Abstract
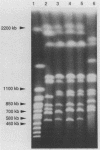
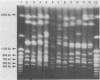
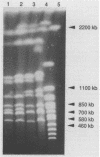
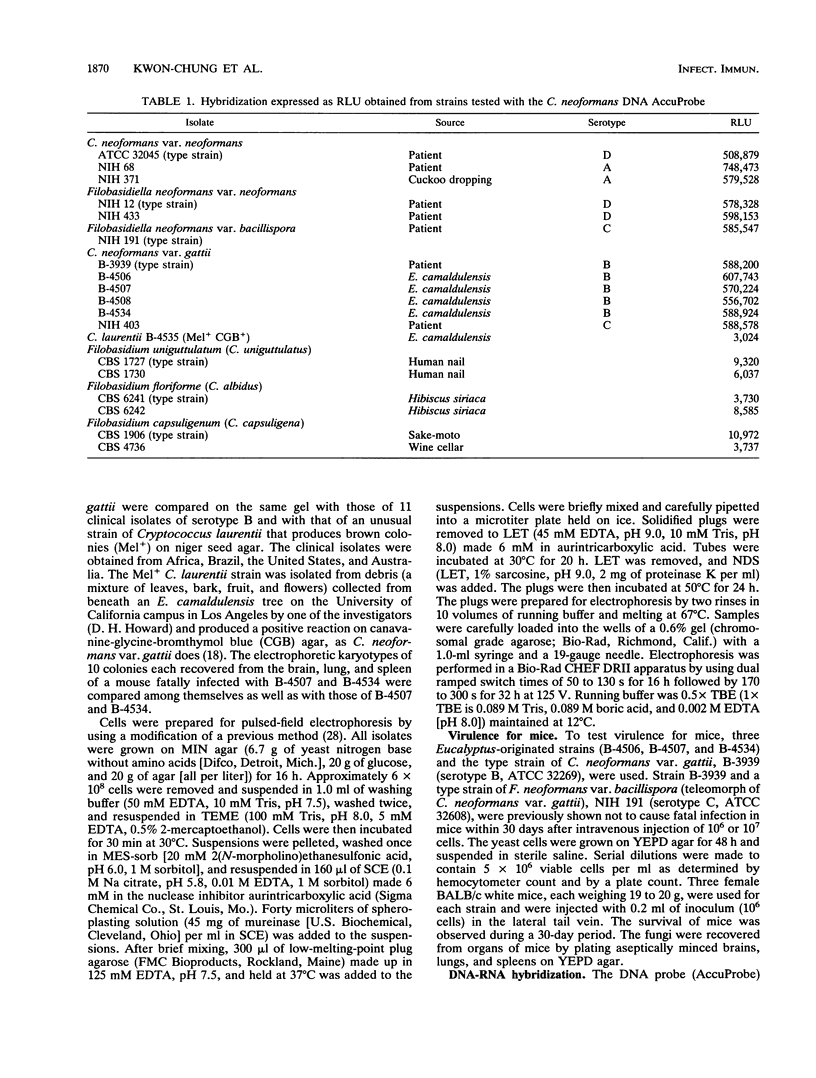
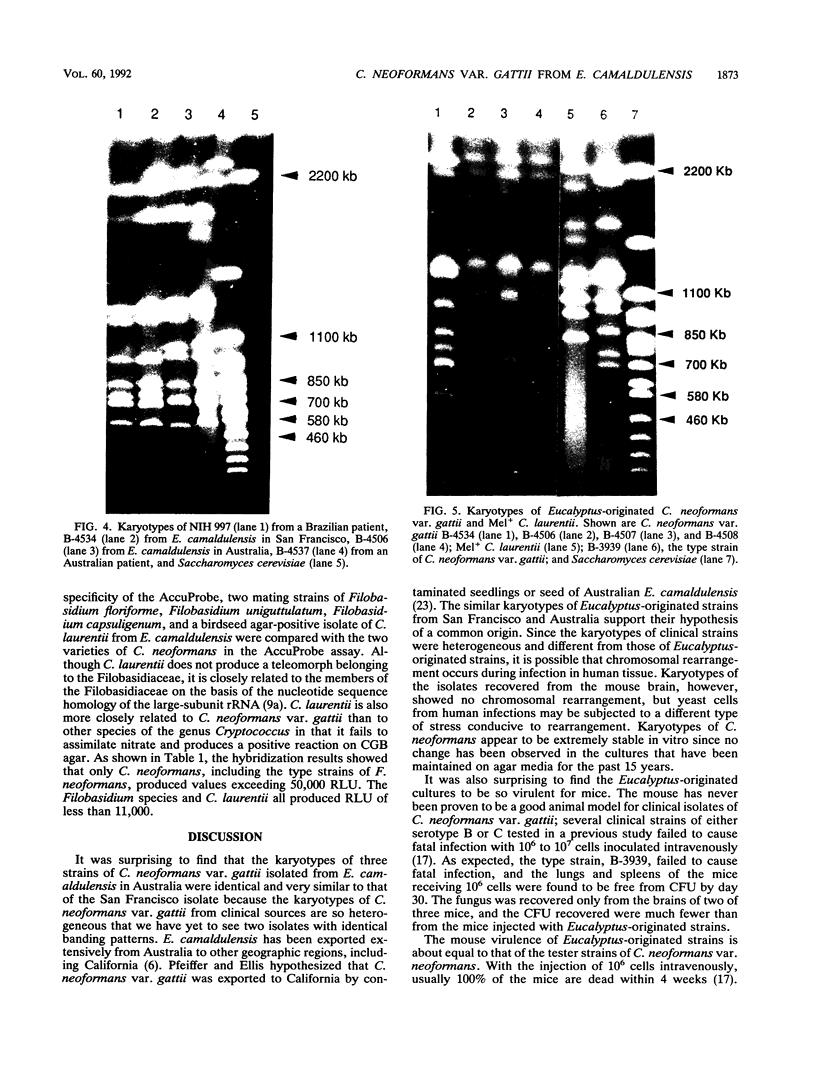
Four strains of Cryptococcus neoformans var. gattii originating from Eucalyptus camaldulensis, three from Australia and one from San Francisco, were tested for their serotype, virulence for mice, and a number of genetic and molecular characteristics. All were found to be serotype B and showed significantly higher virulence for mice than did the type strains of C. neoformans var. gattii and Filobasidiella neoformans var. bacillispora, which were obtained from human cryptococcosis cases. Electrophoretic karyotypes of the strains from Australia were identical, although they were collected from sites at least 15 to 500 km apart. The electrophoretic karyotype of the strain from San Francisco was the same as that of the Australian isolates except for the mobility of one chromosome. On the contrary, no two isolates of serotype B (of a total of 11) from clinical sources were the same, regardless of their geographic origin. Furthermore, none of the clinical isolates showed a chromosomal banding pattern identical to that of Eucalyptus-originated strains. The Eucalyptus-originated strains failed to form dikaryons when crossed with the tester strains of the two varieties of F. neoformans. Hybridization analysis with a nucleic acid probe (AccuProbe C. neoformans Culture Confirmation Test; Gen-Probe Inc., San Diego, Calif.), however, showed signals of equal intensity for clinical strains and the Eucalyptus-originated strains. Various fungi phylogenetically related to C. neoformans, including a phenol oxidase-positive strain of Cryptococcus laurentii obtained from E. camaldulensis, were negative in the nucleic acid hybridization test. These observations confirm that, in spite of karyotypic differences and the lack of dikaryon formation with the tester strains of F. neoformans, Eucalyptus-originated C. neoformans var. gattii is the same organism as those isolated from cases of human infection. Furthermore, the C. neoformans culture confirmation test using a commercial nucleic acid probe is specific for C. neoformans.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AJELLO L. Occurrence of Cryptococcus neoformans in soils. Am J Hyg. 1958 Jan;67(1):72–77. doi: 10.1093/oxfordjournals.aje.a119921. [DOI] [PubMed] [Google Scholar]
- Bauwens L., Swinne D., De Vroey C., De Meurichy W. Isolation of Cryptococcus neoformans var. neoformans in the aviaries of the Antwerp Zoological Gardens. Mykosen. 1986 Jul;29(7):291–294. [PubMed] [Google Scholar]
- Bennett J. E., Kwon-Chung K. J., Howard D. H. Epidemiologic differences among serotypes of Cryptococcus neoformans. Am J Epidemiol. 1977 Jun;105(6):582–586. doi: 10.1093/oxfordjournals.aje.a112423. [DOI] [PubMed] [Google Scholar]
- EMMONS C. W. Isolation of Cryptococcus neoformans from soil. J Bacteriol. 1951 Dec;62(6):685–690. doi: 10.1128/jb.62.6.685-690.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMMONS C. W. Saprophytic sources of Cryptococcus neoformans associated with the pigeon (Columba livia). Am J Hyg. 1955 Nov;62(3):227–232. doi: 10.1093/oxfordjournals.aje.a119775. [DOI] [PubMed] [Google Scholar]
- Ellis D. H. Cryptococcus neoformans var. gattii in Australia. J Clin Microbiol. 1987 Feb;25(2):430–431. doi: 10.1128/jcm.25.2.430-431.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis D. H., Pfeiffer T. J. Natural habitat of Cryptococcus neoformans var. gattii. J Clin Microbiol. 1990 Jul;28(7):1642–1644. doi: 10.1128/jcm.28.7.1642-1644.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensley P. K., Davis C. E., Anderson M. P., Fletcher K. C. Cryptococcosis in a male Beccari's crowned pigeon. J Am Vet Med Assoc. 1979 Nov 1;175(9):992–994. [PubMed] [Google Scholar]
- Kwon-Chung K. J. A new species of Filobasidiella, the sexual state of Cryptococcus neoformans B and C serotypes. Mycologia. 1976 Jul-Aug;68(4):943–946. [PubMed] [Google Scholar]
- Kwon-Chung K. J., Bennett J. E. Distribution of alpha and alpha mating types of Cryptococcus neoformans among natural and clinical isolates. Am J Epidemiol. 1978 Oct;108(4):337–340. doi: 10.1093/oxfordjournals.aje.a112628. [DOI] [PubMed] [Google Scholar]
- Kwon-Chung K. J., Bennett J. E. Epidemiologic differences between the two varieties of Cryptococcus neoformans. Am J Epidemiol. 1984 Jul;120(1):123–130. doi: 10.1093/oxfordjournals.aje.a113861. [DOI] [PubMed] [Google Scholar]
- Kwon-Chung K. J., Bennett J. E. High prevalence of Cryptococcus neoformans var. gattii in tropical and subtropical regions. Zentralbl Bakteriol Mikrobiol Hyg A. 1984 Jul;257(2):213–218. [PubMed] [Google Scholar]
- Kwon-Chung K. J., Bennett J. E., Rhodes J. C. Taxonomic studies on Filobasidiella species and their anamorphs. Antonie Van Leeuwenhoek. 1982;48(1):25–38. doi: 10.1007/BF00399484. [DOI] [PubMed] [Google Scholar]
- Kwon-Chung K. J., Polacheck I., Bennett J. E. Improved diagnostic medium for separation of Cryptococcus neoformans var. neoformans (serotypes A and D) and Cryptococcus neoformans var. gattii (serotypes B and C). J Clin Microbiol. 1982 Mar;15(3):535–537. doi: 10.1128/jcm.15.3.535-537.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon-Chung K. J., Rhodes J. C. Encapsulation and melanin formation as indicators of virulence in Cryptococcus neoformans. Infect Immun. 1986 Jan;51(1):218–223. doi: 10.1128/iai.51.1.218-223.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurudeen T. A., Ahearn D. G. Regulation of melanin production by Cryptococcus neoformans. J Clin Microbiol. 1979 Nov;10(5):724–729. doi: 10.1128/jcm.10.5.724-729.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer T., Ellis D. Environmental isolation of Cryptococcus neoformans gattii from California. J Infect Dis. 1991 Apr;163(4):929–930. doi: 10.1093/infdis/163.4.929. [DOI] [PubMed] [Google Scholar]
- Polacheck I., Hearing V. J., Kwon-Chung K. J. Biochemical studies of phenoloxidase and utilization of catecholamines in Cryptococcus neoformans. J Bacteriol. 1982 Jun;150(3):1212–1220. doi: 10.1128/jb.150.3.1212-1220.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinne-Desgain D. Cryptococcus neoformans of saprophytic origin. Sabouraudia. 1975 Nov;13(3):303–308. [PubMed] [Google Scholar]
- Wickes B. L., Golin J. E., Kwon-Chung K. J. Chromosomal rearrangement in Candida stellatoidea results in a positive effect on phenotype. Infect Immun. 1991 May;59(5):1762–1771. doi: 10.1128/iai.59.5.1762-1771.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]