence of Treponema denticola to fibronectin. Infect Immun. 1990 Dec;58(12):3924–3928. doi: 10.1128/iai.58.12.3924-3928.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehazya P., Coles R. S., Jr Agglutination of human erythrocytes by Fusobacterium nucleatum: factors influencing hemagglutination and some characteristics of the agglutinin. J Bacteriol. 1980 Jul;143(1):205–211. doi: 10.1128/jb.143.1.205-211.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiehn N. E. Enzyme activities from eight small-sized oral spirochetes. Scand J Dent Res. 1986 Apr;94(2):132–140. doi: 10.1111/j.1600-0722.1986.tb01377.x. [DOI] [PubMed] [Google Scholar]
- Fitzgerald T. J., Johnson R. C., Miller J. N., Sykes J. A. Characterization of the attachment of Treponema pallidum (Nichols strain) to cultured mammalian cells and the potential relationship of attachment to pathogenicity. Infect Immun. 1977 Nov;18(2):467–478. doi: 10.1128/iai.18.2.467-478.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J. Adherent interactions which may affect microbial ecology in the mouth. J Dent Res. 1984 Mar;63(3):378–385. doi: 10.1177/00220345840630030401. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J. Bacterial adhesion to oral tissues: a model for infectious diseases. J Dent Res. 1989 May;68(5):750–760. doi: 10.1177/00220345890680050101. [DOI] [PubMed] [Google Scholar]
- Grenier D. Characteristics of hemolytic and hemagglutinating activities of Treponema denticola. Oral Microbiol Immunol. 1991 Aug;6(4):246–249. doi: 10.1111/j.1399-302x.1991.tb00485.x. [DOI] [PubMed] [Google Scholar]
- Inoshita E., Amano A., Hanioka T., Tamagawa H., Shizukuishi S., Tsunemitsu A. Isolation and some properties of exohemagglutinin from the culture medium of Bacteroides gingivalis 381. Infect Immun. 1986 May;52(2):421–427. doi: 10.1128/iai.52.2.421-427.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LISTGARTEN M. A. ELECTRON MICROSCOPIC OBSERVATIONS ON THE BACTERIAL FLORA OF ACUTE NECROTIZING ULCERATIVE GINGIVITIS. J Periodontol. 1965 Jul-Aug;36:328–339. doi: 10.1902/jop.1965.36.4.328. [DOI] [PubMed] [Google Scholar]
- Laughon B. E., Syed S. A., Loesche W. J. API ZYM system for identification of Bacteroides spp., Capnocytophaga spp., and spirochetes of oral origin. J Clin Microbiol. 1982 Jan;15(1):97–102. doi: 10.1128/jcm.15.1.97-102.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Listgarten M. A. Direct microscopy of periodontal pathogens. Oral Microbiol Immunol. 1986 Nov;1(1):31–38. doi: 10.1111/j.1399-302x.1986.tb00314.x. [DOI] [PubMed] [Google Scholar]
- Listgarten M. A., Helldén L. Relative distribution of bacteria at clinically healthy and periodontally diseased sites in humans. J Clin Periodontol. 1978 May;5(2):115–132. doi: 10.1111/j.1600-051x.1978.tb01913.x. [DOI] [PubMed] [Google Scholar]
- Loesche W. J., Giordano J., Hujoel P. P. The utility of the BANA test for monitoring anaerobic infections due to spirochetes (Treponema denticola) in periodontal disease. J Dent Res. 1990 Oct;69(10):1696–1702. doi: 10.1177/00220345900690101301. [DOI] [PubMed] [Google Scholar]
- Mikx F. H. Comparison of peptidase, glycosidase and esterase activities of oral and non-oral Treponema species. J Gen Microbiol. 1991 Jan;137(1):63–68. doi: 10.1099/00221287-137-1-63. [DOI] [PubMed] [Google Scholar]
- Mikx F. H., Maltha J. C., van Campen G. J. Spirochetes in early lesions of necrotizing ulcerative gingivitis experimentally induced in beagles. Oral Microbiol Immunol. 1990 Apr;5(2):86–89. doi: 10.1111/j.1399-302x.1990.tb00233.x. [DOI] [PubMed] [Google Scholar]
- Mikx F. H., de Jong M. H. Keratinolytic activity of cutaneous and oral bacteria. Infect Immun. 1987 Mar;55(3):621–625. doi: 10.1128/iai.55.3.621-625.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Sharon N. Adhesins as lectins: specificity and role in infection. Curr Top Microbiol Immunol. 1990;151:91–113. doi: 10.1007/978-3-642-74703-8_5. [DOI] [PubMed] [Google Scholar]
- Ohta K., Makinen K. K., Loesche W. J. Purification and characterization of an enzyme produced by Treponema denticola capable of hydrolyzing synthetic trypsin substrates. Infect Immun. 1986 Jul;53(1):213–220. doi: 10.1128/iai.53.1.213-220.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen I. Attachment of Treponema denticola to cultured human epithelial cells. Scand J Dent Res. 1984 Feb;92(1):55–63. doi: 10.1111/j.1600-0722.1984.tb00860.x. [DOI] [PubMed] [Google Scholar]
- Oosterwaal P. J., Matee M. I., Mikx F. H., van 't Hof M. A., Renggli H. H. The effect of subgingival debridement with hand and ultrasonic instruments on the subgingival microflora. J Clin Periodontol. 1987 Oct;14(9):528–533. doi: 10.1111/j.1600-051x.1987.tb00995.x. [DOI] [PubMed] [Google Scholar]
- Reijntjens F. M., Mikx F. H., Wolters-Lutgerhorst J. M., Maltha J. C. Adherence of oral treponemes and their effect on morphological damage and detachment of epithelial cells in vitro. Infect Immun. 1986 Feb;51(2):642–647. doi: 10.1128/iai.51.2.642-647.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saglie R., Newman M. G., Carranza F. A., Jr, Pattison G. L. Bacterial invasion of gingiva in advanced periodontitis in humans. J Periodontol. 1982 Apr;53(4):217–222. doi: 10.1902/jop.1982.53.4.217. [DOI] [PubMed] [Google Scholar]
- Steiner B. M., Sell S., Schell R. F. Treponema pallidum attachment to surface and matrix proteins of cultured rabbit epithelial cells. J Infect Dis. 1987 Apr;155(4):742–748. doi: 10.1093/infdis/155.4.742. [DOI] [PubMed] [Google Scholar]
- Theilade E., Wright W. H., Jensen S. B., Löe H. Experimental gingivitis in man. II. A longitudinal clinical and bacteriological investigation. J Periodontal Res. 1966;1:1–13. doi: 10.1111/j.1600-0765.1966.tb01842.x. [DOI] [PubMed] [Google Scholar]
- Weinberg A., Holt S. C. Interaction of Treponema denticola TD-4, GM-1, and MS25 with human gingival fibroblasts. Infect Immun. 1990 Jun;58(6):1720–1729. doi: 10.1128/iai.58.6.1720-1729.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Verseveld H. W., Bakker P., van der Woude T., Terleth C., de Graaf F. K. Production of fimbrial adhesins K99 and F41 by enterotoxigenic Escherichia coli as a function of growth-rate domain. Infect Immun. 1985 Jul;49(1):159–163. doi: 10.1128/iai.49.1.159-163.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]