Abstract
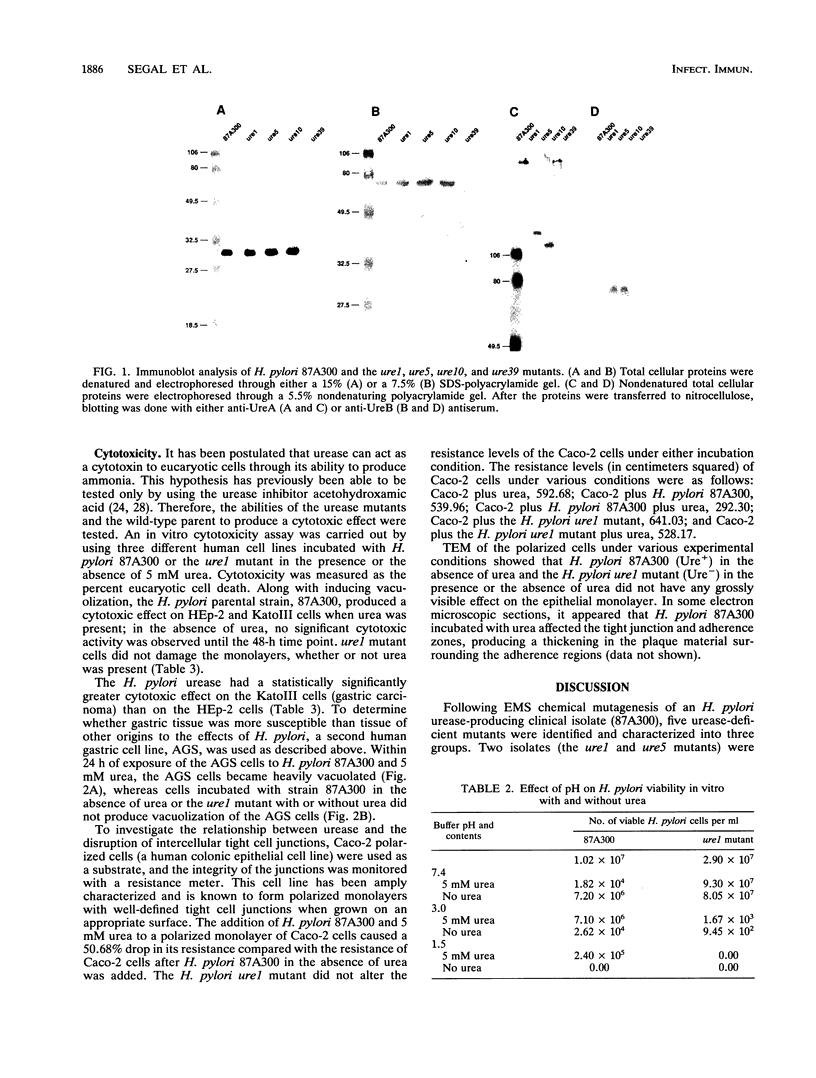
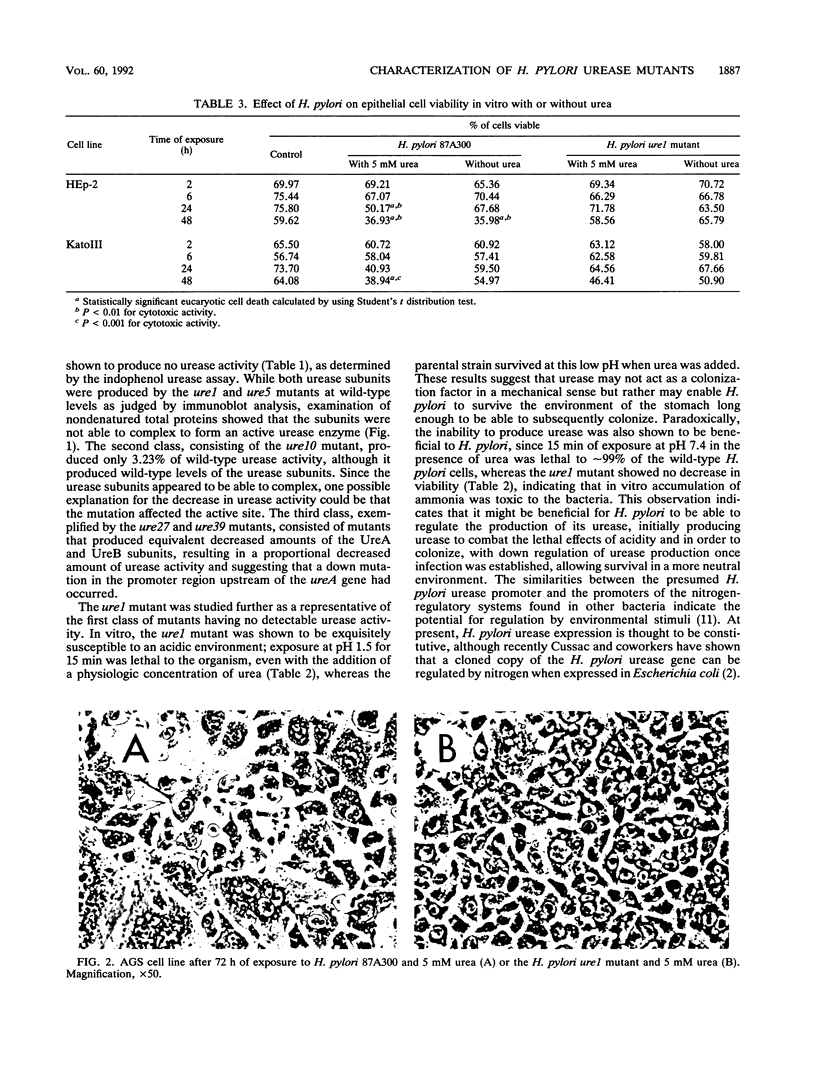
The association between Helicobacter pylori, gastritis, and peptic ulcer is well established, and the association of infection with gastric cancer has been noted in several developing countries. However, the pathogenic mechanism(s) leading to disease states has not been elucidated. The H. pylori urease is thought to be a determinant of pathogenicity, since the enzyme is produced by all H. pylori clinical isolates. Evidence indicates that some H. pylori strains are more cytotoxic than others, with a correlation between the activity of the urease and the presence of a vacuolating cytotoxin having been made. However, the number of cytotoxins remains unknown at this time. The relationship between the urease and cytotoxicity has previously been examined with chemical inhibitors. To examine the role of the urease and its relationship to cytotoxicity, urease-deficient mutants were produced following ethyl methanesulfonate mutagenesis of H. pylori 87A300. Two mutants (the ure1 and ure5 mutants) which were entirely deficient in urease activity (Ure-) were selected. Characterization of the isolates at the protein level showed that the urease subunits lacked the ability to complex and form the active urease enzyme. The ure1 mutant was shown to be sensitive to the effects of low pH in vitro and exhibited no cytotoxicity to eucaryotic cells, whereas the parental strain (Ure+) produced a cytotoxic effect in the presence of urea. Interaction between the H. pylori Ure+ and Ure- strains and Caco-2 cells appeared to be similar in that both bacterial types elicited pedestal formation and actin condensation. These results indicate that the H. pylori urease may have many functions, among them (i) protecting H. pylori against the acidic environment of the stomach, (ii) acting as a cytotoxin, with human gastric cells especially susceptible to its activity, and (iii) disrupting cell tight junctions in such a manner that the cells remain viable but an ionic flow between the cells occurs.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Eaton K. A., Brooks C. L., Morgan D. R., Krakowka S. Essential role of urease in pathogenesis of gastritis induced by Helicobacter pylori in gnotobiotic piglets. Infect Immun. 1991 Jul;59(7):2470–2475. doi: 10.1128/iai.59.7.2470-2475.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figura N., Guglielmetti P., Rossolini A., Barberi A., Cusi G., Musmanno R. A., Russi M., Quaranta S. Cytotoxin production by Campylobacter pylori strains isolated from patients with peptic ulcers and from patients with chronic gastritis only. J Clin Microbiol. 1989 Jan;27(1):225–226. doi: 10.1128/jcm.27.1.225-226.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazell S. L., Lee A., Brady L., Hennessy W. Campylobacter pyloridis and gastritis: association with intercellular spaces and adaptation to an environment of mucus as important factors in colonization of the gastric epithelium. J Infect Dis. 1986 Apr;153(4):658–663. doi: 10.1093/infdis/153.4.658. [DOI] [PubMed] [Google Scholar]
- Hazell S. L., Lee A. Campylobacter pyloridis, urease, hydrogen ion back diffusion, and gastric ulcers. Lancet. 1986 Jul 5;2(8497):15–17. doi: 10.1016/s0140-6736(86)92561-4. [DOI] [PubMed] [Google Scholar]
- Hecht G., Pothoulakis C., LaMont J. T., Madara J. L. Clostridium difficile toxin A perturbs cytoskeletal structure and tight junction permeability of cultured human intestinal epithelial monolayers. J Clin Invest. 1988 Nov;82(5):1516–1524. doi: 10.1172/JCI113760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessey S. J., Spencer J., Wyatt J. I., Sobala G., Rathbone B. J., Axon A. T., Dixon M. F. Bacterial adhesion and disease activity in Helicobacter associated chronic gastritis. Gut. 1990 Feb;31(2):134–138. doi: 10.1136/gut.31.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L. T., Mobley H. L. Purification and N-terminal analysis of urease from Helicobacter pylori. Infect Immun. 1990 Apr;58(4):992–998. doi: 10.1128/iai.58.4.992-998.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labigne A., Cussac V., Courcoux P. Shuttle cloning and nucleotide sequences of Helicobacter pylori genes responsible for urease activity. J Bacteriol. 1991 Mar;173(6):1920–1931. doi: 10.1128/jb.173.6.1920-1931.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leunk R. D., Johnson P. T., David B. C., Kraft W. G., Morgan D. R. Cytotoxic activity in broth-culture filtrates of Campylobacter pylori. J Med Microbiol. 1988 Jun;26(2):93–99. doi: 10.1099/00222615-26-2-93. [DOI] [PubMed] [Google Scholar]
- Madara J. L. Loosening tight junctions. Lessons from the intestine. J Clin Invest. 1989 Apr;83(4):1089–1094. doi: 10.1172/JCI113987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madara J. L., Stafford J. Interferon-gamma directly affects barrier function of cultured intestinal epithelial monolayers. J Clin Invest. 1989 Feb;83(2):724–727. doi: 10.1172/JCI113938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Palomo A. Ultrastructural modifications of intercellular junctions in some epithelial tumors. Lab Invest. 1970 Jun;22(6):605–614. [PubMed] [Google Scholar]
- Mobley H. L., Cortesia M. J., Rosenthal L. E., Jones B. D. Characterization of urease from Campylobacter pylori. J Clin Microbiol. 1988 May;26(5):831–836. doi: 10.1128/jcm.26.5.831-836.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley H. L., Hausinger R. P. Microbial ureases: significance, regulation, and molecular characterization. Microbiol Rev. 1989 Mar;53(1):85–108. doi: 10.1128/mr.53.1.85-108.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura A., Stemmermann G. N., Chyou P. H., Kato I., Perez-Perez G. I., Blaser M. J. Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. N Engl J Med. 1991 Oct 17;325(16):1132–1136. doi: 10.1056/NEJM199110173251604. [DOI] [PubMed] [Google Scholar]
- Parsonnet J., Friedman G. D., Vandersteen D. P., Chang Y., Vogelman J. H., Orentreich N., Sibley R. K. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991 Oct 17;325(16):1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- Parsonnet J., Vandersteen D., Goates J., Sibley R. K., Pritikin J., Chang Y. Helicobacter pylori infection in intestinal- and diffuse-type gastric adenocarcinomas. J Natl Cancer Inst. 1991 May 1;83(9):640–643. doi: 10.1093/jnci/83.9.640. [DOI] [PubMed] [Google Scholar]
- Sarosiek J., Slomiany A., Slomiany B. L. Evidence for weakening of gastric mucus integrity by Campylobacter pylori. Scand J Gastroenterol. 1988 Jun;23(5):585–590. doi: 10.3109/00365528809093916. [DOI] [PubMed] [Google Scholar]
- Simons K., Fuller S. D. Cell surface polarity in epithelia. Annu Rev Cell Biol. 1985;1:243–288. doi: 10.1146/annurev.cb.01.110185.001331. [DOI] [PubMed] [Google Scholar]
- Smoot D. T., Mobley H. L., Chippendale G. R., Lewison J. F., Resau J. H. Helicobacter pylori urease activity is toxic to human gastric epithelial cells. Infect Immun. 1990 Jun;58(6):1992–1994. doi: 10.1128/iai.58.6.1992-1994.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983 Jun 4;1(8336):1273–1275. [PubMed] [Google Scholar]
- Weinstein R. S., Merk F. B., Alroy J. The structure and function of intercellular junctions in cancer. Adv Cancer Res. 1976;23:23–89. doi: 10.1016/s0065-230x(08)60543-6. [DOI] [PubMed] [Google Scholar]
- Xu J. K., Goodwin C. S., Cooper M., Robinson J. Intracellular vacuolization caused by the urease of Helicobacter pylori. J Infect Dis. 1990 Jun;161(6):1302–1304. doi: 10.1093/infdis/161.6.1302. [DOI] [PubMed] [Google Scholar]





