Abstract
Antigen-specific precursor frequency is increasingly being appreciated as an important factor in determining the kinetics, magnitude, and degree of differentiation of T cell responses, and recently was found to play a critical role in determining the relative requirement of CD8+ T cells for CD28- and CD154-mediated costimulatory signals during transplantation. We addressed the possibility that variations in CD4+ T cell precursor frequency following transplantation might affect CD4+ T cell proliferation, effector function, and provision of help for donor-reactive B cell and CD8+ T cell responses. Using a transgenic model system wherein increasing frequencies of donor-reactive CD4+ T cells were transferred into skin graft recipients, we observed that a critical CD4+ T cell threshold precursor frequency was necessary to provide help following blockade of the CD28 and CD154 costimulatory pathways, as measured by increased B cell and CD8+ T cell responses and precipitation of graft rejection. In contrast to high-frequency CD8+ T cell responses, this effect was observed even though the proliferative and cytokine responses of Ag-specific CD4+ T cells were inhibited. Thus, we conclude that an initial high frequency of donor-reactive CD4+ T cells uncouples T cell proliferative and effector cytokine production from the provision of T cell help.
This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (The JI). The American Association of Immunologists, Inc. (AAI), publisher of The JI, holds the copyright to this manuscript. This version of the manuscript has not yet been copyedited or subjected to editorial proofreading by The JI; hence, it may differ from the final version published in The JI (online and in print). AAI (The JI) is not liable for errors or omissions in this author-produced version of the manuscript or in any version derived from it by the U.S. National Institutes of Health or any other third party. The final, citable version of record can be found at www.jimmunol.org
Keywords: Transplantation, precursor frequency, costimulation, CD4+ T-lymphocyte
Introduction
The two-signal model of T cell activation has been a dominant concept in immunology for the past several decades. To generate an effective response in vivo, T cells must receive signal 1 delivered via the T cell receptor (TCR), but also require costimulatory signals (signal 2) (1). The concept of blocking costimulatory pathways to prevent allograft rejection and to possibly achieve donor-specific tolerance has captivated the interest of the transplant community for many years. While a panoply of costimulatory molecules has been described over the years, two of the originally discovered pathways, CD28 and CD40, remain among the most pivotal identified for the execution of rejection. Short-term blockade of the CD28 pathway with the CTLA4-Ig fusion protein has proved to be a potent inhibitor of rejection and was useful as a component of tolerance regimens in many rodent transplant models (2-6). A large Phase II study of belatacept, a second-generation derivative of CTLA-4 Ig, in human recipients of de novo renal allografts demonstrated belatacept to be as effective as cyclosporine without evidence of nephrotoxicity (7). The CD40 pathway also has been a therapeutic target of great interest and of considerable consternation. Interactions between CD40 and its ligand CD154 play a crucial role in many aspects of the T cell response, including induction of B7 molecules and IL-12 on APC, effector functions of macrophages and Ig class switching in B cells (8). Anti-CD154 mAbs are potent inhibitors of rejection and are crucial for tolerance induction in many of the most stringent murine models (9-16). Furthermore, combined blockade of these pathways synergistically inhibits T cell response and allograft rejection in rodents and non-human primates (14, 17, 18).
Despite potent effects in many experimental rejection models, combined blockade of the CD28 and CD40 pathways does not consistently control rejection responses in all situations. For example, BALB/c → C3H skin grafts experience prolonged survival of >120 days following CD28/CD40 blockade, whereas engraftment of BALB/c skin onto B6 recipients treated with the same regimen results in only moderate prolongation of graft survival (14, 19-22). This costimulation blockade-resistant rejection was found to be impervious to increases in the dose or duration of CTLA-4 Ig/anti-CD154 treatment, suggesting that some T cell responses exhibit a fundamental reduction in the requirement for CD28 and CD154-mediated costimulatory signals (19). Studies of the strain-specific genetic factors that promote costimulation blockade-resistant rejection have shown that genes outside of MHC locus were primarily responsible for the differences in the observed efficacy of CD28/CD40 blockade (23). More recently, Kemball et al. found that costimulation blockade inhibits the CD8+ T cell response to polyoma virus in C3H mice, but not in B6 mice, demonstrating that the CD8+ T cell response is costimulation blockade-resistant in B6 mice in both viral and allograft models (24). Several studies have reported data implicating CD8+ T cells as the cell type primarily responsible for this costimulation blockade-resistant (19, 20, 22, 25, 26). Recently, we confirmed these findings with the demonstration that high-frequency CD8+ , with donor-reactive T cell help, were required for costimulation blockade-resistant rejection in a surrogate minor antigen mismatch model (27). The relative resistance of high-frequency CD8+ T cells to the effects of costimulation blockade may be due to differential requirements of CD4+ and CD8+ T cells for costimulatory signals during priming.
The requirement for CD4+ T cell help during allograft rejection is dependent on many factors including the type of tissue being transplanted, activation status of the responding T cells, and the degree of antigenic disparity present between the donor and recipient. In general, rejection of fully allogeneic grafts can be accomplished by donor-reactive CD8+ T cells alone (28). In contrast, rejection of minor antigen mismatched allografts requires the presence of CD4+ T cells to mediate complete rejection of the tissue (29-32). These discrepancies may result from the unique pathway of direct allorecognition that occurs in MHC mismatched situations, in which a high frequency of recipient-derived T cells recognize and respond to donor peptide:MHC complexes. Recent studies in models of pathogen-specific immune responses have characterized a phenotype of “helpless” CD8+ T cells, which can mount an effective primary immune response but have impaired ability differentiate into fully competent effectors, to sustain the response, and to generate potent secondary responses upon recall (33-36). Studies in models of transplantation have corroborated these results, finding that, in certain model systems, CD4-deficient animals develop an alloreactive CD8+ T cell response and reject the graft, yet exhibit reduced CD8+ T cell cytolytic function and memory cell formation (37-40).
As noted above, our previous studies implicated an important role for high-frequency CD8+ T cell populations in mediating costimulation blockade-resistant rejection, in that graft-specific CD8+ T cells stimulated at high frequency proliferated and accumulated even in the presence of CTLA-4 Ig and anti-CD154, resulting in more “high-quality,” multi-cytokine producing effector cells and the precipitation of graft rejection (27). In contrast, graft-specific CD8+ T cells stimulated at lower frequency failed to accumulate and did not differentiate into high quality effectors that were capable of rejecting a skin graft. These experiments were done in the presence of an elevated level of graft-specific CD4+ cells, within the range of physiologic allospecific T cell frequencies, to provide adequate T cell help. In this study, we sought to address the requirement for this antigen-specific CD4+ T cell help in the development of costimulation blockade-resistant rejection, and to determine the impact of CD4+ helper precursor frequency on the development of costimulation blockade-resistant rejection. Specifically, we examined the outcome of graft rejection or acceptance when high frequency CD8+ T cells were stimulated in the presence of high or low frequency CD4+ helper T cells. To accomplish this, we made use of a model system wherein TCR transgenic OT-I (CD8+) and OT-II (CD4+) T cells are adoptively transferred into naïve B6 recipients, raising the precursor frequency of antigen-specific cells prior to engraftment with Act-mOVA skin, which constitutively expresses full length membrane-bound chicken ovalbumin (mOVA) protein under control of the β-actin promoter (29). We observed a critical CD4+ helper T cell threshold precursor frequency that allowed for the provision of T cell help even in the presence of costimulation blockade. Interestingly, high-frequency CD4+ T cell populations were able to provide T cell help, as measured by increased B cell and CD8+ T cell responses, even though the proliferative and cytokine responses of those T cells were completely inhibited by costimulation blockade. Thus, we conclude that an initial high frequency of CD4+ T cells uncouples T cell proliferation and cytokine production from the provision of T cell help.
Methods
Mice
Adult male 6- to 8-week old C57BL/6, BALB/c, C3H/HeJ, 129, BALB.B, and B6.SJL were purchased from the Jackson Laboratory (Bar Harbor, ME). TCR transgenic OT-I and OT-II mice were purchased from Taconic, Inc. and were bred onto RAG-/- and Thy1.1+ backgrounds. Act-mOVA mice were produced and provided by Dr. Marc Jenkins, Univ. of Minnesota (29). Animals received humane care and treatment in accordance with Emory University Institutional Animal Care and Use Committee guidelines.
Skin Grafting and Costimulation Blockade
Full thickness skin grafts (∼1 cm2 ) were transplanted onto the dorsal thorax of recipient mice and secured with a plastic adhesive bandage for 5 days. Graft survival was then monitored by daily visual inspection. Rejection was defined as the complete loss of viable epidermal tissue. Statistical analyses were performed using one-way ANOVA followed by Dunn’s post-test comparing the relevant data sets (GraphPad Prism Software). Where indicated, recipients of skin grafts received treatment with 500 μg each of hamster anti-mouse CD40L mAb (MR-1, Bioexpress) and human CTLA-4 Ig (Bristol-Meyers Squibb) administered i.p. on the day of transplantation (day 0) as well as on post-transplant days 2, 4, and 6.
T Cell Adoptive Transfers
OT-I and OT-II TCR tg T cells were harvested from the spleens of OT-IxThy1.1+ xRAG-/- and OT-IIxThy1.1+ xRAG-/- mice, respectively. Single cell suspensions were prepared and counted, and the frequency of OT-I or OT-II T cells within the splenocytes preparation was determined prior to adoptive transfer by staining with anti-Vα2 (used by both TCRs) and anti-CD8 or anti-CD4, respectively (Pharmingen, San Diego, CA). Cells were labeled with 5 μM CFSE prior to adoptive transfer. Mice received a single i.v. injection of the indicated number of OT-I or OT-II T cells along with syngeneic B6 carrier splenocytes.
Flow Cytometric Analyses for Frequency and Absolute Number
At the indicated timepoints, recipients of OT-I and/or OT-II T cell transfers were sacrificed, spleens and draining axillary lymph nodes were harvested, and single cell suspensions were prepared. Cells were stained with Thy1.1-PerCP, CD8-PacOrange, and CD4-PacBlue (all BD Pharmingen) for flow cytometric analysis on a BD LSRII multi-color flow cytometer. In some cases, absolute number of antigen-specific T cells was determined by TruCount Bead Analysis (Pharmingen) according to manufacturer’s instuctions. Flow cytometric data were analyzed using FlowJo Software (Treestar, San Carlos, CA).
Intracellular Cytokine Staining
For measurement of IFN-γ and TNF-α secreting cells, single cell suspensions of splenocytes or draining axillary LN cells from adoptive transfer/skin graft recipients (1×106 per well) were incubated in a 96 well plate with 10 nM OVA257-264 (SIINFEKL), 10 μM OVA 323-339 (ISQAVHAAHAEINEAGR) (Emory University Microchemical Core Facility), and 10 μg/ml Brefeldin A (Pharmingen, San Diego, CA). After 6 hours in culture, cells were processed using an intracellular staining kit (Pharmingen, San Diego, CA) according to manufacturer’s instructions and stained with anti-TNF-α-PE, anti-IFN-γ-APC, anti-Thy1.1-PerCP, anti-CD8-Pacific Orange, and anti-CD4-Pacific Blue (all from Pharmingen).
Statistical Analyses
Survival times for skin graft experiments were compared by log-rank test, and numbers of donor-specific T cells and antibody responses were compared by Mann-Whitney non-parametric test.
Results
High-frequency CD8+ T cell responses require a critical threshold of CD4+ T cell help in order to mediate costimulation blockade-resistant rejection
Our previous studies implicated an important role for high-frequency CD8+ T cell populations in mediating costimulation blockade-resistant rejection, in that graft-specific CD8+ T cells stimulated at high frequency proliferated and accumulated even in the presence of CTLA-4 Ig and anti-CD154, resulting in more high-quality effector cells and the precipitation of graft rejection (27). In contrast, graft-specific CD8+ T cells stimulated at a lower frequency failed to accumulate and did not differentiate into high quality effectors that were capable of rejecting a skin graft. These experiments were done in the presence of a constant, but elevated, level of graft-specific CD4+ cells to provide adequate T cell help. To address the requirement for antigen-specific CD4+ T cell help in the development of costimulation blockade-resistant rejection in this model, we sought to examine the outcome of graft rejection or acceptance when high frequency CD8+ T cells were stimulated in the presence of high or low frequency CD4+ helper T cells. We used a model system wherein TCR transgenic OT-I (CD8+ ) and OT-II (CD4+ ) T cells were adoptively transferred into naïve B6 recipients, raising the precursor frequency of antigen-specific cells prior to engraftment with Act-mOVA skin, which constitutively expresses full length membrane-bound chicken ovalbumin (mOVA) protein under control of the β-actin promoter (29), and treatment with CTLA-4 Ig/ anti-CD154 mAbs (costimulation blockade). In order to enumerate the precursor frequency of antigen-specific T cells resulting from the adoptive transfer, splenocytes, LN cells, and peripheral blood lymphocytes were isolated from recipients of 105 , 106 , or 107 OT-I and OT-II T cells, and were analyzed for the presence of Thy1.1+ CD4+ (OT-II) or CD8+ (OT-I) T cells 48 h post-transfer. Analysis of this data revealed that the frequencies of Thy1.1+ OT-I and OT-II T cells increased in an incremental manner, with recipients of 105 cells bearing about 0.04% donor specific CD4+ or CD8+ T cells, recipients of 106 cells having a donor-reactive precursor frequency of about 0.4%, and recipients of 107 cells having a donor-reactive precursor frequency of approximately 4% ((27)and data not shown). The experimentally-increased precursor frequencies of these adoptive transfer recipients therefore span the range that is thought to be the physiologically relevant for allogeneic T cell responses (41).
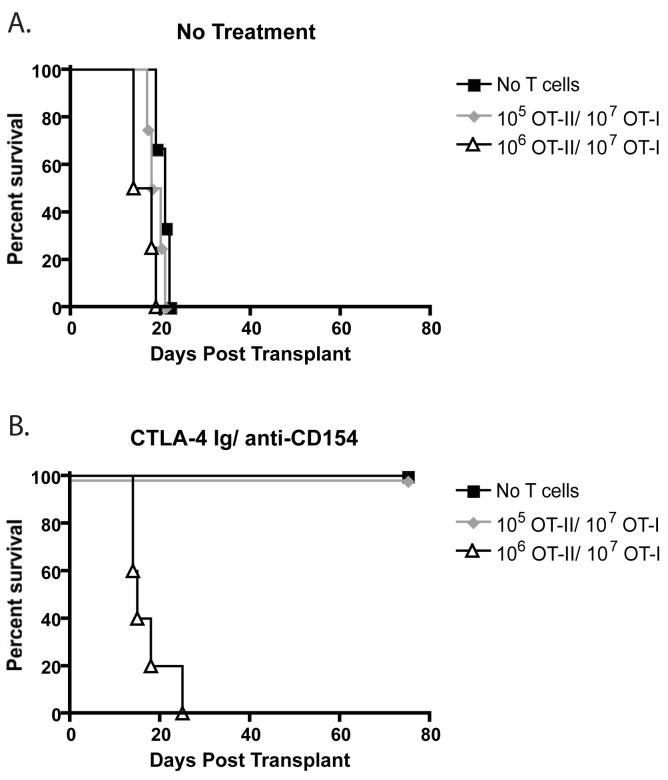
Recipients of 1×105 and 1×106 OT-II T cells (along with 1×107 OT-I T cells) that were not treated with costimulation blockade uniformly rejected their grafts with MSTs of 19 and 16, respectively (Figure 1A). Costimulation blockade-treated recipients of a low frequency (105 ) of OT-IIs along with 107 OT-I T cells exhibited long term graft survival with a MST of >200 days (Figure 1B). In contrast, recipients of 106 OT-II T cells along with 107 OT-I T cells in the presence of costimulation blockade exhibited costimulation blockade-resistant rejection with a MST of 15 days (Figure 1B). These data suggest that a critical threshold of CD4+ T cell help is required for high frequency donor-specific CD8+ T cells to mediate costimulation blockade resistant rejection.
Figure 1. Rejection of skin grafts despite treatment with CTLA-4 Ig and anti-CD154 requires a critical precursory frequency of donor-specific CD4+ T cells.
The indicated frequencies of donor reactive OT-I and OT-II T cells were adoptively transferred into naïve B6 recipients prior to transplantation of an mOVA skin graft. Where indicated, mice received CTLA-4 Ig and anti-CD154 on days 0, 2, 4, and 6 as described in Materials and Methods. Untreated controls uniformly experienced graft rejection (A), whereas costimulation blockade-treated animals receiving no exogenous donor-specific T cells went on to long-term graft survival (B). Recipients of 105 OT-IIs and 107 OT-I cells accepted mOVA skin grafts following treatment with costimulation blockade (B), whereas recipients of 106 OT-II and 107 OT-I experienced costimulation blockade-resistant rejection (B). Results shown are of n=5 mice per group, and are representative of three independent experiments.
Reduced CD4+ T cell response in the presence of costimulation blockade at both high and low precursor frequency
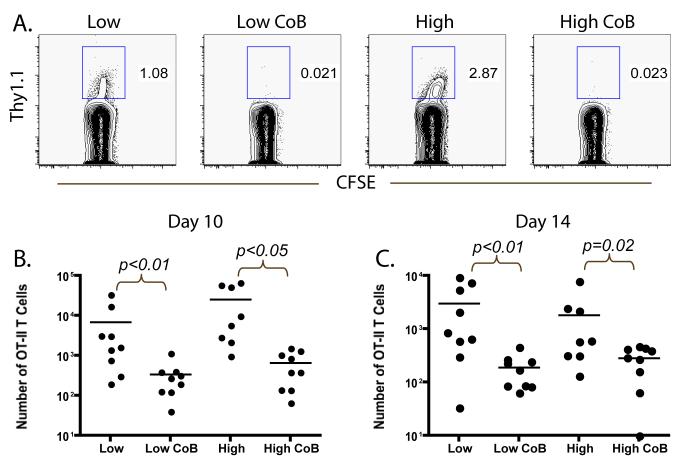
Because of the observed costimulation blockade-resistant rejection of mOVA skin grafts at high donor-specific CD4+ T cell frequency, we reasoned that, like their CD8+ counterparts (27), donor-specific CD4+ T cell populations might evade the effects of costimulation blockade at high frequency. In order to test this hypothesis, we analyzed the expansion and accumulation of the donor-reactive CD4+ T cell response when primed at both lower (105 ) and higher (106 ) frequencies in the presence or absence of costimulation blockade. By tracking CD4+ Thy1.1+ donor-specific T cell responses in the draining LN at days 10 and 14 post-transplant, we observed near complete inhibition of the expansion of OT-II T cells in costimulation blockade-treated recipients of both 105 and 106 OT-II T cells as compared to their untreated controls. This effect was observed in the frequency of Thy1.1+ donor-specific T cells as a percentage of the total CD4+ T cell compartment at days 10 and 14 (Figures 2A and data not shown). In addition, the absolute number of OT-II donor-reactive T cells in the draining lymph node was significantly reduced at days 10 (Figure 2B) and 14 (Figure 2C) in recipients of both 105 and 106 OT-II T cells (p<0.01 and p<0.05 as compared to untreated controls, respectively). These results suggest that the expansion and accumulation of high frequency donor-reactive CD4+ T cells are attenuated by the effects of costimulation blockade, and thus that the observed differences in graft outcomes are not due to the differential expansion and accumulation of high versus low frequency CD4+ populations in the presence of costimulation blockade at these time points.
Figure 2. Accumulation of donor-reactive CD4+ T cells is diminished in the presence of costimulation blockade regardless of initial precursor frequency.
105 (low) or 106 (high) donor-reactive OT-II T cells were adoptively transferred into naïve B6 recipients along with 107 OT-I T cells. Mice were given an mOVA skin graft and treated with costimulation blockade where indicated. A) The frequency of donor-specific CD4+ Thy1.1 effectors found in the axillary LN at day 10 was reduced by treatment with CTLA-4 Ig and anti-CD154 in groups stimulated at either 105 (low) or 106 (high) antigen-specific CD4+ T cells, relative to untreated controls. Data shown are gated on CD4+ cells, and are representative of three independent experiments with 2-3 mice per group per experiment. B) The absolute number of responding CD4+ Thy1.1+ effectors at day 10 post-transplant was diminished following treatment with costimulation blockade as compared to untreated controls in recipients of both 105 (p<0.01) and 106 (p<0.05) initial OT-II T cells. C) The absolute number of responding CD4+ Thy1.1+ effectors was also diminished at day 14 post-transplant following treatment with costimulation blockade as compared to untreated controls in recipients of both 105 (p<0.01) and 106 (p<0.02) initial OT-II T cells. Data shown are cumulative results from three independent experiments with 2-3 mice per group, per experiment.
High frequency helpers do not exhibit early expansion or produce inflammatory cytokines following treatment with costimulation blockade
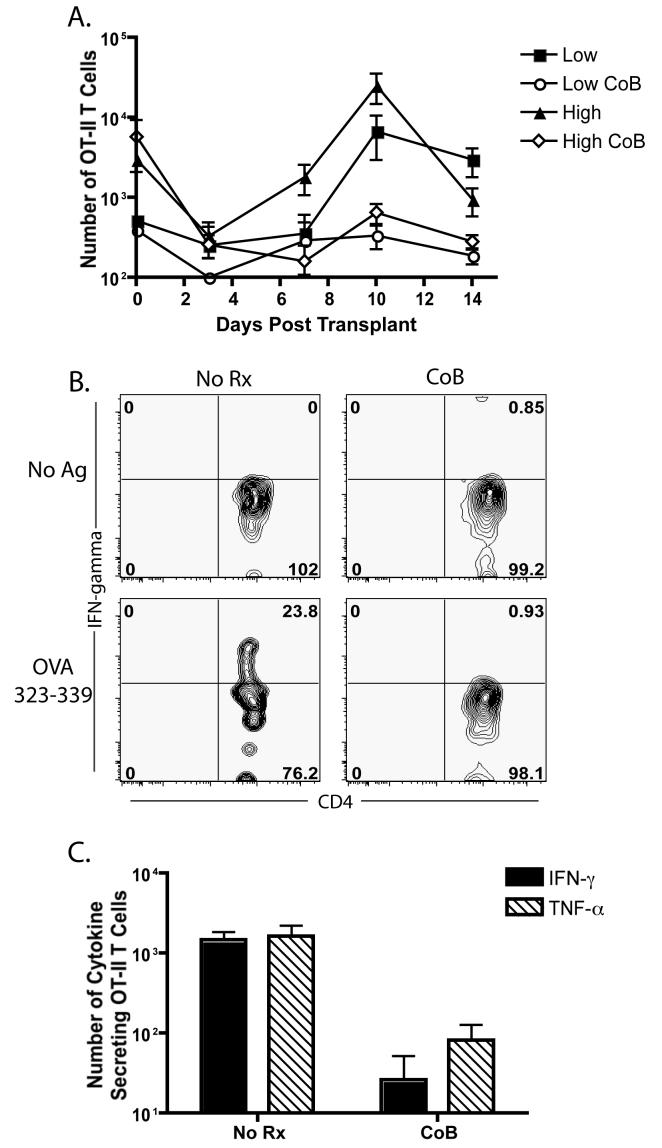
We reasoned that high frequency donor-specific CD4+ T cells could be expanding early in the course of the immune response (< day 10) and providing help prior to the diminution of the response by day 10. In order to address this issue, we examined the kinetics of expansion of CD4+ donor-specific OT-II T cells at days 0, 3, 7, 10, and 14 post-transplant, in the presence and absence of costimulation blockade. In untreated mice receiving either high or low frequencies of CD4+ help, the OT-II T cells, after an initial decline in numbers, underwent rapid 10-20 fold expansion that peaked on post-operative day 10 (Figure 3A). In contrast, OT-II in mice receiving costimulation blockade showed no evidence of significant clonal expansion at any time point, regardless of their initial precursor frequency (Figure 3A). In addition, we assessed whether donor-reactive CD4+ T cells at high frequency showed evidence of escape from costimulation blockade as evidenced by differentiation into either IFNγ or TNFα producing cells. DLN cells from recipients of high-frequency donor-reactive OT-II cells were harvested at day 10 post-transplant, restimulated in vitro and analyzed for their ability to produce ex vivo IFN-γ or TNFα. While OT-II cells isolated from the DLN of untreated recipients of 106 OT-II T cells and 107 OT-I cells exhibited an ability to produce IFN-γ following a 5 hour in vitro stimulation, recipients of these cells that were treated with costimulation blockade exhibited a profound inability to produce IFN-γ (Figure 3B). This deficit in the ability to secrete inflammatory cytokines was also evidenced in the dramatic reduction of absolute numbers of IFN-γ-secreting or TNF-α-secreting effectors (p<0.05 for both cytokines when compared to untreated controls) (Figure 3C). Taken together, these results demonstrate that although a high frequency of CD4+ donor-reactive T cells appeared to be critical for the execution of the costimulation blockade resistant rejection, their role as mediators of this process does not require their expansion, accumulation or differentiation into cells which expresses effector cytokines.
Figure 3. No evidence for enhanced early expansion or cytokine secretion in donor-reactive CD4+ T cells stimulated at high frequency.
105 or 106 donor-reactive Thy1.1+ OT-II T cells and 107 Thy1.1+ OT-I T cells were adoptively transferred into naïve B6 recipients. Mice were given an mOVA skin graft and treated with costimulation blockade where indicated. Donor-reactive CD4+ OT-II T cells were analyzed for the kinetics and magnitude of expansion at days 0, 3, 7, 10, and 14 post-transplant (A). There was no detectable transient increase in the frequency of CD4+ Thy1.1+ cells in the high frequency group prior to day 10. Draining LN cells from mice that were primed at 106 donor reactive CD4+ T cells were harvested at day 10, restimulated in vitro with OVA 323-339 peptide for 5 hours, and analyzed for the presence of intracellular IFN-γ (B,C) or TNF-α (C). Data shown are gated on CD4+ Thy1.1+ cells, and are representative of two independent experiments with 2-3 mice per group. Results indicated both the frequency (B) and absolute number (C, p<0.05) of cytokine-secreting cells is reduced in donor-reactive CD4+ populations even when stimulated at high frequency. Data shown are cumulative results from at least two independent experiments with 2-3 mice per group, per experiment.
Diminished expansion and accumulation of CD8+ effectors primed in the presence of low frequency CD4+ helpers and costimulation blockade
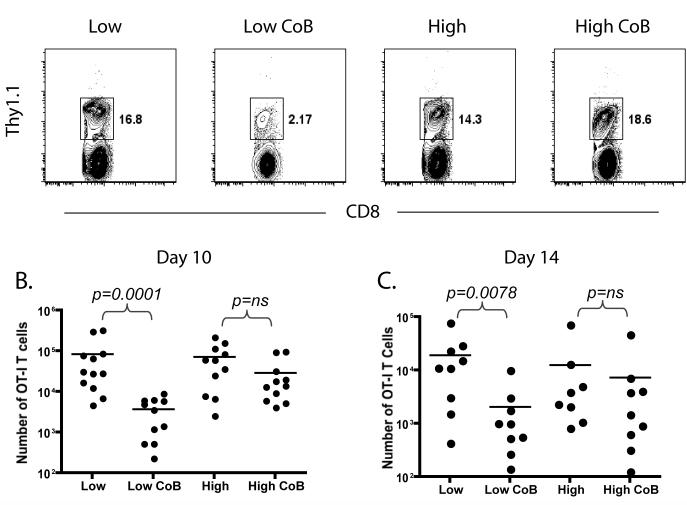
Our analysis of the CD4+ donor-reactive population primed at high or low frequency failed to reveal any demonstrable differences on the proliferation and effector function of the graft-specific CD4+ response. Thus, we reasoned that the high frequency CD4+ donor-reactive cells might manifest their effects indirectly, by affecting the donor-reactive CD8+ population. We analyzed the graft-specific OT-I T cell response in mice that had received high frequency OT-I T cells (107 ) along with high frequency (106 ) or low frequency (105 ) OT-II helper cells. Results demonstrated that while the accumulation of donor-reactive OT-I T cells was greatly diminished in the presence of costimulation blockade in mice receiving 105 OT-II helper T cells there was only a modest diminution of the donor-reactive CD8+ T cell response in the recipients of high-frequency helpers (Figure 4A). Analysis of the absolute number of graft-specific OT-I T cells per draining LN demonstrated that in the presence of low-frequency help, costimulation blockade led to a >20-fold reduction in the expansion of donor-reactive CD8+ OT-I T cells at day 10 (p=0.0001, Figure 4B) and a >10-fold reduction at day 14 (p=0.0078, Figure 4C). In contrast, in the presence of high frequency help, costimulation blockade did not significantly impair CD8+ T cell expansion at either time-point. These data suggest that the high frequency CD4+ helper T cells, while inhibited in their proliferation and effector function, can potentiate the high-frequency CD8+ T cell response even in the presence of costimulation blockade.
Figure 4. Expansion of donor-reactive CD8+ T cells is reduced in the presence of costimulation blockade when primed in the presence of low, but not high, frequencies of antigen-specific CD4+ helper cells.
105 or 106 donor-reactive Thy1.1+ OT-II T cells were adoptively transferred into naïve B6 recipients along with 107 Thy1.1+ OT-I T cells. Mice were given an mOVA skin graft and treated with costimulation blockade where indicated. A) The frequency of donor-specific CD8+ Thy1.1 effectors found in the brachial LN at day 10 was more potently reduced by treatment with CTLA-4 Ig and anti-CD154 in recipients of 105 than in recipients of 106 antigen-specific CD4+ T cells (A). Data shown are gated on CD8+ cells, and are representative of four independent experiments with 2-3 mice per group per experiment. The absolute number of responding CD8+ Thy1.1+ effectors at day 10 post-transplant was statistically significantly diminished following treatment with costimulation blockade as compared to untreated controls in recipients of 105 (p=0.001) but not 106 (p=ns) initial OT-II T cells. C) The absolute number of responding CD8+ Thy1.1+ effectors was also diminished at day 14 post-transplant following treatment with costimulation blockade in recipients of 105 (p=0.0078) but not in recipients of 106 (p=ns) initial OT-II T cells. Data shown are cumulative results from three independent experiments with 2-3 mice per group, per experiment.
High frequency donor-reactive CD4+ T cells can provide help for donor-specific antibody production
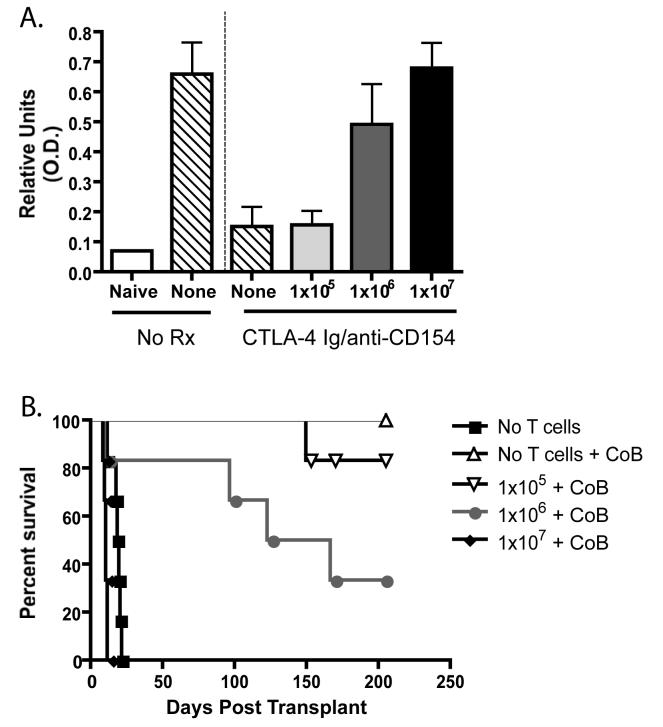
Given the findings suggesting that 106 donor-specific CD4+ T cells could provide help for CD8+ T cell responses in the presence of costimulation blockade, we hypothesized that high-frequency CD4+ T cell responses may also provide help for class-switched donor-reactive IgG antibody production. Serum from recipients of 105 , 106 , or 107 OT-I and OT-II T cells that had received an mOVA skin graft and been treated with costimulation blockade was analyzed on day 60 post-transplant for the presence of anti-OVA IgG by ELISA. Controls demonstrated that while mice receiving an mOVA skin graft and no treatment had appreciable titers of anti-OVA IgG, mice receiving an mOVA skin graft and costimulation blockade exhibited greatly diminished anti-OVA serum antibody levels (Figure 5A, p<0.05). Interestingly, the addition of 105 graft-specific OTI and OT-II T cells at the time of transplant did not result in an increase in anti-OVA antibody; however, the addition of 106 OT-I and OT-II T cells resulted in a sharp increase in the level of anti-OVA antibody (Figure 5A, p<0.05). This level of serum anti-OVA IgG approached the levels observed in either untreated recipients or in recipients of 107 OT-I and OT-II T cells; however in both of these conditions mOVA skin grafts will undergo acute rejection, whereas mOVA skin grafts are not acutely rejected in costimulation blockade-treated recipients of 106 OT- and OT-IIs. We reasoned that the increase in anti-donor antibody precipitated by the increase in CD4+ helpers might play a role in chronic rejection at later time points. In order to further examine the clinical impact of the increase in anti-OVA antibody in recipients of high-frequency donor-specific CD4+ T cells, we monitored the kinetics of graft rejection at > 80 days post transplant. We observed that recipients of 106 OT-I and OT-II T cells that had elevated serum anti-OVA IgG exhibited an MST of 148 days, whereas recipients of 105 OT-I and OT-II T cells demonstrated long-term graft survival with an undefined MST of >200 days (Figure 5B, p=0.05). Taken together, these results demonstrate that an increase in the frequency of donor-reactive CD4+ helpers in the presence of costimulation blockade treatment can provide increased help for the generation of class-switched anti-OVA antibody responses that may be involved in chronic rejection of the mOVA skin graft at later time points.
Figure 5. A critical frequency of antigen-specific CD4+ T cells can provide help for donor-reactive antibody production in the absence of CD80/86 and CD40-mediated costimulation.
The indicated numbers of donor-reactive Thy1.1+ CD4+ or CD8+ T cells were adoptively transferred into naïve B6 recipients, which then received an mOVA skin graft and costimulation blockade where indicated. At day 60 post transplant, serum was analyzed for the presence of anti-OVA IgG (A). Results indicated that costimulation blockade effectively reduced the titer of anti-OVA antibody as compared to untreated controls (p<0.05). Transfer of 105 OT-II T cells did not reconstitute antibody production, but transfer of 106 cells resulted in the generation of anti-OVA antibody at levels near to those observed in untreated controls. Recipients of 106 but not 105 OT-II T cells were also more likely to undergo graft loss due to chronic rejection (p<0.05)(B). Results shown are cumulative data from two independent experiments with a total of 6 mice per group.
Discussion
Antigen-specific precursor frequency is beginning to be appreciated as a critical factor in determining in the eventual outcome of a T cell response. Seminal studies have defined an important role for precursor frequency in the kinetics, magnitude, homeostatic expansion, and degree of memory cell generation in infectious model systems (42-45). Furthermore, we recently implicated a role for CD8+ T cell precursor frequency in determining the degree of proliferation and differentiation of responding donor-reactive T cell populations during transplantation, and in mediating costimulation blockade-resistant rejection (27). The issue of responding T cell precursor frequency is an especially relevant one for the field of transplantation, as the range of physiologically relevant frequencies may span a much greater range for alloantigens than for conventional antigens. For example, the precursor frequency of T cells responding to fully allogeneic tissue has been reported to be 1-10% (41), whereas the frequency of T cells responding to a minor antigen could be as low as 1 in 106. Therefore, the precursor frequency of donor-ractive T cells for a given donor-recipient pair depends heavily on the degree of MHC matching between donor and recipient.
In the current study we addressed the issue of CD4+ T cell precursor frequency and how variations in this parameter during T cell priming during transplantation could affect CD4+ T cell proliferation, effector cytokine production, and provision of help for donor-reactive B cell and CD8+ T cell responses. We observed a critical CD4+ helper T cell threshold precursor frequency that precipitated costimulation blockade-resistant rejection when combined with high-frequency CD8+ T cells even in the presence of costimulation blockade (Figure 1). Interestingly, high-frequency CD4+ T cell populations were able to provide T cell help, as measured by increased B cell and CD8+ T cell responses (Figures 4 and 5), even though the proliferative and cytokine responses of those T cells were significantly inhibited by costimulation blockade (Figures 2 and 3). Thus, we conclude that an initial high frequency of CD4+ T cells uncouples T cell proliferative and effector function from the provision of T cell help.
The results of this study, combined with previous findings, highlight important differences in the requirements for activation in the absence of costimulation between CD4+ and CD8+ T cells. Specifically, our prior study demonstrated that a high precursor frequency of CD8+ T cells obviates the need for costimulation (27). In contrast, our current results reveal that donor-specific CD4+ T cells require costimulation for proliferation and the acquisition of effector function even at high frequencies where they are able to provide T cell help to donor-reactive CD8+ T cells. While we cannot rule out the possibility that differences in affinities of OT-I T cells (high) versus OT-II T cells (lower) could contribute to these observations, increased reliance of CD4+ relative to CD8+ T cells on costimulatory signals for proliferation and differentiation has been well documented in the literature (46-49). For example, T cell responses that “break-through” CD28/CD154 blockade and induce graft rejection have been shown to be composed primarily of CD8+ T cells (19). In addition, memory CD4+ T cells appear to require costimulatory signals for secondary response, whereas memory CD8+ T cells appear to respond independently of CD28 and CD154 signals (25, 46, 50, 51). These studies have focused primarily on proliferation and cytokine production as measures of donor-reactive CD4+ T responses. Our results would suggest that helper function can become uncoupled from proliferation and effector function under conditions of high frequency, and may therefore be less reliant on costimulatory signals.
While our results suggest that an increase in the frequency of donor-reactive CD4+ helpers in the presence of costimulation blockade can provide increased help for the generation of class-switched anti-OVA antibody responses, we cannot formally exclude the possibility that sub-clincial graft damage due to an increased T cell response in recipients of 106 OT-I/OT-II T cells could lead to antigen shedding and the augmentation of antibody responses. Similarly, the augmented early immune response in these hosts may directly cause graft damage that may be involved in chronic rejection of the mOVA skin graft at later time points (Figure 5B). The impact of CD4+ T cell precursor frequency on the generation of anti-donor B cell responses therefore warrants further study.
Analysis of the kinetics of donor-specific CD4+ T cell expansion in our model revealed that cells stimulated at both low and high frequency in the presence of costimulation blockade undergo contraction following transplantation (Figure 3A). These results demonstrate that there is no transient increase in the number of donor-reactive CD4+ effector cells in the mice stimulated at high frequency. Instead, we see a contraction in the number of donor-reactive T cells present in the high-frequency groups between days 0 and 3, when the number of donor-reactive CD4+ T cells begins to increase in the untreated groups. We hypothesize that the increased frequency of donor-reactive CD4+ T cells present during this early phase of the response may be sufficient to provide help for donor-specific B cell and CD8+ T cell responses. This could be accomplished via increased secretion of cytokines such as IL-2, which can be elaborated very early following stimulation in vivo (52), or via the early upregulation of alterative costimulatory molecules (discussed further below). Indeed, there is evidence in the literature that CD4+ T cell help is required early during the response in order to generate optimal antigen-specific CD8+ T cell responses (53). For example, a recent report found that blocking alloreactive CD4+ T cells on the day of transplantation reduced and delayed the expansion of alloreactive CD8+ effectors, whereas blocking alloreactive CD4+ T cells two days post-transplant had no effect (40).
Another possible explanation for the increased CD8+ T cell responses in the presence of high frequency help is the possibility that high-frequency CD4+ T cell responses enhance DC activation more than low-frequency T cells. We have analyzed CD11c+ DC in the draining LN and did not find a difference in the expression of MHC class I or class II, CD80, CD86, or CD40 (data not shown). This data is consistent with the findings of Ingulli and colleagues, who found that the presence of donor-reactive CD4+ T cells did not enhance peptide:MHC complex or costimulatory molecule expression by DC (37). Ingulli and colleagues did find an increase in IL-12 secretion by DC in the presence of donor-reactive CD4+ T cells, but this increased production required the CD40/CD154 pathway (37). Therefore, it is unlikely that high-frequency CD4+ T cells enhance CD8+ T cell responses through increased DC-derived IL-12 in the presence of CD154 and CD28 blockade in our model. We hypothesize that the expression of additional costimulatory molecules on the surface of the donor-reactive CD4+ T cells during days 0-3 post transplant could provide sufficient costimulation to generate anti-donor antibody and CD8+ effector responses. For example, combined in vivo blockade of CD154 and TRANCE during allogeneic murine cardiac transplantation resulted in significantly enhanced graft survival relative to blockade of CD154 alone (54). Alternatively, several other costimulatory pathways have been shown to enhance anti-donor immune responses in a CD28 and/or CD154-independent manner, including ICOS/B7RP-1 (11), CD27/CD70 (55), 4-1BB/4-1BBL (56), and OX-40/OX-40L (57). The contribution of these alternative costimulatory molecules to the ability of high-frequency donor-reactive CD4+ T cells to augment donor-specific CD8+ T cell and B cell responses is the subject of ongoing investigation.
The results from our study also suggest a difference in the requirements for costimulatory signals between donor-reactive CD8+ T cells that do or do not receive sufficient CD4+ T cell help. This is demonstrated by the fact that CD8+ T cell populations that receive sufficient help (high CD4+ T cell precursor frequency) are able to obviate the need for CD28/CD154 mediated costimulatory signals, whereas CD8+ T cells that receive insufficient help are dependent on these signals for expansion and accumulation (Figure 4). This finding is corroborated in the literature by several recent reports suggesting that helpless CD8+ alloreactive T cells are indeed more reliant on costimulation for proliferation and differentiation (40, 58-60). These results have potential implications for clinical application of costimulation blockade-based therapies. As stated above, the degree of MHC class II matching may determine whether costimulation blockade is sufficient to produce graft acceptance. As such, immunomodulatory therapies could be tailored to fit the precursor frequency of the recipient for a given donor. For example, in settings of class II MHC matching, the donor-reactive CD4+ T cell precursor frequency would be predicted to be low, and, based on the results of our studies, costimulation blockade would be predicted to be sufficient to control rejection. This therapy could then be rationally implemented without exposing the patient to more aggressive immunosuppressive agents such as steroids or calcineurin inhibitors, which possess unwanted side effects. In contrast, where donor-reactive CD4+ precursor frequencies are high, adjuvant therapies may be required to control rejection.
In summary, our findings suggest that a critical threshold of donor-specific CD4+ T cell help exists in order to facilitate high frequency CD8+ T cell-mediated costimulation blockade-resistant rejection. While alloreactive CD8+ T cell responses have long been considered responsible for “breakthrough” rejection, our findings now suggest that targeting the alloreactive CD4+ T cells may also be an effective method of controlling the CD8+ T cell response. In addition, future studies will endeavor to define the pathways used by high frequency donor-specific CD4+ T cells to provide help in a CD40L- and CD28-independent manner, with the hopes that targeting additional pathways may facilitate further control of the breakthrough CD8+ donor-specific response.
Acknowledgements
The authors would like to thank Dr. Shivaprakash Gangappa for helpful discussions. This work was supported by NIH grant AI to C.P.L. M.L.F. was supported by a Ruth L. Kirschstein NRSA.
Abbreviations
- mOVA
membrane-bound chicken ovalbumin
- CoB
costimulation blockade
References
- 1.Jenkins MK. The ups and downs of T cell costimulation. Immunity. 1994;1:443–446. doi: 10.1016/1074-7613(94)90086-8. [DOI] [PubMed] [Google Scholar]
- 2.Turka LA, Linsley PS, Lin H, Brady W, Leiden JM, Wei R, Gibson ML, Zheng X, Myrdal S, Gordon D, Bailey T, Bolling SF, Thompson CB. T-cell activation by the CD28 ligand B7 is required for cardiac allograft rejection in vivo. Proc. Natl. Acad. Sci. USA. 1992;89:11102–11105. doi: 10.1073/pnas.89.22.11102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pearson TC, Alexander DZ, Corbascio M, Hendrix R, Ritchie SC, Linsley PS, Faherty D, Larsen CP. Analysis of the B7 costimulatory pathway in allograft rejection. Transplantation. 1997;63:1463–1469. doi: 10.1097/00007890-199705270-00016. [DOI] [PubMed] [Google Scholar]
- 4.Pearson TC, Alexander DZ, Winn KJ, Linsley PS, Lowry RP, Larsen CP. Transplantation tolerance induced by CTLA4-Ig. Transplantation. 1994;57:1701–1706. [PubMed] [Google Scholar]
- 5.Sayegh MH, Akalin E, Hancock WW, Russell ME, Carpenter CB, Linsley PS, Turka LA. CD28-B7 blockade after alloantigenic challenge in vivo inhibits Th1 cytokines but spares Th2. J. Exp. Med. 1995;181:1869–1874. doi: 10.1084/jem.181.5.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lenschow D, Zeng Y, Thistlethwaite J, Montag A, Brady W, Gibson M, Linsley P, Bluestone J. Long-term survival of xenogeneic pancreatic islet grafts induced by CTLA4Ig. Science. 1992;257:789–792. doi: 10.1126/science.1323143. [DOI] [PubMed] [Google Scholar]
- 7.Vincenti F, Larsen C, Durrbach A, Wekerle T, Nashan B, Blancho G, Lang P, Grinyo J, Halloran PF, Solez K, Hagerty D, Levy E, Zhou W, Natarajan K, Charpentier B. Costimulation blockade with belatacept in renal transplantation. N Engl J Med. 2005;353:770–781. doi: 10.1056/NEJMoa050085. [DOI] [PubMed] [Google Scholar]
- 8.Quezada SA, Jarvinen LZ, Lind EF, Noelle RJ. CD40/CD154 interactions at the interface of tolerance and immunity. Annual review of immunology. 2004;22:307–328. doi: 10.1146/annurev.immunol.22.012703.104533. [DOI] [PubMed] [Google Scholar]
- 9.Nanji SA, Hancock WW, Luo B, Schur CD, Pawlick RL, Zhu LF, Anderson CC, Shapiro AM. Costimulation blockade of both inducible costimulator and CD40 ligand induces dominant tolerance to islet allografts and prevents spontaneous autoimmune diabetes in the NOD mouse. Diabetes. 2006;55:27–33. [PubMed] [Google Scholar]
- 10.Nanji SA, Hancock WW, Anderson CC, Adams AB, Luo B, Schur CD, Pawlick RL, Wang L, Coyle AJ, Larsen CP, Shapiro AM. Multiple combination therapies involving blockade of ICOS/B7RP-1 costimulation facilitate long-term islet allograft survival. Am J Transplant. 2004;4:526–536. doi: 10.1111/j.1600-6143.2004.00384.x. [DOI] [PubMed] [Google Scholar]
- 11.Ozkaynak E, Gao W, Shemmeri N, Wang C, Gutierrez-Ramos JC, Amaral J, Qin S, Rottman JB, Coyle AJ, Hancock WW. Importance of ICOS-B7RP-1 costimulation in acute and chronic allograft rejection. Nat Immunol. 2001;2:591–596. doi: 10.1038/89731. [DOI] [PubMed] [Google Scholar]
- 12.Rothstein DM, Livak MF, Kishimoto K, Ariyan C, Qian HY, Fecteau S, Sho M, Deng S, Zheng XX, Sayegh MH, Basadonna GP. Targeting signal 1 through CD45RB synergizes with CD40 ligand blockade and promotes long term engraftment and tolerance in stringent transplant models. Journal of Immunology. 2001;166:322–329. doi: 10.4049/jimmunol.166.1.322. [DOI] [PubMed] [Google Scholar]
- 13.Kishimoto K, Dong VM, Issazadeh S, Fedoseyeva EV, Waaga AM, Yamada A, Sho M, Benichou G, Auchincloss H, Jr., Grusby MJ, Khoury SJ, Sayegh MH. The role of CD154-CD40 versus CD28-B7 costimulatory pathways in regulating allogeneic Th1 and Th2 responses in vivo. J Clin Invest. 2000;106:63–72. doi: 10.1172/JCI9586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larsen CP, Elwood ET, Alexander DZ, Ritchie SC, Hendrix R, Tucker-Burden C, Cho HR, Aruffo A, Hollenbaugh D, Linsley PS, Winn KJ, Pearson TC. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. 1996;381:434–438. doi: 10.1038/381434a0. [DOI] [PubMed] [Google Scholar]
- 15.Parker DC, Greiner DL, Phillips NE, Appel MC, Steele AW, Durie FH, Noelle RJ, Mordes JP, Rossini AA. Survival of mouse pancreatic islet allografts in recipients treated with allogeneic small lymphocytes and antibody to CD40 ligand. Proc. Natl. Acad. Sci. USA. 1995;92:9560–9564. doi: 10.1073/pnas.92.21.9560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hancock WW, Sayegh MH, Zheng XG, Peach R, Linsley PS, Turka LA. Costimulatory function and expression of CD40 ligand, CD80, and CD86 in vascularized murine cardiac allograft rejection. Proc. Natl. Acad. Sci. USA. 1996;93:13967–13972. doi: 10.1073/pnas.93.24.13967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adams AB, Shirasugi N, Jones TR, Durham MM, Strobert EA, Cowan S, Rees P, Hendrix R, Price K, Kenyon NS, Hagerty D, Townsend R, Hollenbaugh D, Pearson TC, Larsen CP. Development of a chimeric anti-CD40 monoclonal antibody that synergizes with LEA29Y to prolong islet allograft survival. J Immunol. 2005;174:542–550. doi: 10.4049/jimmunol.174.1.542. [DOI] [PubMed] [Google Scholar]
- 18.Pearson TC, Trambley J, Odom K, Anderson DC, Cowan S, Bray R, Lin A, Hollenbaugh D, Aruffo A, Siadak AW, Strobert E, Hennigar R, Larsen CP. Anti-CD40 therapy extends renal allograft survival in rhesus macaques. Transplantation. 2002;74:933–940. doi: 10.1097/00007890-200210150-00006. [DOI] [PubMed] [Google Scholar]
- 19.Trambley J, Bingaman AW, Lin A, Elwood ET, Waitze SY, Ha J, Durham MM, Corbascio M, Cowan SR, Pearson TC, Larsen CP. Asialo GM1(+) CD8(+) T cells play a critical role in costimulation blockade-resistant allograft rejection. J Clin Invest. 1999;104:1715–1722. doi: 10.1172/JCI8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo Z, Meng L, Kim O, Wang J, Hart J, He G, Alegre M-L, Thistlethwaite JR, Pearson TC, Larsen CP, Newell KA. CD8-T cell-mediated rejection of intestinal allografts is resistant to inhibition of the CD40/CD154 costimulatory pathway. Transplantation. 2001;71:1351–1354. doi: 10.1097/00007890-200105150-00033. [DOI] [PubMed] [Google Scholar]
- 21.Makhlouf L, Kishimoto K, Smith RN, Abdi R, Koulmanda M, Winn HJ, Auchincloss H, Jr., Sayegh MH. The role of autoimmunity in islet allograft destruction: major histocompatibility complex class II matching is necessary for autoimmune destruction of allogeneic islet transplants after T-cell costimulatory blockade. Diabetes. 2002;51:3202–3210. doi: 10.2337/diabetes.51.11.3202. [DOI] [PubMed] [Google Scholar]
- 22.Jones ND, Van Maurik A, Hara M, Spriewald BM, Witzke O, Morris PJ, Wood KJ. CD40-CD40 ligand-independent activation of CD8+ T cells can trigger allograft rejection. J Immunol. 2000;165:1111–1118. doi: 10.4049/jimmunol.165.2.1111. [DOI] [PubMed] [Google Scholar]
- 23.Williams MA, Trambley J, Ha J, Adams AB, Durham MM, Rees P, Cowan SR, Pearson TC, Larsen CP. Genetic characterization of strain differences in the ability to mediate CD40/CD28-independent rejection of skin allografts. J Immunol. 2000;165:6849–6857. doi: 10.4049/jimmunol.165.12.6849. [DOI] [PubMed] [Google Scholar]
- 24.Kemball CC, Lee ED, Szomolanyi-Tsuda E, Pearson TC, Larsen CP, Lukacher AE. Costimulation Requirements for Antiviral CD8+ T Cells Differ for Acute and Persistent Phases of Polyoma Virus Infection. J Immunol. 2006;176:1814–1824. doi: 10.4049/jimmunol.176.3.1814. [DOI] [PubMed] [Google Scholar]
- 25.Newell KA, He G, Guo Z, Kim O, Szot GL, Rulifson I, Zhou P, Hart J, Thistlethwaite JR, Bluestone JA. Cutting edge: blockade of the CD28/B7 costimulatory pathway inhibits intestinal allograft rejection mediated by CD4+ but not CD8+ T cells. J Immunol. 1999;163:2358–2362. [PubMed] [Google Scholar]
- 26.Markees TG, Phillips NE, Gordon EJ, Noelle RJ, Shultz LD, Mordes JP, Greiner DL, Rossini AA. Long-term survival of skin allografts induced by donor splenocytes and anti-CD154 antibody in thymectomized mice requires CD4(+) T cells, interferon-gamma, and CTLA4. J Clin Invest. 1998;101:2446–2455. doi: 10.1172/JCI2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ford ML, Koehn BH, Wagener ME, Jiang W, Gangappa S, Pearson TC, Larsen CP. Antigen-specific precursor frequency impacts T cell proliferation, differentiation, and requirement for costimulation. The Journal of experimental medicine. 2007;204:299–309. doi: 10.1084/jem.20062319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenberg AS, Singer A. Cellular basis of skin allograft rejection: an in vivo model of immune-mediated tissue destruction. Ann. Rev. Immunol. 1992;10:333–358. doi: 10.1146/annurev.iy.10.040192.002001. [DOI] [PubMed] [Google Scholar]
- 29.Ehst BD, Ingulli E, Jenkins MK. Development of a novel transgenic mouse for the study of interactions between CD4 and CD8 T cells during graft rejection. Am J Transplant. 2003;3:1355–1362. doi: 10.1046/j.1600-6135.2003.00246.x. [DOI] [PubMed] [Google Scholar]
- 30.Valujskikh A, Matesic D, Heeger PS. Characterization and manipulation of T cell immunity to skin grafts expressing a transgenic minor antigen. Transplantation. 1999;68:1029–1036. doi: 10.1097/00007890-199910150-00022. [DOI] [PubMed] [Google Scholar]
- 31.Wettstein PJ, Korngold R. T cell subsets required for in vivo and in vitro responses to single and multiple minor histocompatibility antigens. Transplantation. 1992;54:296–307. doi: 10.1097/00007890-199208000-00020. [DOI] [PubMed] [Google Scholar]
- 32.Roopenian DC, Davis AP, Christianson GJ, Mobraaten LE. The functional basis of minor histocompatibility loci. J Immunol. 1993;151:4595–4605. [PubMed] [Google Scholar]
- 33.Matloubian M, Concepcion RJ, Ahmed R. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. Journal of virology. 1994;68:8056–8063. doi: 10.1128/jvi.68.12.8056-8063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cardin RD, Brooks JW, Sarawar SR, Doherty PC. Progressive loss of CD8+ T cell-mediated control of a gamma-herpesvirus in the absence of CD4+ T cells. The Journal of experimental medicine. 1996;184:863–871. doi: 10.1084/jem.184.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Janssen EM, Droin NM, Lemmens EE, Pinkoski MJ, Bensinger SJ, Ehst BD, Griffith TS, Green DR, Schoenberger SP. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature. 2005;434:88–93. doi: 10.1038/nature03337. [DOI] [PubMed] [Google Scholar]
- 36.Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–856. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 37.Filatenkov AA, Jacovetty EL, Fischer UB, Curtsinger JM, Mescher MF, Ingulli E. CD4 T cell-dependent conditioning of dendritic cells to produce IL-12 results in CD8-mediated graft rejection and avoidance of tolerance. J Immunol. 2005;174:6909–6917. doi: 10.4049/jimmunol.174.11.6909. [DOI] [PubMed] [Google Scholar]
- 38.Horne PH, Koester MA, Jayashankar K, Lunsford KE, Dziema HL, Bumgardner GL. Disparate primary and secondary allospecific CD8+ T cell cytolytic effector function in the presence or absence of host CD4+ T cells. J Immunol. 2007;179:80–88. doi: 10.4049/jimmunol.179.1.80. [DOI] [PubMed] [Google Scholar]
- 39.Lunsford KE, Horne PH, Koester MA, Eiring AM, Walker JP, Dziema HL, Bumgardner GL. Activation and maturation of alloreactive CD4-independent, CD8 cytolytic T cells. Am J Transplant. 2006;6:2268–2281. doi: 10.1111/j.1600-6143.2006.01479.x. [DOI] [PubMed] [Google Scholar]
- 40.Zhai Y, Wang Y, Wu Z, Kupiec-Weglinski JW. Defective alloreactive CD8 T cell function and memory response in allograft recipients in the absence of CD4 help. J Immunol. 2007;179:4529–4534. doi: 10.4049/jimmunol.179.7.4529. [DOI] [PubMed] [Google Scholar]
- 41.Suchin EJ, Langmuir PB, Palmer E, Sayegh MH, Wells AD, Turka LA. Quantifying the frequency of alloreactive T cells in vivo: new answers to an old question. J Immunol. 2001;166:973–981. doi: 10.4049/jimmunol.166.2.973. [DOI] [PubMed] [Google Scholar]
- 42.Marzo AL, Klonowski KD, Le Bon A, Borrow P, Tough DF, Lefrancois L. Initial T cell frequency dictates memory CD8+ T cell lineage commitment. Nat Immunol. 2005;6:793–799. doi: 10.1038/ni1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hataye J, Moon JJ, Khoruts A, Reilly C, Jenkins MK. Naive and memory CD4+ T cell survival controlled by clonal abundance. Science. 2006;312:114–116. doi: 10.1126/science.1124228. [DOI] [PubMed] [Google Scholar]
- 44.Badovinac VP, Haring JS, Harty JT. Initial T cell receptor transgenic cell precursor frequency dictates critical aspects of the CD8(+) T cell response to infection. Immunity. 2007;26:827–841. doi: 10.1016/j.immuni.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blair DA, Lefrancois L. Increased competition for antigen during priming negatively impacts the generation of memory CD4 T cells. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:15045–15050. doi: 10.1073/pnas.0703767104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Whitmire JK, Murali-Krishna K, Altman J, Ahmed R. Antiviral CD4 and CD8 T-cell memory: differences in the size of the response and activation requirements. Philos Trans R Soc Lond B Biol Sci. 2000;355:373–379. doi: 10.1098/rstb.2000.0577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shahinian A, Pfeffer K, Lee KP, Kundig TM, Kishihara K, Wakeham A, Kawai K, Ohashi PS, Thompson CB, Mak TW. Differential T cell costimulatory requirements in CD28-deficient mice. Science. 1993;261:609–612. doi: 10.1126/science.7688139. [DOI] [PubMed] [Google Scholar]
- 48.Yang Y, Wilson JM. CD40 ligand-dependent T cell activation: requirement of B7-CD28 signaling through CD40. Science. 1996;273:1862–1864. doi: 10.1126/science.273.5283.1862. [DOI] [PubMed] [Google Scholar]
- 49.Brooks JW, Hamilton-Easton AM, Christensen JP, Cardin RD, Hardy CL, Doherty PC. Requirement for CD40 ligand, CD4(+) T cells, and B cells in an infectious mononucleosis-like syndrome. Journal of virology. 1999;73:9650–9654. doi: 10.1128/jvi.73.11.9650-9654.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adams AB, Williams MA, Jones TR, Shirasugi N, Durham MM, Kaech SM, Wherry EJ, Onami T, Lanier JG, Kokko KE, Pearson TC, Ahmed R, Larsen CP. Heterologous immunity provides a potent barrier to transplantation tolerance. J Clin Invest. 2003;111:1887–1895. doi: 10.1172/JCI17477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Honey K, Cobbold SP, Waldmann H. CD40 ligand blockade induces CD4+ T cell tolerance and linked suppression. J Immunol. 1999;163:4805–4810. [PubMed] [Google Scholar]
- 52.Sojka DK, Bruniquel D, Schwartz RH, Singh NJ. IL-2 secretion by CD4+ T cells in vivo is rapid, transient, and influenced by TCR-specific competition. J Immunol. 2004;172:6136–6143. doi: 10.4049/jimmunol.172.10.6136. [DOI] [PubMed] [Google Scholar]
- 53.Castellino F, Germain RN. Cooperation between CD4+ and CD8+ T cells: when, where, and how. Annual review of immunology. 2006;24:519–540. doi: 10.1146/annurev.immunol.23.021704.115825. [DOI] [PubMed] [Google Scholar]
- 54.Guillonneau C, Louvet C, Renaudin K, Heslan JM, Heslan M, Tesson L, Vignes C, Guillot C, Choi Y, Turka LA, Cuturi MC, Anegon I, Josien R. The role of TNF-related activation-induced cytokine-receptor activating NF-kappa B interaction in acute allograft rejection and CD40L-independent chronic allograft rejection. J Immunol. 2004;172:1619–1629. doi: 10.4049/jimmunol.172.3.1619. [DOI] [PubMed] [Google Scholar]
- 55.Yamada A, Salama AD, Sho M, Najafian N, Ito T, Forman JP, Kewalramani R, Sandner S, Harada H, Clarkson MR, Mandelbrot DA, Sharpe AH, Oshima H, Yagita H, Chalasani G, Lakkis FG, Auchincloss H, Jr., Sayegh MH. CD70 signaling is critical for CD28-independent CD8+ T cell-mediated alloimmune responses in vivo. J Immunol. 2005;174:1357–1364. doi: 10.4049/jimmunol.174.3.1357. [DOI] [PubMed] [Google Scholar]
- 56.Wang J, Guo Z, Dong Y, Kim O, Hart J, Adams A, Larsen CP, Mittler RS, Newell KA. Role of 4-1BB in allograft rejection mediated by CD8+ T cells. Am J Transplant. 2003;3:543–551. doi: 10.1034/j.1600-6143.2003.00088.x. [DOI] [PubMed] [Google Scholar]
- 57.Demirci G, Amanullah F, Kewalaramani R, Yagita H, Strom TB, Sayegh MH, Li XC. Critical role of OX40 in CD28 and CD154-independent rejection. J Immunol. 2004;172:1691–1698. doi: 10.4049/jimmunol.172.3.1691. [DOI] [PubMed] [Google Scholar]
- 58.Fischbein MP, Ardehali A, Yun J, Schoenberger S, Laks H, Irie Y, Dempsey P, Cheng G, Fishbein MC, Bonavida B. CD40 signaling replaces CD4+ lymphocytes and its blocking prevents chronic rejection of heart transplants. J Immunol. 2000;165:7316–7322. doi: 10.4049/jimmunol.165.12.7316. [DOI] [PubMed] [Google Scholar]
- 59.Zhan Y, Brady JL, Sutherland RM, Lew AM. Without CD4 help, CD8 rejection of pig xenografts requires CD28 costimulation but not perforin killing. J Immunol. 2001;167:6279–6285. doi: 10.4049/jimmunol.167.11.6279. [DOI] [PubMed] [Google Scholar]
- 60.Lunsford KE, Koester MA, Eiring AM, Horne PH, Gao D, Bumgardner GL. Targeting LFA-1 and cd154 suppresses the in vivo activation and development of cytolytic (cd4-Independent) CD8+ T cells. J Immunol. 2005;175:7855–7866. doi: 10.4049/jimmunol.175.12.7855. [DOI] [PubMed] [Google Scholar]