Abstract
Ever since investigations in the field of behavioral endocrinology were hatched with experiments on roosters, birds have provided original insights into issues of fundamental importance for all vertebrate groups. Here we focus on more recent advances that continue this tradition, including 1) environmental regulation of neuroendocrine and behavioral systems, 2) steroidogenic enzyme functions that are related to intracrine processes and de novo production of neurosteroids, and 3) hormonal regulation of neuroplasticity. We also review recent findings on the anatomical and functional organization of steroid-sensitive circuits in the basal forebrain and midbrain. A burgeoning body of data now demonstrates that these circuits comprise an evolutionarily conserved network, thus numerous novel insights obtained from birds can be used (in a relatively straightforward manner) to generate predictions for other taxa as well. We close by using birdsong as an example that links these areas together, thereby highlighting the exceptional opportunities that birds offer for integrative studies of behavioral neuroendocrinology, and behavioral biology in general.
Keywords: aggression, reproduction, song, limbic system, vasotocin, photoperiod, neurosteroid, DHEA, aromatase, adult neurogenesis, estrogen, testosterone, thyroxin, hypothalamus, hippocampus, HVC, septum, GnRH, SCN, season, neuroplasticity
Birds offer excellent opportunities for behavioral and neuroendocrine experiments in both the field and the laboratory, and studies conducted at this interface have historically placed avian models on the cutting edge of research (Konishi et al., 1989; also see Wingfield, this issue). Indeed, wild birds offer a combination of accessibility (being diurnal and terrestrial) and diversity (social and ecological) that makes them invaluable models for studying mechanistic and evolutionary questions that are not as tractable in other vertebrate groups. This diversity can be studied within the context of neural and endocrine systems that share many features with those of other taxa (thereby increasing the general relevance of avian studies) (Goodson, 2005), although these features are often more pronounced in birds. Such examples include exceptionally robust and widespread neural aromatase activity, and adult neurogenesis in the forebrain, both of which are considered further below (also see Ball and Balthazart, 2004; Balthazart et al., 2004). Thus, in keeping with its rich history, avian behavioral neuroendocrinology continues to offer an unparalleled proving ground for new and broadly relevant ideas.
Steroid-sensitive circuits of the basal forebrain and midbrain: New ideas on an old system
The social behavior network
During the 1970's, the neural distribution of sex steroid receptors was described for a variety of vertebrate species (Martinez-Vargas et al., 1976; Morrell and Pfaff, 1978). Steroid receptors were localized to many of the same areas across a broad range of taxa, suggesting that the different vertebrate groups possess a common set of core brain structures that coordinate a variety of steroid-dependent behaviors. In mammals, most of these brain regions are now known to comprise a “social behavior network,” which consists of six, bidirectionally connected nodes – the extended medial amygdala (i.e., the medial amygdala and the medial bed nucleus of stria terminalis, BSTm), the lateral septum (LS), the preoptic area (POA), the anterior hypothalamus (AH), the ventromedial hypothalamus (VMH) and midbrain areas such as the periaqueductal gray (PAG) and various regions of the tegmentum. Each of these areas is involved in multiple forms of social behavior, including aggression and sexual behavior (Newman, 1999).
In birds, recent tracing studies demonstrate reciprocal connectivity throughout the same suite of steroid-sensitive areas (identically named, with the exception of the avian medial amygdala, which is known as the nucleus taeniae) (Balthazart et al., 1994; Briganti et al., 1996; Balthazart and Absil, 1997; Cheng et al., 1999; Montagnese et al., 2004; Riters and Alger, 2004), and a variety of functional data are now available regarding their roles in overt aggression, agonistic communication, appetitive and consummatory sexual behavior, parental behavior, and non-sexual affiliation (Balthazart et al., 1998; Thompson et al., 1998; Goodson et al., 1999, 2005; Riters and Ball, 1999; Absil et al., 2002; Goodson and Evans, 2004; Riters et al., 2004; Ruscio and Adkins-Regan, 2004; Charlier et al., 2005). These functional studies have established extensive similarities with other taxa. For instance, investigations of immediate early gene responses in birds (using antibodies for Fos and Zenk, also known as egr-1) have shown that birds and mammals exhibit similar patterns of activation following agonistic encounters (Goodson and Evans, 2004), appetitive sexual behavior and copulation (Ball et al., 1997; Meddle et al., 1997, 1999; Tlemçani et al., 2000; Riters et al., 2004; Charlier et al., 2005). Notably, recent studies have provided much-needed information on various behavioral functions of the avian PAG and adjacent tegmentum (Shaw, 2000; Maney and Ball, 2003; Charlier et al., 2005). These tracings and functional studies have been conducted concomitantly with a large number of immunocytochemical studies, particularly focused on the distribution of neuropeptides and/or their regulation by sex steroids (Aste et al., 1991, 1995, 1997, 1998a; Panzica et al., 1998, 1999b; Goodson et al., 2004a; Plumari et al., 2004). Overall, the data strongly support the proposal that birds possess a “social behavior network” that is homologous to the network identified for mammals (Goodson, 2005; for a more general consideration of the avian forebrain, see Reiner et al., 2004).
Vasotocin, sex steroids and aggression
One of these peptidergic components that has been intensively studied is the arginine vasotocin (AVT) system of the BSTm and LS, and the data generated thus far have both confirmed and extended multiple findings in mammals (review: Goodson and Bass, 2001). As in mammals (which express a homologous neuropeptide, arginine vasopressin), the AVT neuronal population of the BSTm is often sexually dimorphic and innervates the LS in a male-biased fashion (Viglietti-Panzica et al., 1992, 1994; Jurkevich et al., 1999). In most species examined, this system is strongly regulated by testosterone (T) (Voorhuis et al., 1988; Viglietti-Panzica et al., 1992; Aste et al., 1997; Panzica et al., 1999a; Plumari et al., 2004), and data from Japanese quail (Coturnix japonica) show that T regulates AVT primarily via its aromatization to estradiol (E2) (Viglietti-Panzica et al., 2001). Also similar to mammals, AVT acts within the septum to modulate stress responsivity and agonistic behavior (Goodson, 1998a; Goodson and Evans, 2004).
Studies of birds have yielded multiple novel insights into this system, as well. For instance, AVT modulates overt male-male aggression in a species-specific manner (Goodson, 1998a,b; Goodson and Adkins-Regan, 1999; Goodson et al., 2004b), and AVT infusions into the septum have been shown to exert context-specific effects on territorial behavior: AVT facilitates spontaneous, agonistic dawn song in male field sparrows (Spizella pusilla), but inhibits overt male-male aggression (Goodson, 1998a). These species- and context-specific effects likely reflect the fact that AVT acts most strongly on LS responses to stress, not agonistic stimuli per se, as recently shown for male song sparrows (Melospiza melodia) (Goodson and Evans, 2004). In these experiments, AVT receptor blockade was combined with presentation of a severe nonsocial stressor and/or simulated territorial intrusion (STI), followed by analyses of Zenk responses within specific chemoarchitectonic subdivisions of the LS (which are very similar to LS subdivisions in rats; see Goodson et al., 2004a). The results indicate that AVT's effects on agonistic behaviors are secondary to its modulation of broader affective states (e.g., stress or anxiety); thus species differences in AVT's effects may reflect a context-specific relationship between stress and aggression.
Sexual behavior, the POA and aromatase
Birds also offer distinct advantages for the study of male sexual behavior, and a large amount of information is now available on the functional properties of the avian POA, which is essential for male sexual behavior, and its neuroendocrine constituents such as aromatase (Foidart et al., 1994; Balthazart et al., 1996, 1998; Riters et al., 1998, 2004; Castagna et al., 1999; Riters and Ball, 1999). In both mammals and birds, aromatase (estrogen synthetase) mRNA is localized to a variety of areas in the social behavior network, but is most dense within the POA (Balthazart et al., 1990b,c; Foidart et al., 1995; Aste et al., 1998b; for a review of both the mammalian and avian literatures, see Balthazart et al., 2004). However, some aspects of aromatase function may be more easily addressed in birds: Although it is difficult to compare findings generated using different assays (and in different vertebrate groups), the data demonstrate that there is higher aromatase activity in the avian diencephalon compared to mammals. Perhaps because of this relative abundance, aromatase immunolocalization has proven easier in birds, permitting a more detailed cellular inspection of the role of aromatase in sexual behavior (see Balthazart et al., 2004).
Birds also exhibit patterns of appetitive and consummatory sexual behavior that are easily quantified. In quail, these components of sexual behavior are dependent upon different subregions of the medial preoptic nucleus (Balthazart et al., 1998), a sexually-dimorphic structure of the POA (Adkins-Regan and Watson, 1990; Panzica et al., 1996). Quail therefore offer offer good opportunities to investigate specific components of sexual behavior in relation to specific subdivisions of the POA. Both appetitive and consummatory sexual behavior are T-dependent in quail and require the aromatization of T to E2 (Balthazart et al., 1995, 1997), and the POM is an essential target for the activation of behavior (e.g., Riters et al., 1998; for reviews, see Panzica et al., 1996; Balthazart et al., 2004). In addition, recent studies show that pharmacological blockade of aromatase activity produces deficits in both appetitive and consummatory sexual behavior within 15-45 minutes (Cornil et al., 2003; also see Ball and Balthazart, 2004; Balthazart et al., 2004).
Neural and neuroendocrine correlates of sociality
Unlike other vertebrate groups, in which species-typical group size (“sociality”) is typically confounded with other behavioral and ecological variables (e.g., mating system, patterns of parental care, and habitat; see Goodson, 2005), birds offer the opportunity to study sociality while controlling for most other relevant factors. Recently, this advantage has been exploited to determine how differences in species-typical group size relate to immediate early gene activity within the social behavior network (Goodson et al., 2005). Four estrildid finch and waxbill species (one territorial, one modestly gregarious and two highly colonial) were exposed to a same-sex conspecific (or a control condition) in a paradigm that elicited little overt social behavior, thereby allowing an examination of Zenk and Fos responses that are related primarily to social perception and/or motivation. Responses of the extended medial amygdala (nucleus taeniae and BSTm) were negatively correlated with sociality, and showed stepwise variation across species. In addition, the territorial species showed significantly greater responses in the ventrolateral LS, AH and lateral VMH than did all three gregarious species. Importantly, the pattern of response that distinguishes the territorial species is characteristic of animals (birds and mammals) that are exposed to aversive or stressful social stimuli (for discussion, see Goodson, 2005).
Finally, multiple peptidergic systems may coordinate these species-specific responses. Recent experiments (which include an additional territorial species) show that receptor distributions for corticotropin releasing factor, vasoactive intestinal polypeptide, and AVT parallel social structure within numerous brain regions. These include three of the areas listed above -- the LS, VMH and BSTm (J.L. Goodson, A.K. Evans and Y. Wang, unpubl. obs.).
Environmental effects on avian brain and behavior: Tractability and diversity
An issue relevant to all of the topics presented here is the major influence of environmental factors (e.g., changing photoperiod) on brain and behavior (Dawson et al., 2001; Ball et al., 2002). Indeed, the activity of steroid-sensitive circuits (first section above), brain steroidogenesis (next section), and processes related to neural plasticity (second section below) are all seasonally regulated. Although steady advances in our understanding of the environmental regulation of avian behavior and physiology have occurred since pioneering work in the 1920s (Rowan, 1925), recent progress has been particularly remarkable.
Photoperiodic states
Birds undergo pronounced annual changes in physiology, morphology and behavior that facilitate survival and reproduction in varying environments (Murton and Westwood, 1977). This results from both changing external environmental cues, and changing internal responsiveness to those cues (Dawson et al., 2001; Ball, 1993). Seasonally breeding birds pass annually through three physiological states that underlie annual reproductive cycles: sensitivity, stimulation, and refractoriness (Ball and Hahn, 1997). Brains of sensitive birds can mount a reproduction-related neuroendocrine response to environmental cues. Lack of stimulatory external cues in early winter, in combination with sex steroid feedback, hold reproductive development in check (Cockrem, 1995). In late winter and early spring, external cues stimulate the secretion of gonadotropin releasing hormone (GnRH) and gonadotropin (Follett, 1984). Refractoriness to stimulatory cues causes gonadal collapse, and declines in reproductive behavior (Nicholls et al., 1988; Ball et al., 2002). Although large changes in behavior, gonadal physiology, and peripheral endocrine physiology occur in a variety of animals, birds are unusual in the degree to which these changes can be underlain by similarly dramatic changes within the neuroendocrine machinery itself. During absolute photorefractoriness, the down-regulation of the septo-infundibular GnRH system is profound (Ball and Hahn, 1997; Hahn et al., 1997; Dawson et al., 2001). In the few temperate zone taxa that do not become absolutely photorefractory (i.e., Japanese quail, crossbills; Robinson and Follett, 1982; Hahn et al., 2004), down-regulation of the GnRH system does not occur, even though the birds may be relatively refractory (Foster et al., 1987; Pereyra et al., in press; see Nicholls et al., 1988). Stimulation of the reproductive axis can enhance activity of the GnRH system (e.g., Saldanha et al., 1994a), but the most pronounced changes occur during development and dissipation of absolute refractoriness (Ball and Hahn, 1997; Hahn et al., 1997; Sharp, 1996).
The medial-basal hypothalamus, brain deiodinase and photoperiodism
The avian medial-basal hypothalamus (MBH) plays an important role in transducing environmental cues into reproductive signals (Sharp and Follett, 1969; Davies and Follett, 1975; Juss, 1993), and recent evidence indicates that the clock responsible for circadian measurement of daylength is located in the MBH of Japanese quail (Yasuo et al., 2003; Ball and Balthazart, 2003). Although clock genes are expressed rhythmically in the quail SCN and pineal, only in the MBH does the rhythm remain constant under experimental light-dark regimes. The critical role of the MBH in avian photoperiodism (Ball and Balthazart, 2003) is further underscored by the facts that 1) this region probably also contains the deep-brain photoreceptors that are responsible for reproductive photoinduction (Silver et al., 1988; Saldanha et al., 1994b, 2001), and 2) the MBH exhibits significant Fos responses to photic cues (Meddle and Follett, 1997).
The MBH also figures prominently in the effects of thyroid hormones in avian photoperiodism. Thyroid hormones may act either directly (cf. Wilson and Reinert, 1995, 2000) or permissively (cf. Bentley et al., 1997) in photoinduction, photorefractoriness, or both (see Dawson et al., 2001). Central effects (Wilson and Reinert, 2000) likely depend on production of physiologically active thyroid hormone, 3,5,3′-triiodothyronine (T3), which occurs in the MBH (Yoshimura et al., 2003). Light pulses during the photoinducible phase specifically activate transcription of the gene Dio2, which codes for the type 2 iodothyronine deiodinase enzyme responsible for catalyzing conversion of thyroxine (T4) to T3 in the quail MBH. Numerous lines of evidence, including photoinduced changes in MBH T3 and T4 levels, intraventricular infusion of thyroid hormones, and central blocking of the T4 to T3 conversion, all point to a MBH-mediated role of thyroid hormones in avian photoperiodism (Yoshimura et al., 2003).
These findings also dovetail nicely with evidence supporting a role for glia in avian photoperiodism, given that astroglia (specifically, astrocytes and tanycytes) express Dio2 in mammals (Garcia-Segura et al., 1996; Garcia-Segura and McCarthy, 2004). Astroglia may therefore be key in the conversion of T4 to T3 in the avian MBH. Furthermore, in the quail median eminence, glial endfeet apparently create greater separation between GnRH terminals and the basal lamina during short days than during long days, suggesting that the glia may obstruct GnRH secretion (Yamamura et al., 2004). Earlier studies on European starlings (Sturnis vulgaris) suggest that glia may affect synaptic input to GnRH neurons as well (Parry and Goldsmith, 1993).
Diversity of photoperiodism
Although quail have served as a proving ground for many ideas in avian behavioral endocrinology (Balthazart and Ball, 1995), including mechanisms of photoperiodism (e.g., Follett, 1984; Ball and Balthazart, 2003), exploration of the diversity of avian reproductive cycles, from strictly seasonal through temporally opportunistic, is required to clarify the adaptive significance of a given mechanism. For example, seasonal plasticity of the GnRH system is greater in taxa that become absolutely photorefractory, such as European starlings and house finches (Carpodacus mexicanus), than in those that do not, such as quail and crossbills (Loxia sp.) (Foster et al., 1987, 1988; Cho et al., 1998; MacDougall-Shackleton et al., 2001; Pereyra et al., in press). These differences are correlated with the degree of reproductive flexibility displayed (Hahn et al., 1997; MacDougall-Shackleton et al., 2005). Comparisons among taxa that vary in reproductive flexibility, and in the form of refractoriness that develops, may help reveal the extent to which processes such as glial ensheathment and deiodinase activity contribute to short-term modification of GnRH release versus long-term down-regulation of GnRH production. Studying temporally opportunistic taxa (cf. Hahn et al., 1995), such as crossbills, that rely heavily on non-photic cues to time changes in reproductive condition (Hahn, 1995; Hahn et al., 1995, 2005) should clarify whether mechanisms underlying processing of photic cues apply to other kinds of cues, such as food supply, as well.
Neural steroidogenic enzymes: Intracrinology and neurosteroids
The central nervous system has traditionally been considered to be a receiver of sex steroids produced by the gonads and adrenals. Over time, this idea has been modified, with increasing emphasis on the brain's ability to synthesize sex steroids independently. Such considerations have important consequences for interpreting correlations (or lack thereof) between behavior and plasma sex steroid levels, and interpreting effects (or lack thereof) of castration on behavior.
Aromatase
Aromatase catalyzes the conversion of AE to E1, and of T to E2 (Fig. 1). Aromatase was initially characterized in the ovary and then detected in multiple areas of the basal forebrain in mammals (Naftolin et al., 1971, 1972), raising the hypothesis that some effects of gonadal T on sexual and aggressive behavior are dependent upon local conversion of T to E2 by brain aromatase. Indeed, a variety of data now show that brain aromatase is a key mediator of the link between T and behavior, and research on birds has provided seminal insights into 1) the cellular localization of aromatase protein and mRNA in neurons (Balthazart et al., 1990c; Shen et al., 1994), 2) the presence of aromatase at the synapse (Schlinger and Callard, 1989; Naftolin et al., 1996; Peterson et al., submitted), 3) hippocampal aromatase expression (Vockel et al., 1990; Saldanha et al., 2000), 4) the expression of aromatase in glia (Schlinger et al., 1994), 5) the regulation of aromatase by endocrine and environmental factors (Hutchison et al., 1986; Balthazart et al., 1990a; Schlinger and Callard, 1990), and 6) behavioral functions of aromatase (Adkins 1975; Adkins and Nock, 1976; Schumacher et al., 1984; and see first section).
Figure 1.
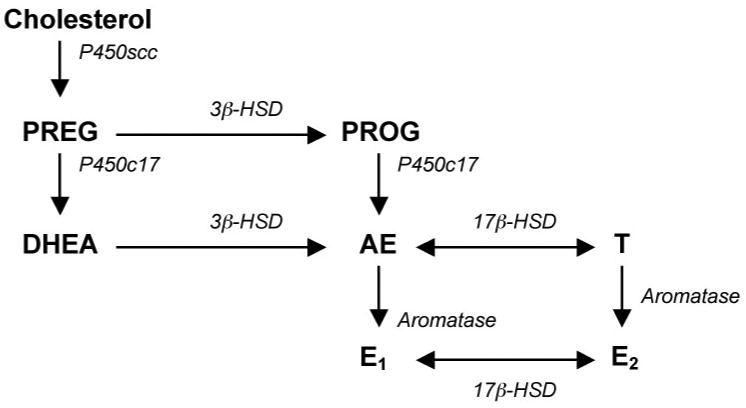
Simplified diagram of sex steroid synthesis. Steroids are in bold; enzymes are in italics. Steroids: PREG=pregnenolone; PROG=progesterone; DHEA=dehydroepiandrosterone; AE=androstenedione; T=testosterone; E1=estrone; E2=17β-estradiol. Enzymes: P450scc=Cytochrome P450 side chain cleavage; P450c17=Cytochrome P450 17α-hydroxylase/C17,20 lyase; 3β-HSD=3β-hydroxysteroid dehydrogenase/isomerase; 17β-HSD=17β-hydroxysteroid dehydrogenase; Aromatase=Cytochrome P450 aromatase.
Several examples of recent work demonstrate that avian studies continue to make novel contributions to this field. First, most studies of aromatase focus on the brain, but aromatase was recently detected in the spinal cord of quail (Evrard et al., 2000; Evrard and Balthazart, 2004a). Interestingly, spinal cord aromatase modulates nociception in quail, indicating a novel function for this enzyme (Evrard and Balthazart, 2004b, 2004a). Second, studies in quail also indicate that aromatase activity is rapidly regulated within minutes by calcium-dependent phosphorylation (Balthazart et al., 2001a,b, 2003). Traditionally, aromatase has been thought to be regulated on a longer time scale, involving gene transcription. Rapid modulation opens new directions for research and has major implications for understanding the functions of aromatase. Third, in songbirds, recent ultrastructural studies have examined the regulation of aromatase in terminals within the hippocampus, hypothalamus, and song nuclei (Saldanha et al., 2000; Peterson et al., submitted). Such studies are critical to understanding the function of aromatase at the synapse, which has remained enigmatic. Importantly, this line of inquiry is greatly facilitated by the relatively high levels of aromatase in the avian nervous system.
Intracrinology
The “aromatase hypothesis” no longer portrayed the brain as a passive recipient of gonadal sex steroids, but in general researchers still assumed that the brain could only aromatize T that was produced in the gonad. However, it is becoming increasingly clear that the adrenal glands can play a major role in supplying sex steroids to the nervous system. For example, the sex steroid precursor DHEA (Fig. 1) and its sulfated ester (DHEA-S) are secreted in abundant quantities by the human adrenal cortex (Thijssen and Nieuwenhuyse, 1999). Circulating DHEA can be locally metabolized to sex steroids within peripheral target tissues (e.g., prostate, skin), a phenomenon Labrie and colleagues termed “intracrinology” (Labrie, 1991). Interestingly, DHEA metabolism to sex steroids also occurs within the human brain (Milewich et al., 1991) and rodent brain (Zhao et al., 1991; Zwain and Yen, 1999; Jellinck et al., 2001).
Recent studies in birds suggest that DHEA plays an important role in the control of territorial aggression. For example, in field studies of male song sparrows, territorial behavior is robustly expressed during non-breeding season (autumn and early winter) (Wingfield and Hahn, 1994; Soma and Wingfield, 1999), although plasma T, 5α-DHT, AE, E2 and E1 are non-detectable at this time (Wingfield and Hahn, 1994; Soma and Wingfield, 1999). The testes are also completely regressed during the autumn (Soma and Wingfield, 1999) and castration does not decrease aggression in the non-breeding season (Wingfield, 1994). In contrast, aromatase inhibition does decrease aggression in autumn (Soma et al., 1999, 2000a,b), despite the fact that plasma levels of aromatizable androgens (AE and T) are non-detectable (<0.1 ng/ml). However, plasma levels of DHEA are detectable (on average ∼0.8 ng/ml, up to 1.7 ng/ml) (Soma and Wingfield, 2001), and DHEA levels in the adrenals and regressed testes are also high. In autumn, DHEA treatment increases territorial singing and the size of the song nucleus HVC, similar to the effects of T and E2 (Soma et al., 2002, 2004b; Tramontin et al., 2003). DHEA treatment, unlike T, does not stimulate growth of the cloacal protuberance, a peripheral secondary sex character (Soma et al., 2002), and does not suppress immune function in non-breeding sparrows (Owen-Ashley et al., 2004).
Recently, DHEA metabolism by 3β-HSD (Fig. 1) in songbird brain has been characterized in zebra finches (Soma et al., 2004a). In song sparrows, brain tissue metabolizes DHEA to AE and estrogen, and DHEA metabolism is higher in autumn than spring across several forebrain regions (K. Soma, D. Wacker, J. Wingfield and B. Schlinger, unpub. obs.). Interestingly, brain 3β-HSD activity is rapidly modulated (Soma et al., 2004a), similar to brain aromatase (see above). Taken together, these data support the hypothesis that non-breeding song sparrows combine adrenal and/or gonadal DHEA synthesis with neural DHEA metabolism to maintain territorial behavior in the absence of gonadal T secretion. Moreover, it remains possible that the brain itself is a significant site of DHEA synthesis (see below).
Similar results have been obtained in field studies of the spotted antbird (Hylophylax n. naevioides), a tropical bird in which both sexes aggressively defend territories year-round. Spotted antbirds have basal plasma T levels throughout the year, but nonetheless, a combined treatment of aromatase inhibitor plus androgen receptor antagonist decreases male aggression in the breeding season (Hau et al., 2000). In the non-breeding season, both sexes show high levels of aggression, primarily to intruders of the same sex (Hau et al., 2004). Both males and females have elevated levels of plasma DHEA in the non-breeding season, and in males, plasma DHEA levels are positively correlated with aggressive vocalizations and/or the length of territorial intrusions (Hau et al., 2004). These data suggest that DHEA regulates aggression in this tropical species, possibly in both males and females.
Neurosteroids
Groundbreaking studies by Baulieu and colleagues suggested that the brain can synthesize DHEA and other steroids de novo from cholesterol (designated as “neurosteroids”) (Corpechot et al., 1981). Despite great advances in understanding the biochemistry of neurosteroid synthesis, much remains unknown regarding the physiological functions of neurosteroids and the selective forces that favor evolution of such local endocrine signalling.
Recent studies in birds suggest that sex steroids can be synthesized entirely within the brain, both during development and adulthood. Studies by Tsutsui and colleagues have identified P450scc, P450c17, and 3β-HSD (Fig. 1) in the brain of quail (Tsutsui and Yamazaki, 1995; Tsutsui et al., 2003). These novel experiments have successfully combined measurements of enzyme mRNA, protein, and activity with measurements of steroids in plasma and brain tissue. Regional analyses of neural steroidogenic enzymes reveal high expression in the telencephalon and diencephalon, as well as Purkinje neurons of the cerebellum (Ukena et al., 1999; Matsunaga et al., 2002). The functions of neurosteroids in Purkinje neurons are currently under investigation.
In the zebra finch (Taeniopygia guttata), profound male-biased sex differences in the telencephalic song control system (Nottebohm and Arnold, 1976) arise during early development (Gurney and Konishi, 1980). Estrogen treatment of female zebra finches during development masculinizes the song system (Gurney and Konishi, 1980), but experimental manipulations that produce functional testes in genetic females do not masculinize the song system (Wade and Arnold, 1996). Recent data suggest that neurosteroids, but perhaps not gonadal steroids, are important for the development of a male pathway from the song nucleus HVC to the robust nucleus of the arcopallium (RA) in the song circuit (Holloway and Clayton, 2001; Schlinger et al., 2001). In organotypic slice cultures from developing animals, innervation of the song nucleus RA by HVC neurons occurs in slices from males but not females (Holloway and Clayton, 2001), and treatment of male slices with an aromatase inhibitor reduces the innervation of RA. This is striking because the slices were cultured in a medium with low sex steroids. In addition, measurements of E2 in the conditioned medium indicate that estrogen is produced de novo in the slices, with higher E2 production in male slices than female slices. Studies in vivo demonstrate neural expression of mRNA for P450c17 during development, although no sex differences in P450c17 mRNA were detected in brain (London et al., 2003). Taken together, these studies suggest that neurosteroids regulate formation of the HVC-RA pathway in males, but the role of neurosteroids in song nucleus size dimorphism requires further investigation (Arnold, 2004).
Adult neurogenesis and steroid-mediated neuroplasticity: Bird is the word
Although overt changes in adult neuron number and morphology were previously relegated to the realm of “lower” vertebrates, the realization that such changes occur in mammals has rejuvenated inquiry into the structural reorganization of the CNS. This perspective has increased our appreciation of the structural indices whereby stimuli (including hormones) alter neurons and neuronal networks. Here we discuss the contribution of avian models to our understanding of how changes in the size, number, and projections of neurons may modulate adult CNS function.
Adult neurogenesis
Although neurogenesis was reported in the brains of adult mammals beginning in the early 1960s, these studies were viewed with considerable skepticism (see Gould and Gross, 2002, for review). However, based on ultrastructural, peptidergic, and electrophysiological characteristics, several landmark studies later reported abundant neurogenesis in the brains of adult canaries (Serinus canaria; Goldman & Nottebohm, 1983; Paton & Nottebohm, 1984; Alvarez-Buylla & Nottebohm, 1988). These observations changed our appreciation of plasticity in the adult vertebrate brain: Not only was the abundance of these mitotic neurons higher than previously suggested, but more rigorous criteria were used to support the inference that the mitotic cells were truly neurons (see above). In only two decades, we have learned that precursor cells located in the subventricular zone (SVZ; Goldman & Nottebohm, 1983; Goldman et al., 1996) give rise to radial glia and neurons within the SVZ itself (Alvarez-Buylla et al., 1990). These new neurons migrate along radial glia (Alvarez-Buylla & Nottebohm, 1988) into HVC, a pallial song control nucleus critical for sensorimotor integration (Goldman et al., 1993). The observation that precursor cells from adult canaries can differentiate into neurons (Goldman, 1990; Goldman et al., 1992), migrate along radial glia (Goldman et al., 1993) and become functionally active (Goldman & Nedergaard, 1992) in vitro, strongly underscores the inherently robust nature of adult neurogensis in birds. Indeed, in vivo, the canary HVC exhibits an orchestrated balance of neuronal turnover with peaks of cell death in August-September and January, each of which is followed by peaks of neurogenesis in October and March (Kirn et al., 1994). Although the precise functional role of neuronal replacement remains enigmatic, it is clear that newly born neurons in the adult canary brain project from HVC to RA (Kirn & Nottebohm, 1993) and may underlie changes in song learning and production (Alvarez-Buylla et al., 1990; Kirn et al., 1994). Presumably, this rich history of adult neurogenesis in passerines and other birds will provide important touchstones for the developing topic of adult neurogenesis in mammals, including humans. In particular, the balance between cell death and neurogenesis, and the possible modulation of cell birth by cell death (Scharff et al., 2000) provide exciting avenues of research thar are easily applicable to clinical situations in humans.
Seasonal adult neuroplasticity in the song system
The dynamic nature of the avian brain is perhaps most obvious in the oscine suborder of the family Passeriformes, which contains the true songbirds (i.e., perching birds that are known to learn their songs). Specific portions of the adult songbird brain undergo dramatic changes in volume across seasons. These changes have been exhaustively described in nuclei of the song circuit (Tramontin & Brenowitz, 2000) and, to a lesser extent in the hippocampus (Smulders et al., 1995; 2000). Several photoperiodic passerines from different families demonstrate increases in the volume of the song nuclei HVC, RA, Area X and MAN in the summer relative to the winter. These changes are not subtle: HVC volume changes 2-fold in some songbirds. Changes in song nuclei do not reflect non-specific changes in the size of the telencephalon, and for most of these brain nuclei, the volume increase is due to a combination of increased cell number, cell size, cell spacing, dendritic arborization, and/or synaptic density. These changes are believed to underlie the considerable increase in singing behavior observed in the summer, and may mediate vernal changes in song structure and repertoire in some species (Nottebohm, 2004).
Circulating steroids, particularly T, are implicated in the regulation of song circuit plasticity. Administration of T, or its metabolite E2, increases neuronal number and size in HVC and Area X in song sparrows, white-crowned sparrows (Zonotrichia leucophrys gambelii), and dark-eyed juncos (Junco hyemalis), and increases neuron size in RA (Gulledge & Deviche, 1995; Tramontin et al., 2003; Soma et al., 2004). The arborization of neurons within RA is profoundly affected by E2 in the adult canary, with the number of dendrites and synaptic profiles increasing with steroid administration (DeVoogd & Nottebohm, 1981). Surprisingly, the steroidal influence on the song system can be extremely rapid, as significant increases in HVC neuron number are detectable within a week of T administration (Brenowitz, 2004). Importantly, non-steroidal factors also influence neuroplasticity within the adult song circuit. For instance, Gulledge & Deviche (1998) described independent effects of steroids and photoperiod on the growth of HVC in the juvenile junco. Melatonin, a photoperiodically regulated indoleamine, inhibits the growth of HVC in European starlings (Bentley & Ball, 1999), demonstrating that non-steroidal hormones can also affect the plasticity of the avian brain. Thus, the response of the adult passerine brain to hormones is rapid and robust, as exemplified by the recruitment of new neurons into specific neural circuits during adulthood.
Hippocampal plasticity
Although less dramatic, recruitment of neurons into the adult hippocampus is also observed for food caching songbirds, and the hippocampus is critical for accurate cache retrieval (Sherry & Vaccarino, 1989). Scatter-hoarding species are found within a number of songbird families, including the Corvids (crows and jays), Parids (tits and chickadees), and Sittids (nuthatches; Krebs et al., 1990), and of these, the neuroanatomy of the black-capped chickadee (Poecile atricapillus), has been studied the most. In this species, mitotic neurons are known to migrate into the adult hippocampus (Barnea & Nottebohm, 1996), thereby increasing hippocampal volume via changes in neuron number, not neuron size (Smulders et al., 1995; 2000; but see Hoshooley & Sherry, 2004). Interestingly, in contrast to what is observed in the song system, new neurons invade the passerine hippocampus during the autumn and winter. This suggests the possibility that different parts of the adult songbird brain recruit new neurons in a season- and behavior-specific manner. Indeed, of chickadees examined in October, February or June, cell number in the hippocampus (which is necessary for food retrieval) is maximal in October, whereas cell numbers in HVC and Area X (necessary for singing) are maximal in June (Saldanha et al., 2004). Although the endocrine mediators of hippocampal plasticity are unknown, its marked seasonal nature suggests the involvement of hormones. These combined data on the song system and hippocampus point to the exaggerated capability of the adult passerine brain to reorganize behaviorally relevant circuitry during adulthood.
Responses to neural injury
Hormone-mediated neural reorganization is also revealed in pathological conditions. In mammals and birds, recent studies on the role of cell-specific hormone production in response to neural injury reveal a critical role for steroids in the mitigation of damage. Brain damage results in an upregulation of aromatase in reactive glia, a cell-type that does not express aromatase in the intact homeotherm brain (Garcia-Segura et al., 1999, Peterson et al., 2001; 2004). These data suggest that glia switch on the ability to synthesize E2 in response to neural injury, and indicate a functional role for the glial aromatase expression that was first described in cell cultures of songbird telencephalon (Schlinger et al., 1994). Indeed localized administration of an aromatase inhibitor increases neural damage and increases apoptosis relative to localized saline administration (Wynne & Saldanha, 2004) an effect rescued by replacement with E2 in the zebra finch (Saldanha et al., in press). These data point to the clinical relevance of avian studies and highlight important similarities between avian and mammalian neurophysiology.
Multi-system integration: Birdsong and neuroendocrine cascades
Perhaps more than any other vertebrate group, birds offer the opportunity to conduct examinations of behavioral neuroendocrinology in a manner that integrates components of appetitive and consummatory behavioral states, environmental regulation, and neuroplasticity -- with each component being mediated by a diversity of endocrine mechanisms (e.g., by actions of gonadal, adrenal and thyroid hormones, neuropeptide modulators, intracrine processes and/or de novo steroid synthesis in the brain).
Birdsong in particular provides an excellent window into mechanistic integration (Ball et al., 2002), and in many male birds that breed and display song seasonally, the cascade of mechanistic events that initiates vernal song can be traced back to an increase in photoperiod. Increasing photoperiod is detected by the MBH (Saldanha et al., 1994b, 2001; Meddle & Follett, 1997), and gonadal recrudescence is subsequently triggered by release of GnRH and gonadotropin (Follett, 1984; Dawson et al., 2001). The accompanying rise in circulating T coordinates a variety of processes in the brain, many of which require that T be aromatized to E2. These include, but are not limited to, increased expression of neuropeptides (e.g., AVT within the BSTm-LS system) (Viglietti-Panzica et al., 2001; Plumari et al., 2004), dramatic restructuring and growth of telencephalic song nuclei (Tramontin & Brenowitz, 2000; Nottebohm, 2004), and an increase in aromatase activity within the VMH and POA (Vockel et al., 1990).
While the rise in circulating hormones directly influences the song circuitry, it can also influence singing via components of the social behavior network that are involved in sexual and aggressive behaviors (see first section). These areas express sex steroid receptors (Balthazart et al., 1992; Metzdorf et al., 1999), and a variety of data show that they participate in the control of song. For instance, lesions of the medial preoptic nucleus (POM) decrease courtship singing in starlings (Riters and Ball, 1999), and the expression of song is correlated with POM size, Zenk immunoreactivity and Fos immunoreactivity (Riters et al., 2000, 2004); the midbrain central gray may additionally play a role in territorial singing (see below). The social behavior network also plays a role in the selection of what to sing: In male field sparrows, AVT infusions into the septum selectively promote the use of an agonistic song type, but do not influence the use of a multipurpose song type (Goodson, 1998a).
This influence of the social behavior network on the telencephalic song system may be mediated by a variety of connections, particularly those through the midbrain central gray. All forebrain components of the social behavior network project to the midbrain central gray, as do preoptic aromatase neurons (Cheng et al., 1999; Absil et al., 2001; Montagnese et al., 2004; Riters and Alger, 2004). The central gray in turn provides direct input to the telencephalic song system (i.e., monosynaptic projections are observed for HVC and RA) (Appeltants et al., 2000). Neurons in the central gray exhibit a significant Fos response to agonistic challenge in free-living male song sparrows, and the number of songs sung correlates positively with the number of Fos-immunoreactive neurons (Maney and Ball, 2003).
Importantly, the central gray is a major site of catecholamine production, and the catecholaminergic innervation of HVC and RA is modulated by T (Appeltants et al., 2003). Recent data from Japanese quail (a non-songbird) also demonstrate that catecholamine neurons of the central gray are responsive to sexual interactions (Charlier et al., 2005). Thus, the combined findings suggest that the midbrain central gray integrates a variety of inputs, and then influences the song system in a manner that is appropriate to the behavioral, hormonal, seasonal and motivational context.
It is important to note how many components of this multifaceted cascade are exceptionally pronounced in birds, such as the degree of neuroplasticity and the expression of aromatase. At the same time, these variables can be studied within a context that is meaningful for a wide range of taxa, given that other vertebrates 1) often coordinate their social behavior and reproductive physiology based upon the same environmental variables (e.g., photoperiod and food availability) and 2) possess neuroendocrine and social behavior circuits of the basal forebrain and midbrain that are strongly evolutionarily conserved. In songbirds, these features are juxtaposed to a highly derived system that is dedicated to the learning and production of song. While a “song system” is obviously absent in other vertebrates, songbirds offer highly tractable opportunities to study the conserved aspects of behavioral regulation (i.e., functions dependent upon the social behavior network) as they relate to the telencephalic coordination of learning and performance (functions that are served by the song system). Hence, song can be viewed as a proxy for many kinds of complex behavior, and by examining the interactions between the song system and other steroid-sensitive circuits, we should obtain much information that is novel, important, and broadly relevant.
References
- Absil P, Riters LV, Balthazart J. Preoptic aromatase cells project to the mesencephalic central gray in the male Japanese quail (Coturnix japonica) Horm Behav. 2001;40:369–383. doi: 10.1006/hbeh.2001.1702. [DOI] [PubMed] [Google Scholar]
- Absil P, Braquenier JB, Balthazart J, Ball GF. Effects of lesions of nucleus taeniae on appetitive and consummatory aspects of male sexual behavior in Japanese quail. Brain Behav Evol. 2002;60:13–35. doi: 10.1159/000064119. [DOI] [PubMed] [Google Scholar]
- Adkins EK. Hormonal basis of sexual differentiation in the Japanese quail. J Comp Physiol Psychol. 1975;89:61–71. doi: 10.1037/h0076406. [DOI] [PubMed] [Google Scholar]
- Adkins EK, Nock BL. The effects of the antiestrogen CI-628 on sexual behavior activated by androgen or estrogen in quail. Horm Behav. 1976;7:417–429. doi: 10.1016/0018-506x(76)90013-1. [DOI] [PubMed] [Google Scholar]
- Adkins-Regan E, Watson JT. Sexual dimorphism in the avian brain is not limited to the song system of songbirds: a morphometric analysis of the brain of the quail (Coturnix japonica) Brain Res. 1990;514:320–326. doi: 10.1016/0006-8993(90)91427-i. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Kirn JR, Nottebohm F. Birth of projection neurons in adult avian brain may be related to perceptual or motor learning. Science. 1990;249:1444–1446. doi: 10.1126/science.1698312. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Nottebohm F. Migration of young neurons in adult avian brain. Nature. 1988;335:353–354. doi: 10.1038/335353a0. [DOI] [PubMed] [Google Scholar]
- Appeltants D, Absil P, Balthazart J, Ball GF. Identification of the origin of catecholaminergic inputs to HVc in canaries by retrograde tract tracing combined with tyrosine hydroxylase immunocytochemistry. J Chem Neuroanat. 2000;18:117–133. doi: 10.1016/s0891-0618(99)00054-x. [DOI] [PubMed] [Google Scholar]
- Appeltants D, Ball GF, Balthazart J. Song activation by testosterone is associated with an increased catecholaminergic innervation of the song control system in female canaries. Neuroscience. 2003;121:801–814. doi: 10.1016/s0306-4522(03)00496-2. [DOI] [PubMed] [Google Scholar]
- Arnold AP. Sex chromosomes and brain gender. Nat Rev Neurosci. 2004;5:701–708. doi: 10.1038/nrn1494. [DOI] [PubMed] [Google Scholar]
- Aste N, Balthazart J, Absil P, Grossmann R, Mulhbauer E, Viglietti-Panzica C, Panzica GC. Anatomical and neurochemical definition of the nucleus of the stria terminalis in Japanese quail (Coturnix japonica) J Comp Neurol. 1998a;396:141–157. [PubMed] [Google Scholar]
- Aste N, Panzica GC, Viglietti-Panzica C, Harada N, Balthazart J. Distribution and effects of testosterone on aromatase mRNA in the quail forebrain: A non-radioactive in situ hybridization study. J Chem Neuroanat. 1998b;14:103–115. doi: 10.1016/s0891-0618(97)10023-0. [DOI] [PubMed] [Google Scholar]
- Aste N, Viglietti-Panzica C, Balthazart J, Panzica GC. Testosterone modulation of peptidergic pathways in the septo-preoptic region of male Japanese quail. Poult Avian Biol Rev. 1997;8:77–93. [Google Scholar]
- Aste N, Viglietti-Panzica C, Fasolo A, Andreone C, Vaudry H, Pelletier G, Panzica GC. Localization of neuropeptide Y-immunoreactive cells and fibres in the brain of the Japanese quail. Cell Tissue Res. 1991;265:219–230. doi: 10.1007/BF00398070. [DOI] [PubMed] [Google Scholar]
- Aste N, Viglietti-Panzica C, Fasolo A, Panzica GC. Mapping of neurochemical markers in quail central nervous system: VIP- and SP-like immunoreactivity. J Chem Neuroanat. 1995;8:87–102. doi: 10.1016/0891-0618(94)00031-n. [DOI] [PubMed] [Google Scholar]
- Atoji Y, Wild JM. Fiber connections of the hippocampal formation and septum and subdivisions of the hippocampal formation in the pigeon as revealed by tract tracing and kainic acid lesions. J Comp Neurol. 2004;475:426–461. doi: 10.1002/cne.20186. [DOI] [PubMed] [Google Scholar]
- Atoji Y, Wild JM, Yamamoto Y, Suzuki Y. Intratelencephalic connections of the hippocampus in pigeons (Columba livia) J Comp Neurol. 2002;447:177–199. doi: 10.1002/cne.10239. [DOI] [PubMed] [Google Scholar]
- Ball GF, Balthazart J. Birds return every spring like clockwork, but where is the clock? Endocrinology. 2003;144:3739–3741. doi: 10.1210/en.2003-0781. [DOI] [PubMed] [Google Scholar]
- Ball GF, Balthazart J. Hormonal regulation of brain circuits mediating male sexual behavior in birds. Physiol Behav. 2004;83:329–346. doi: 10.1016/j.physbeh.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Ball GF, Hahn TP. GnRH neuronal systems in birds and their relation to the control of seasonal reproduction. In: Parhar IS, Sakuma Y, editors. GnRH Neurons: Gene to Behavior. Brain Shuppan Publishers; Tokyo: 1997. pp. 325–342. [Google Scholar]
- Ball GF, Riters LV, Balthazart J. Neuroendocrinology of song behavior and avian brain plasticity: multiple sites of action of sex steroid hormones. Front Neuroendocrinol. 2002;23:137–178. doi: 10.1006/frne.2002.0230. [DOI] [PubMed] [Google Scholar]
- Ball GF, Tlemçani O, Balthazart J. Induction of the Zenk protein after sexual interactions in male Japanese quail. Neuroreport. 1997;8:2965–2770. doi: 10.1097/00001756-199709080-00032. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Absil P. Identification of catecholaminergic inputs to and outputs from aromatase-containing brain areas of the Japanese quail by tract tracing combined with tyrosine hydroxylase immunocytochemistry. J Comp Neurol. 1997;382:401–428. [PubMed] [Google Scholar]
- Balthazart J, Absil P, Foidart A, Houbart M, Harada N, Ball Gregory F. Distribution of aromatase-immunoreactive cells in the forebrain of zebra finches (Taeniopygia guttata): Implications for the neural action of steroids and nuclear definition in the avian hypothalamus. J Neurobiol. 1996;31:129–148. doi: 10.1002/(SICI)1097-4695(199610)31:2<129::AID-NEU1>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Absil P, Gerard M, Appeltants D, Ball GF. Appetitive and consummatory male sexual behavior in Japanese quail are differentially regulated by subregions of the preoptic medial nucleus. J Neurosci. 1998;18:6512–6527. doi: 10.1523/JNEUROSCI.18-16-06512.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthazart J, Baillien M, Ball GF. Phosphorylation processes mediate rapid changes of brain aromatase activity. J Steroid Biochem Mol Biol. 2001a;79:261–277. doi: 10.1016/s0960-0760(01)00143-1. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Baillien M, Ball GF. Rapid and reversible inhibition of brain aromatase activity. J Neuroendocrinol. 2001b;13:63–73. doi: 10.1046/j.1365-2826.2001.00598.x. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Baillien M, Charlier TD, Ball GF. Calcium-dependent phosphorylation processes control brain aromatase in quail. Eur J Neurosci. 2003;17:1591–1606. doi: 10.1046/j.1460-9568.2003.02598.x. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Baillien M, Cornil CA, Ball GF. Preoptic aromatase modulates male sexual behavior: slow and fast mechanisms of action. Physiol Behav. 2004;83:247–270. doi: 10.1016/j.physbeh.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Ball GF. Sexual differentiation of brain and behavior in birds. Trends Endocrinol Metab. 1995;6:21–29. doi: 10.1016/1043-2760(94)00098-o. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Castagna C, Ball GF. Aromatase inhibition blocks the activation and sexual differentiation of appetitive male sexual behavior in Japanese quail. Behav Neurosci. 1997;111:381–397. [PubMed] [Google Scholar]
- Balthazart J, Dupiereux V, Aste N, Viglietti-Panzica C, Barrese M, Panzica GC. Afferent and efferent connections of the sexually dimorphic medial preoptic nucleus of the male quail revealed by in vitro transport of DiI. Cell Tissue Res. 1994;276:455–475. doi: 10.1007/BF00343944. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Evrard L, Surlemont C. Effects of the nonsteroidal inhibitor R76713 on testosterone-induced sexual behavior in the Japanese quail (Coturnix coturnix japonica) Horm Behav. 1990a;24:510–531. doi: 10.1016/0018-506x(90)90039-z. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Foidart A, Harada N. Immunocytochemical localization of aromatase in the brain. Brain Res. 1990b;514:327–333. doi: 10.1016/0006-8993(90)91428-j. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Foidart A, Surlemont C, Vockel A, Harada N. Distribution of aromatase in the brain of the Japanese quail, ring dove, and zebra finch: an immunocytochemical study. J Comp Neurol. 1990c;301:276–288. doi: 10.1002/cne.903010210. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Foidart A, Wilson EM, Ball GF. Immunocytochemical localization of androgen receptors in the male songbird and quail brain. J Comp Neurol. 1992;317:407–420. doi: 10.1002/cne.903170407. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Reid J, Absil P, Foidart A, Ball GF. Appetitive as well as consummatory aspects of male sexual behavior in quail are activated by androgens and estrogens. Behav Neurosci. 1995;109:485–501. [PubMed] [Google Scholar]
- Barnea A, Nottebohm F. Recruitment and replacement of hippocampal neurons in young and adult chickadees: an addition to the theory of hippocampal learning. Proc Natl Acad Sci U S A. 1996;93:714–718. doi: 10.1073/pnas.93.2.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley GE, Van't Hof TJ, Ball GF. Seasonal neuroplasticity in the songbird telencephalon: a role for melatonin. Proc Natl Acad Sci U S A. 1999;96:4674–4679. doi: 10.1073/pnas.96.8.4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenowitz EA. Plasticity of the adult avian song control system. Ann N Y Acad Sci. 2004;101:560–585. doi: 10.1196/annals.1298.006. [DOI] [PubMed] [Google Scholar]
- Briganti F, Beani L, Panzica GC. Connections of the dorsomedial part of the nucleus intercollicularis in a male non-songbird, the grey partridge: A tract-tracing study. Neurosci Lett. 1996;221:61–65. doi: 10.1016/s0304-3940(96)13261-4. [DOI] [PubMed] [Google Scholar]
- Castagna C, Obole A, Viglietti-Panzica C, Balthazart J, Panzica GC. Effects of testosterone on the synaptology of the medial preoptic nucleus of male Japanese quail. Brain Res Bull. 1999;50:241–249. doi: 10.1016/s0361-9230(99)00193-8. [DOI] [PubMed] [Google Scholar]
- Charlier TD, Ball GF, Balthazart J. Sexual behavior activates the expression of the immediate early genes c-fos and Zenk (egr-1) in catecholaminergic neurons of male Japanese quail. Neuroscience. 2005;131:13–30. doi: 10.1016/j.neuroscience.2004.09.068. [DOI] [PubMed] [Google Scholar]
- Cheng M, Chaiken M, Zuo M, Miller H. Nucleus taenia of the amygdala of birds: Anatomical and functional studies in ring doves (Streptopelia risoria) and European starlings (Sturnus vulgaris) Brain Behav Evol. 1999;53:243–270. doi: 10.1159/000006597. [DOI] [PubMed] [Google Scholar]
- Cho RN, Hahn TP, MacDougall-Shackleton SA, Ball GF. Seasonal variation in brain GnRH in free-living breeding and photorefractory house finches (Carpodacus mexicanus) Gen Comp Endocrinol. 1998;109:244–250. doi: 10.1006/gcen.1997.7027. [DOI] [PubMed] [Google Scholar]
- Cockrem JA. Timing of seasonal breeding in birds, with particular reference to New Zealand birds. Reprod Fertil Dev. 1995;7:1–19. doi: 10.1071/rd9950001. [DOI] [PubMed] [Google Scholar]
- Cornil CA, Evrard HC, Ball GF, Balthazart J. Rapid effects of 17β-estradiol and vorozole, an aromatase inhibitor, on male sexual behavior in Japanese quail. Soc Neurosci Abstr. 2003;33:726.5. [Google Scholar]
- Corpechot C, Robel P, Axelson M, Sjovall J, Baulieu EE. Characterization and measurement of dehydroepiandrosterone sulfate in rat brain. Proc Natl Acad Sci U S A. 1981;78:4704–4707. doi: 10.1073/pnas.78.8.4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies DT, Follett BK. The neuroendocrine control of gonadotrophin release in Japanese quail. I. The role of the tuberal hypothalamus. Proc Roy Soc Lond B. 1975;191:303–315. doi: 10.1098/rspb.1975.0129. [DOI] [PubMed] [Google Scholar]
- Dawson A, King VM, Bentley GE, Ball GF. Photoperiodic control of seasonality in birds. J Biol Rhythms. 2001;16:366–381. doi: 10.1177/074873001129002079. [DOI] [PubMed] [Google Scholar]
- DeVoogd T, Nottebohm F. Gonadal hormones induce dendritic growth in the adult avian brain. Science. 1981;214:202–204. doi: 10.1126/science.7280692. [DOI] [PubMed] [Google Scholar]
- Evrard H, Baillien M, Foidart A, Absil P, Harada N, Balthazart J. Localization and controls of aromatase in the quail spinal cord. J Comp Neurol. 2000;423:552–564. doi: 10.1002/1096-9861(20000807)423:4<552::aid-cne2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Evrard HC, Balthazart J. Aromatization of androgens into estrogens reduces response latency to a noxious thermal stimulus in male quail. Horm Behav. 2004a;45:181–189. doi: 10.1016/j.yhbeh.2003.09.014. [DOI] [PubMed] [Google Scholar]
- Evrard HC, Balthazart J. Rapid regulation of pain by estrogens synthesized in spinal dorsal horn neurons. J Neurosci. 2004b;24:7225–7229. doi: 10.1523/JNEUROSCI.1638-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foidart A, Harada N, Balthazart J. Effects of steroidal and non steroidal aromatase inhibitors on sexual behavior and aromatase-immunoreactive cells and fibers in the quail brain. Brain Res. 1994;657:105–123. doi: 10.1016/0006-8993(94)90958-x. [DOI] [PubMed] [Google Scholar]
- Foidart A, Reid J, Absil P, Yhoshimura N, Harada N, Balthazart J. Critical re-examination of the distribution of aromatase- immunoreactive cells in the quail forebrain using antibodies raised against human placental aromatase and against the recombinant quail, mouse or human enzyme. J Chem Neuroanat. 1995;8:267–282. doi: 10.1016/0891-0618(95)00054-b. [DOI] [PubMed] [Google Scholar]
- Follett BK. Birds. In: Lamming GE, editor. Marsha'll's Physiology of Reproduction. Vol. 1. Longman Green; Edinburgh: 1984. pp. 283–350. [Google Scholar]
- Foster RG, Panzica GC, Parry DM, Viglietti-Panzica C. Immunocytochemical studies on the LHRH system of the Japanese quail: Influence by photoperiod and aspects of sexual differentiation. Cell Tissue Res. 1988;253:327–335. doi: 10.1007/BF00222289. [DOI] [PubMed] [Google Scholar]
- Foster RG, Plowman G, Goldsmith AR, Follett BK. Immunocytochemical demonstration of marked changes in the luteinizing hormone-releasing hormone system of photosensitive and photorefractory European starlings. J Endocrinol. 1987;115:211–220. doi: 10.1677/joe.0.1150211. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, Chowen JA, Naftolin F. Endocrine Glia: Roles of glial cells in the brain actions of steroid and thyroid hormones and in the regulation of hormone secretion. Front Neuroendocrinol. 1996;17:180–211. doi: 10.1006/frne.1996.0005. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, McCarthy MM. Role of glia in neuroendocrine function. Endocrinology. 2004;145:1082–1086. doi: 10.1210/en.2003-1383. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, Wozniak A, Azcoitia I, Rodriguez JR, Hutchison RE, Hutchison JB. Aromatase expression by astrocytes after brain injury: implications for local estrogen formation in brain repair. Neuroscience. 1999;89:567–578. doi: 10.1016/s0306-4522(98)00340-6. [DOI] [PubMed] [Google Scholar]
- Goldman SA. Neuronal development and migration in explant cultures of the adult canary forebrain. J Neurosci. 1990;10:2931–2939. doi: 10.1523/JNEUROSCI.10-09-02931.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman SA, Lemmon V, Chin SS. Migration of newly generated neurons upon ependymally derived radial guide cells in explant cultures of the adult songbird forebrain. Glia. 1993;8:150–160. doi: 10.1002/glia.440080303. [DOI] [PubMed] [Google Scholar]
- Goldman SA, Nedergaard M. Newly generated neurons of the adult songbird brain become functionally active in long-term culture. Dev Brain Res. 1992;68:217–223. doi: 10.1016/0165-3806(92)90063-3. [DOI] [PubMed] [Google Scholar]
- Goldman SA, Nottebohm F. Neuronal production, migration, differentiation in a vocal control nucleus of the adult female canary brain. Proc Natl Acad Sci U S A. 1983;80:2390–2394. doi: 10.1073/pnas.80.8.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman SA, Zaremba A, Niedzwiecki D. In vitro neurogenesis by neuronal precursor cells derived from the adult songbird brain. J Neurosci. 1992;12:2532–2541. doi: 10.1523/JNEUROSCI.12-07-02532.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman SA, Zukhar A, Barami K, Mikawa T, Niedzwiecki D. Ependymal/subependymal zone cells of postnatal and adult songbird brain generate both neurons and nonneuronal siblings in vitro and in vivo. J Neurobiol. 1996;30:505–520. doi: 10.1002/(SICI)1097-4695(199608)30:4<505::AID-NEU6>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Goodson JL. Territorial aggression and dawn song are modulated by septal vasotocin and vasoactive intestinal polypeptide in male field sparrows (Spizella pusilla) Horm Behav. 1998a;34:67–77. doi: 10.1006/hbeh.1998.1467. [DOI] [PubMed] [Google Scholar]
- Goodson JL. Vasotocin and vasoactive intestinal polypeptide modulate aggression in a territorial songbird, the violet-eared waxbill (Estrildidae: Uraeginthus granatina) Gen Comp Endocrinol. 1998b;111:233–244. doi: 10.1006/gcen.1998.7112. [DOI] [PubMed] [Google Scholar]
- Goodson JL. The vertebrate social behavior network: evolutionary themes and variations. Horm Behav. 2005 doi: 10.1016/j.yhbeh.2005.02.003. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Adkins-Regan E. Effect of intraseptal vasotocin and vasoactive intestinal polypeptide infusions on courtship song and aggression in the male zebra finch (Taeniopygia guttata) J Neuroendocrinol. 1999;11:19–25. doi: 10.1046/j.1365-2826.1999.00284.x. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Bass AH. Social behavior functions and related anatomical characteristics of vasotocin/vasopressin systems in vertebrates. Brain Res Rev. 2001;35:246–265. doi: 10.1016/s0165-0173(01)00043-1. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Eibach R, Sakata J, Adkins-Regan E. Effect of septal lesions on male song and aggression in the colonial zebra finch (Taeniopygia guttata) and the territorial field sparrow (Spizella pusilla) Behav Brain Res. 1999;98:167–180. [PubMed] [Google Scholar]
- Goodson JL, Evans AK. Neural responses to territorial challenge and nonsocial stress in male song sparrows: segregation, integration, and modulation by a vasopressin V1 antagonist. Horm Behav. 2004;46:371–381. doi: 10.1016/j.yhbeh.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Evans AK, Lindberg L. Chemoarchitectonic subdivisions of the songbird septum and a comparative overview of septum chemical anatomy in jawed vertebrates. J Comp Neurol. 2004a;473:293–314. doi: 10.1002/cne.20061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Evans AK, Lindberg L, Allen CD. Neuro-evolutionary patterning of sociality. Proc R Soc Lond B. 2005;272:227–235. doi: 10.1098/rspb.2004.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Lindberg L, Johnson P. Effects of central vasotocin and mesotocin manipulations on social behavior in male and female zebra finches. Horm Behav. 2004b;45:136–143. doi: 10.1016/j.yhbeh.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Gould E, Gross CG. Neurogenesis in adult mammals: some progress and problems. J Neurosci. 2002;22:619–623. doi: 10.1523/JNEUROSCI.22-03-00619.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulledge CC, DeViche P. Autoradiographic localization of opioid receptors in vocal control regions of a male passerine bird Junco hyemalis. J Comp Neurol. 1995;356:408–417. doi: 10.1002/cne.903560308. [DOI] [PubMed] [Google Scholar]
- Gulledge CC, Deviche P. Photoperiod and testosterone independently affect vocal control region volumes in adolescent male songbirds. J Neurobiol. 1998;36:550–558. [PubMed] [Google Scholar]
- Gurney ME, Konishi M. Hormone-induced sexual differentiation of brain and behavior in zebra finches. Science. 1980;208:1380–1383. doi: 10.1126/science.208.4450.1380. [DOI] [PubMed] [Google Scholar]
- Hahn TP. Integration of photoperiodic and food cues to time changes in reproductive physiology by an opportunistic breeder, the red crossbill, Loxia curvirostra (Aves: Carduelinae) J Exp Zool. 1995;272:213–226. [Google Scholar]
- Hahn TP, Boswell T, Wingfield JC, Ball GF. Temporal flexibility in avian reproduction: Patterns and mechanisms. In: Nolan V Jr, Ketterson ED, Thompson CF, editors. Curr Ornithol. Vol. 14. Plenum; New York and London: 1997. pp. 39–80. [Google Scholar]
- Hahn TP, Pereyra ME, Katti M, Ward GM, MacDougall-Shackleton SA. Effects of food availability on the reproductive system. In: Dawson A, Sharp PJ, editors. Functional Avian Endocrinology. Narosa Publishing House; New Delhi, India: 2005. in press. [Google Scholar]
- Hahn TP, Pereyra ME, Sharbaugh SM, Bentley GE. Physiological responses to photoperiod in three cardueline finch species. Gen Comp Endocrinol. 2004;137:99–108. doi: 10.1016/j.ygcen.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Hahn TP, Wingfield JC, Mullen R, Deviche PJ. Endocrine bases of spatial and temporal opportunism in arctic breeding birds. Am Zool. 1995;35:259–273. [Google Scholar]
- Hau M, Stoddard ST, Soma KK. Territorial aggression and hormones during the non-breeding season in a tropical bird. Horm Behav. 2004;45:40–49. doi: 10.1016/j.yhbeh.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Hau M, Wikelski M, Soma KK, Wingfield JC. Testosterone and year-round territorial aggression in a tropical bird. Gen Comp Endocrinol. 2000;117:20–33. doi: 10.1006/gcen.1999.7390. [DOI] [PubMed] [Google Scholar]
- Holloway CC, Clayton DF. Estrogen synthesis in the male brain triggers development of the avian song control pathway in vitro. Nat Neurosci. 2001;4:170–175. doi: 10.1038/84001. [DOI] [PubMed] [Google Scholar]
- Hoshooley JS, Sherry DF. Neuron production, neuron number, structure size are seasonally stable in the hippocampus of the food-storing black-capped chickadee Poecile atricapillus. Behav Neurosci. 2004;118:345–355. doi: 10.1037/0735-7044.118.2.345. [DOI] [PubMed] [Google Scholar]
- Hutchison JB, Steimer TJ, Hutchison RE. Formation of behaviorally active estrogen in the dove brain - induction of preoptic aromatase by intracranial testosterone. Neuroendocrinology. 1986;43:416–427. doi: 10.1159/000124558. [DOI] [PubMed] [Google Scholar]
- Jellinck PH, Lee SJ, McEwen BS. Metabolism of dehydroepiandrosterone by rat hippocampal cells in culture: possible role of aromatization and 7-hydroxylation in neuroprotection. J Steroid Biochem Mol Biol. 2001;78:313–317. doi: 10.1016/s0960-0760(01)00106-6. [DOI] [PubMed] [Google Scholar]
- Jurkevich A, Barth SW, Kuenzel VJ, Kohler A, Grossmann R. Development of sexually dimorphic vasotocinergic system in the bed nucleus of stria terminalis in chickens. J Comp Neurol. 1999;408:46–60. doi: 10.1002/(sici)1096-9861(19990524)408:1<46::aid-cne4>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Juss TS. Neuroendocrine and neural changes associated with the photoperiodic control of reproduction. In: Sharp PJ, editor. Avian Endocrinology. Journal of Endocrinology Ltd; Bristol: 1993. pp. 47–60. [Google Scholar]
- Kirn J, O'Loughlin B, Kasparian S, Nottebohm F. Cell death and neuronal recruitment in the high vocal center of adult male canaries are temporally related to changes in song. Proc Natl Acad Sci U S A. 1994;91:7844–7848. doi: 10.1073/pnas.91.17.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirn JR, Nottebohm F. Direct evidence for loss and replacement of projection neurons in adult canary brain. J Neurosci. 1993;13:1654–1663. doi: 10.1523/JNEUROSCI.13-04-01654.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi M, Emlen ST, Ricklefs RE, Wingfield JC. Contributions of bird studies to biology. Science. 1989;246:465–472. doi: 10.1126/science.2683069. [DOI] [PubMed] [Google Scholar]
- Krebs JR. Food-storing birds: adaptive specialization in brain and behaviour? Philos Trans R Soc Lond B. 1990;329:153–160. doi: 10.1098/rstb.1990.0160. [DOI] [PubMed] [Google Scholar]
- Labrie F. Intracrinology. Mol Cell Endocrinol. 1991;78:C113–C118. doi: 10.1016/0303-7207(91)90116-a. [DOI] [PubMed] [Google Scholar]
- London SE, Boulter J, Schlinger BA. Cloning of the zebra finch androgen synthetic enzyme CYP17: A study of its neural expression throughout posthatch development. J Comp Neurol. 2003;467:496–508. doi: 10.1002/cne.10936. [DOI] [PubMed] [Google Scholar]
- MacDougall-Shackleton SA, Deviche PJ, Crain RD, Ball GF, Hahn TP. Seasonal changes in brain GnRH immunoreactivity and song-control nuclei volumes in an opportunistically breeding songbird. Brain Behav Evol. 2001;58:38–48. doi: 10.1159/000047260. [DOI] [PubMed] [Google Scholar]
- MacDougall-Shackleton SA, Pereyra ME, Hahn TP. GnRH, photorefractoriness and breeding schedules of cardueline finches. In: Dawson A, Sharp PJ, editors. Functional Avian Endocrinology. Narosa Publishing House; New Delhi, India: 2005. in press. [Google Scholar]
- Maney DL, Ball GF. Fos-like immunoreactivity in catecholaminergic brain nuclei after territorial behavior in free-living song sparrows. J Neurobiol. 2003;56:163–170. doi: 10.1002/neu.10227. [DOI] [PubMed] [Google Scholar]
- Martinez-Vargas MC, Stumpf WE, Sar M. Anatomical distribution of estrogen target cells in the avian CNS: A comparison with the mammalian CNS. J Comp Neurol. 1976;167:83–103. doi: 10.1002/cne.901670106. [DOI] [PubMed] [Google Scholar]
- Matsunaga M, Ukena K, Tsutsui K. Androgen biosynthesis in the quail brain. Brain Res. 2002;948:180–185. doi: 10.1016/s0006-8993(02)03147-5. [DOI] [PubMed] [Google Scholar]
- Meddle SL, Foidart A, Wingfield JC, Ramenofskyand M, Balthazart J. Effects of sexual interactions with a male on fos-like immunoreactivity in the female quail brain. J Neuroendocrinol. 1999;11:771–784. doi: 10.1046/j.1365-2826.1999.00384.x. [DOI] [PubMed] [Google Scholar]
- Meddle SL, Follett BK. Photoperiodically driven changes in Fos expression within the basal tuberal hypothalamus and median eminence of Japanese quail. J Neurosci. 1997;17:8909–8919. doi: 10.1523/JNEUROSCI.17-22-08909.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meddle SL, King VM, Follett BK, Wingfield JC, Ramenofsky M, Foidart A, Balthazart J. Copulation activates Fos-like immunoreactivity in the male quail forebrain. Behav Brain Res. 1997;85:143–159. doi: 10.1016/s0166-4328(97)87581-x. [DOI] [PubMed] [Google Scholar]
- Metzdorf R, Gahr M, Fusani L. Distribution of aromatase, estrogen receptor, and androgen receptor mRNA in the forebrain of songbirds and nonsongbirds. J Comp Neurol. 1999;407:115–129. [PubMed] [Google Scholar]
- Milewich L, Shaw CE, Doody KM, Rainey WE, Mason JI, Carr BR. 3β-hydroxysteroid dehydrogenase activity in glandular and extraglandular human fetal tissues. J Clin Endocrinol Metab. 1991;73:1134–1140. doi: 10.1210/jcem-73-5-1134. [DOI] [PubMed] [Google Scholar]
- Montagnese CM, Szekely AD, Adam A, Csillag A. Efferent connections of septal nuclei of the domestic chick (Gallus domesticus): an anterograde pathway tracing study with a bearing on functional circuits. J Comp Neurol. 2004;469:437–456. doi: 10.1002/cne.11018. [DOI] [PubMed] [Google Scholar]
- Morrell JI, Pfaff DW. A neuroendocrine approach to brain function: Localization of sex steroid concentrating cells in vertebrate brains. Am Zool. 1978;18:447–460. [Google Scholar]
- Murton R, Westwood N. Avian breeding cycles. Clarendon Press; Oxford: 1977. [Google Scholar]
- Naftolin F, Horvath TL, Jakab RL, Leranth C, Harada N, Balthazart J. Aromatase immunoreactivity in axon terminals of the vertebrate brain: An immunocytochemical study on quail, rat, monkey and human tissues. Neuroendocrinology. 1996;63:149–155. doi: 10.1159/000126951. [DOI] [PubMed] [Google Scholar]
- Naftolin F, Ryan KJ, Petro Z. Aromatization of androstenedione by limbic system tissue from human foetuses. J Endocrinol. 1971;51:795–796. doi: 10.1677/joe.0.0510795. [DOI] [PubMed] [Google Scholar]
- Naftolin F, Ryan KJ, Petro Z. Aromatization of androstenedione by anterior hypothalamus of adult male and female rat. Endocrinology. 1972;90:295–298. doi: 10.1210/endo-90-1-295. [DOI] [PubMed] [Google Scholar]
- Newman SW. The medial extended amygdala in male reproductive behavior: A node in the mammalian social behavior network. Ann N Y Acad Sci. 1999;877:242–257. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- Nicholls TJ, Goldsmith AR, Dawson A. Photorefractoriness in birds and comparison with mammals. Physiol Rev. 1988;68:133–176. doi: 10.1152/physrev.1988.68.1.133. [DOI] [PubMed] [Google Scholar]
- Nottebohm F. The road we travelled: discovery, choreography, significance of brain replaceable neurons. Ann N Y Acad Sci. 2004;101:628–658. doi: 10.1196/annals.1298.027. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Arnold AP. Sexual dimorphism in vocal control areas of songbird brain. Science. 1976;194:211–213. doi: 10.1126/science.959852. [DOI] [PubMed] [Google Scholar]
- Owen-Ashley NT, Hasselquist D, Wingfield JC. Androgens and the immunocompetence handicap hypothesis: Unraveling direct and indirect pathways of immunosuppression in song sparrows. Am Nat. 2004;164:490–505. doi: 10.1086/423714. [DOI] [PubMed] [Google Scholar]
- Panzica G, Pessatti M, Viglietti-Panzica C, Grossmann R, Balthazart J. Effects of testosterone on sexually dimorphic parvocellular neurons expressing vasotocin mRNA in the male quail brain. Brain Res. 1999a;850:55–62. doi: 10.1016/s0006-8993(99)02098-3. [DOI] [PubMed] [Google Scholar]
- Panzica GC, Castagna C, Viglietti-Panzica C, Russo C, Tlemçani O, Balthazart J. Organizational effects of estrogens on brain vasotocin and sexual behavior in quail. J Neurobiol. 1998;37:684–699. doi: 10.1002/(sici)1097-4695(199812)37:4<684::aid-neu15>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Panzica GC, Plumari L, Garcia-Ojeda E, Deviche P. Central vasotocin-immunoreactive system in a male passerine bird (Junco hyemalis) J Comp Neurol. 1999b;409:105–117. [PubMed] [Google Scholar]
- Panzica GC, Viglietta-Panzica C, Balthazart J. The sexually dimorphic medial preoptic nucleus of quail: A key brain area mediating steroid action on male sexual behavior. Front Neuroendocrinol. 1996;17:51–125. doi: 10.1006/frne.1996.0002. [DOI] [PubMed] [Google Scholar]
- Parry D, Goldsmith AR. Ultrastructural evidence for changes in synaptic input to the hypothalamic luteinizing hormone-releasing hormone neurons in photosensitive and photorefractory starlings. J Neuroendocrinol. 1993;5:387–395. doi: 10.1111/j.1365-2826.1993.tb00499.x. [DOI] [PubMed] [Google Scholar]
- Paton JA, Nottebohm FN. Neurons generated in the adult brain are recruited into functional circuits. Science. 1984;225:1046–1048. doi: 10.1126/science.6474166. [DOI] [PubMed] [Google Scholar]
- Pereyra ME, Sharbaugh SM, Hahn TP. Interspecific variation in photo-induced GnRH plasticity among nomadic cardueline finches. Brain, Behavior and Evolution. 2005;497 doi: 10.1159/000085046. in press. [DOI] [PubMed] [Google Scholar]
- Peterson RS, Lee DW, Fernando G, Schlinger BA. Radial glia express aromatase in the injured zebra finch brain. J Comp Neurol. 2004;475:261–269. doi: 10.1002/cne.20157. [DOI] [PubMed] [Google Scholar]
- Peterson RS, Saldanha CJ, Schlinger BA. Rapid upregulation of aromatase mRNA and protein following neural injury in the zebra finch Taeniopygia guttata. J Neuroendocrinol. 2001;134:317–323. doi: 10.1046/j.1365-2826.2001.00647.x. [DOI] [PubMed] [Google Scholar]
- Peterson RS, Yarram L, Schlinger BA, Saldanha CJ. Aromatase in presynaptic and sexually dimorphic in the adult zebra finch brain. doi: 10.1098/rspb.2005.3181. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plumari L, Plateroti S, Deviche P, Panzica GC. Region-specific testosterone modulation of the vasotocin-immunoreactive system in male dark-eyed junco, Junco hyemalis. Brain Res. 2004;999:1–8. doi: 10.1016/j.brainres.2003.10.037. [DOI] [PubMed] [Google Scholar]
- Reiner A, Perkel DJ, Bruce LL, Butler AB, Csillag A, Kuenzel W, Medina L, Paxinos G, Shimizu T, Striedter G, Wild M, Ball GF, Durand S, Gunturkun O, Lee DW, Mello CV, Powers A, White SA, Hough G, Kubikova L, Smulders TV, Wada K, Dugas-Ford J, Husband S, Yamamoto K, Yu J, Siang C, Jarvis ED, Guturkun O. Revised nomenclature for avian telencephalon and some related brainstem nuclei. J Comp Neurol. 2004;473:377–414. doi: 10.1002/cne.20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risold PY, Swanson LW. Connections of the rat lateral septal complex. Brain Res Rev. 1997;24:115–195. doi: 10.1016/s0165-0173(97)00009-x. [DOI] [PubMed] [Google Scholar]
- Riters LV, Absil P, Balthazart J. Effects of brain testosterone implants on appetitive and consummatory components of male sexual behavior in Japanese quail. Brain Res Bull. 1998;47:69–79. doi: 10.1016/s0361-9230(98)00064-1. [DOI] [PubMed] [Google Scholar]
- Riters LV, Alger SJ. Neuroanatomical evidence for indirect connections between the medial preoptic nucleus and the song control system: possible neural substrates for sexually motivated song. Cell Tissue Res. 2004;316:35–44. doi: 10.1007/s00441-003-0838-6. [DOI] [PubMed] [Google Scholar]
- Riters LV, Ball GF. Lesions to the medial preoptic area affect singing in the male European starling (Sturnus vulgaris) Horm Behav. 1999;36:276–286. doi: 10.1006/hbeh.1999.1549. [DOI] [PubMed] [Google Scholar]
- Riters LV, Eens M, Pinxten R, Duffy DL, Balthazart J, Ball GF. Seasonal changes in courtship song and the medial preoptic area in male European starlings (Sturnus vulgaris) Horm Behav. 2000;38:250–261. doi: 10.1006/hbeh.2000.1623. [DOI] [PubMed] [Google Scholar]
- Riters LV, Teague DP, Schroeder MB, Cummings SE. Vocal production in different social contexts relates to variation in immediate early gene immunoreactivity within and outside of the song control system. Behav Brain Res. 2004;155:307–318. doi: 10.1016/j.bbr.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Robinson JE, Follett BK. Photoperiodism in Japanese Quail: The termination of seasonal breeding by photorefractoriness. Proc Roy Soc Lond B. 1982;215:95–116. doi: 10.1098/rspb.1982.0030. [DOI] [PubMed] [Google Scholar]
- Rowan W. Relation of light to bird migration and developmental changes. Nature. 1925;115:494–495. [Google Scholar]
- Ruscio MG, Adkins-Regan E. Immediate early gene expression associated with induction of brooding behavior in Japanese quail. Horm Behav. 2004;46:19–29. doi: 10.1016/j.yhbeh.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Saldanha CJ, Deviche PJ, Silver R. Increased VIP and decreased GnRH expression in photorefractory dark-eyed juncos (Junco hyemalis) Gen Comp Endocrinol. 1994a;93:128–136. doi: 10.1006/gcen.1994.1015. [DOI] [PubMed] [Google Scholar]
- Saldanha CJ, Leak RK, Silver R. Detection and transduction of daylength in birds. Psychoneuroendocrinology. 1994b;19:641–656. doi: 10.1016/0306-4530(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Saldanha CJ, Patel NP, Kullar RK. Orthogonal patterns of neuroplasticity in the hippocampus and song system of a food storing songbird. Soc Neurosci Abstr. 2004;554:18. [Google Scholar]
- Saldanha CJ, Rohmann KN, Coomaralingam L, Wynne RD. Estrogen delivery by reactive glia decreases apoptosis in the zebra finch Taeniopygia guttata. J Neurobiol. doi: 10.1002/neu.20147. In press. [DOI] [PubMed] [Google Scholar]
- Saldanha CJ, Silverman AJ, Silver R. Direct innervation of GnRH neurons by encephalic photoreceptors in birds. J Biol Rhythms. 2001;16:39–49. doi: 10.1177/074873040101600105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldanha CJ, Tuerk MJ, Kim YH, Fernandes AO, Arnold AP, Schlinger BA. Distribution and regulation of telencephalic aromatase expression in the zebra finch revealed with a specific antibody. J Comp Neurol. 2000;423:619–630. doi: 10.1002/1096-9861(20000807)423:4<619::aid-cne7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Scharff C, Kirn JR, Grossman M, Macklis JD, Nottebohm F. Targeted neuronal death affects neuronal replacement and vocal behavior in adult songbirds. Neuron. 2000;25:481–492. doi: 10.1016/s0896-6273(00)80910-1. [DOI] [PubMed] [Google Scholar]
- Schlinger BA, Amur-Umarjee S, Shen P, Campagnoni AT, Arnold AP. Neuronal and non-neuronal aromatase in primary cultures of developing zebra finch telencephalon. J Neurosci. 1994;14:7541–7552. doi: 10.1523/JNEUROSCI.14-12-07541.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlinger BA, Callard GV. Localization of aromatase in synaptosomal and microsomal subfractions of quail (Coturnix coturnix japonica) brain. Neuroendocrinology. 1989;49:434–441. doi: 10.1159/000125149. [DOI] [PubMed] [Google Scholar]
- Schlinger BA, Callard GV. Aromatization mediates aggressive behavior in quail. Gen Comp Endocrinol. 1990;79:39–53. doi: 10.1016/0016-6480(90)90086-2. [DOI] [PubMed] [Google Scholar]
- Schlinger BA, Soma KK, London SE. Neurosteroids and brain sexual differentiation. Trends Neurosci. 2001;24:429–431. doi: 10.1016/s0166-2236(00)01855-5. [DOI] [PubMed] [Google Scholar]
- Schumacher M, Contenti E, Balthazart J. Partial characterization of testosterone-metabolizing enzymes in the quail brain. Brain Res. 1984;305:51–59. doi: 10.1016/0006-8993(84)91118-1. [DOI] [PubMed] [Google Scholar]
- Sharp PJ. Strategies in avian breeding cycles. Anim Reprod Sci. 1996;42:505–513. [Google Scholar]
- Sharp PJ, Follett BK. The effect of hypothalamic lesions on gonadotrophin release in Japanese quail (Coturnix coturnix japonica) Neuroendocrinology. 1969;5:205–218. doi: 10.1159/000121861. [DOI] [PubMed] [Google Scholar]
- Shaw BK. Involvement of a midbrain vocal nucleus in the production of both the acoustic and postural components of crowing behavior in Japanese quail. J Comp Physiol [A] 2000;186:747–757. doi: 10.1007/s003590000128. [DOI] [PubMed] [Google Scholar]
- Shen P, Campagnoni CW, Kampf K, Schlinger BA, Arnold AP, Campagnoni AT. Isolation and characterization of a zebra finch aromatase cDNA: in situ hybridization reveals high aromatase expression in brain. Mol Brain Res. 1994;24:227–237. doi: 10.1016/0169-328x(94)90136-8. [DOI] [PubMed] [Google Scholar]
- Sherry DF, Vaccarino AL, Buckenham K, Herz RS. The hippocampal complex of food-storing birds. Brain Behav Evol. 1989;34:308–317. doi: 10.1159/000116516. [DOI] [PubMed] [Google Scholar]
- Silver R, Witkowsky P, Horvath P, Alones V, Barnstable CJ, Lehman MN. Coexpression of opsin- and VIP-like immunoreactivity in CSF-contacting neurons of the avian brain. Cell Tissue Res. 1988;253:189–198. doi: 10.1007/BF00221754. [DOI] [PubMed] [Google Scholar]
- Smulders TV, Sasson AD, DeVoogd TJ. Seasonal variation in hippocampal volume in a food-storing bird, the black-capped chickadee. J Neurobiol. 1995;27:15–25. doi: 10.1002/neu.480270103. [DOI] [PubMed] [Google Scholar]
- Smulders TV, Shiflett MW, Sperling AJ, DeVoogd TJ. Seasonal changes in neuron numbers in the hippocampal formation of a food-hoarding bird: the black-capped chickadee. J Neurobiol. 2000;44:414–422. doi: 10.1002/1097-4695(20000915)44:4<414::aid-neu4>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Soma KK, Alday NA, Hau M, Schlinger BA. Dehydroepiandrosterone metabolism by 3β-hydroxysteroid dehydrogenase/Δ 5-Δ 4 isomerase in adult zebra finch brain: Sex difference and rapid effect of stress. Endocrinology. 2004a;145:1668–1677. doi: 10.1210/en.2003-0883. [DOI] [PubMed] [Google Scholar]
- Soma KK, Sullivan K, Wingfield J. Combined aromatase inhibitor and antiandrogen treatment decreases territorial aggression in a wild songbird during the nonbreeding season. Gen Comp Endocrinol. 1999;115:442–453. doi: 10.1006/gcen.1999.7334. [DOI] [PubMed] [Google Scholar]
- Soma KK, Sullivan KA, Tramontin AD, Saldanha CJ, Schlinger BA, Wingfield JC. Acute and chronic effects of an aromatase inhibitor on territorial aggression in breeding and nonbreeding male song sparrows. J Comp Physiol A. 2000a;186:759–769. doi: 10.1007/s003590000129. [DOI] [PubMed] [Google Scholar]
- Soma KK, Tramontin AD, Featherstone J, Brenowitz EA. Estrogen contributes to seasonal plasticity of the adult avian song control system. J Neurobiol. 2004b;58:413–422. doi: 10.1002/neu.10288. [DOI] [PubMed] [Google Scholar]
- Soma KK, Tramontin AD, Wingfield JC. Oestrogen regulates male aggression in the non-breeding season. Proc R Soc Lond B. 2000b;267:1089–1096. doi: 10.1098/rspb.2000.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soma KK, Wingfield JC. Endocrinology of aggression in the nonbreeding season. 22nd International Ornithological Congress; 1999. pp. 1606–1620. [Google Scholar]
- Soma KK, Wingfield JC. Dehydroepiandrosterone in songbird plasma: Seasonal regulation and relationship to territorial aggression. Gen Comp Endocrinol. 2001;123:144–155. doi: 10.1006/gcen.2001.7657. [DOI] [PubMed] [Google Scholar]
- Soma KK, Wissman AM, Brenowitz EA, Wingfield JC. Dehydroepiandrosterone (DHEA) increases territorial song and the size of an associated brain region in a male songbird. Horm Behav. 2002;41:203–212. doi: 10.1006/hbeh.2001.1750. [DOI] [PubMed] [Google Scholar]
- Thijssen JHH, Nieuwenhuyse H, editors. DHEA: A Comprehensive Review. Parthenon Publishing Group; New York: 1999. [Google Scholar]
- Thompson RR, Goodson JL, Ruscio MG, Adkins-Regan E. Role of the archistriatal nucleus taeniae in the sexual behavior of male Japanese quail (Coturnix japonica): A comparison of function with the medial nucleus of the amygdala in mammals. Brain Behav Evol. 1998;51:215–229. doi: 10.1159/000006539. [DOI] [PubMed] [Google Scholar]
- Tlemçani O, Ball GF, D'Hondt E, Vandesande F, Sharp PJ, Balthazart J. Fos induction in the Japanese quail brain after expression of appetitive and consummatory aspects of male sexual behavior. Brain Res Bull. 2000;52:249–262. doi: 10.1016/s0361-9230(00)00233-1. [DOI] [PubMed] [Google Scholar]
- Tramontin AD, Wingfield JC, Brenowitz EA. Androgens and estrogens induce seasonal-like growth of song nuclei in the adult songbird brain. J Neurobiol. 2003;57:130–140. doi: 10.1002/neu.10263. [DOI] [PubMed] [Google Scholar]
- Tramontin AD, Brenowitz EA. Seasonal plasticity in the adult brain. Trends Neurosci. 2000;23:251–258. doi: 10.1016/s0166-2236(00)01558-7. [DOI] [PubMed] [Google Scholar]
- Tsutsui K, Matsunaga M, Ukena K. Biosynthesis and biological actions of neurosteroids in the avian brain. Avian Poult Biol Rev. 2003;14:63–78. [Google Scholar]
- Tsutsui K, Yamazaki T. Avian neurosteroids. I. Pregnenolone biosynthesis in the quail brain. Brain Res. 1995;678:1–9. doi: 10.1016/0006-8993(95)00116-8. [DOI] [PubMed] [Google Scholar]
- Ukena K, Honda Y, Inai Y, Kohchi C, Lea RW, Tsutsui K. Expression and activity of 3β-hydroxysteroid dehydrogenase/Δ5-Δ4 isomerase in different regions of the avian brain. Brain Res. 1999;818:536–542. doi: 10.1016/s0006-8993(98)01296-7. [DOI] [PubMed] [Google Scholar]
- Viglietti-Panzica C, Anselmetti GC, Balthazart J, Aste N, Panzica GC. Vasotocinergic innervation of the septal region in the Japanese quail: sexual differences and the influence of testosterone. Cell Tissue Res. 1992;267:261–265. [Google Scholar]
- Viglietti-Panzica C, Aste N, Balthazart J, Panzica GC. Vasotocinergic innervation of sexually dimorphic medial preoptic nucleus of the male Japanese quail: Influence of testosterone. Brain Res. 1994;657:171–184. doi: 10.1016/0006-8993(94)90965-2. [DOI] [PubMed] [Google Scholar]
- Viglietti-Panzica C, Balthazart J, Plumari L, Fratesi S, Absil P, Panzica GC. Estradiol mediates effects of testosterone on vasotocin immunoreactivity in the adult quail brain. Horm Behav. 2001;40:445–461. doi: 10.1006/hbeh.2001.1710. [DOI] [PubMed] [Google Scholar]
- Vockel A, Prove E, Balthazart J. Effects of castration and testosterone treatment on the activity of testosterone-metabolizing enzymes in the brain of male and female zebra finches. J Neurobiol. 1990;21:808–825. doi: 10.1002/neu.480210514. [DOI] [PubMed] [Google Scholar]
- Voorhuis TAM, Kiss JZ, De Kloet ER, De Wied D. Testosterone-sensitive vasotocin-immunoreactive cells and fibers in the canary brain. Brain Res. 1988;442:139–146. doi: 10.1016/0006-8993(88)91441-2. [DOI] [PubMed] [Google Scholar]
- Wade J, Arnold AP. Functional testicular tissue does not masculinize development of the zebra finch song system. Proc Natl Acad Sci U S A. 1996;93:5264–5268. doi: 10.1073/pnas.93.11.5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Yasuo S, Watanabe T, Yamamura T, Nakao N, Ebihara S, Yoshimura T. Photoperiod regulation of type 2 deiodinase gene in Djungarian hamster: Possible homologies between avian and mammalian photoperiodic regulation of reproduction. Endocrinology. 2004;145:2546–2549. doi: 10.1210/en.2003-1593. [DOI] [PubMed] [Google Scholar]
- Wilson FE, Reinert BD. Thyroid hormones acts centrally to programme photostimulated male American tree sparrows (Spizella arborea) J Neuroendocrinol. 2000;12:87–95. doi: 10.1046/j.1365-2826.2000.00437.x. [DOI] [PubMed] [Google Scholar]
- Wingfield JC. Regulation of territorial behavior in the sedentary song sparrow, Melospiza melodia morphna. Horm Behav. 1994;28:1–15. doi: 10.1006/hbeh.1994.1001. [DOI] [PubMed] [Google Scholar]
- Wingfield JC, Hahn TP. Testosterone and territorial behavior in sedentary and migratory sparrows. Anim Behav. 1994;47:77–89. [Google Scholar]
- Wynne RD, Saldanha CJ. Glial aromatization decreases neural injury in the zebra finch Taeniopygia guttata: influence on apoptosis. J Neuroendocrinol. 2004;16:676–683. doi: 10.1111/j.1365-2826.2004.01217.x. [DOI] [PubMed] [Google Scholar]
- Yamamura T, Hirunagi K, Ebihara S, Yoshimura T. Seasonal morphological changes in the neuro-glial interaction between gonadotropin-releasing hormone nerve terminals and glial endfeet in Japanese quail. Endocrinology. 2004;145:4264–4267. doi: 10.1210/en.2004-0366. [DOI] [PubMed] [Google Scholar]
- Yasuo S, Watanabe M, Okabayashi N, Ebihara S, Yoshimura T. Circadian clock genes and photoperiodism: Comprehensive analysis of clock gene expression in the mediobasal hypothalamus, the suprachiasmatic nucleus, and the pineal gland of Japanese Quail under various light schedules. Endocrinology. 2003;144:3742–3748. doi: 10.1210/en.2003-0435. [DOI] [PubMed] [Google Scholar]
- Yoshimura T, Yasuo S, Watanabe M, Iigo M, Yamamura T, Hirunagi K, Ebihara S. Light-induced hormone conversion of T4 to T3 regulates photoperiod response of gonads in birds. Nature. 2003;426:178–181. doi: 10.1038/nature02117. [DOI] [PubMed] [Google Scholar]
- Zhao HF, Labrie C, Simard J, Delaunoit Y, Trudel C, Martel C, Rheaume E, Dupont E, Luuthe V, Pelletier G, Labrie F. Characterization of rat 3β-hydroxysteroid dehydrogenase Δ5-Δ-4 isomerase cdnas and differential tissue-specific expression of the corresponding messenger rnas in steroidogenic and peripheral tissues. J Biol Chem. 1991;266:583–593. [PubMed] [Google Scholar]
- Zwain IH, Yen SSC. Dehydroepiandrosterone: biosynthesis and metabolism in the brain. Endocrinology. 1999;140:880–887. doi: 10.1210/endo.140.2.6528. [DOI] [PubMed] [Google Scholar]


