Abstract
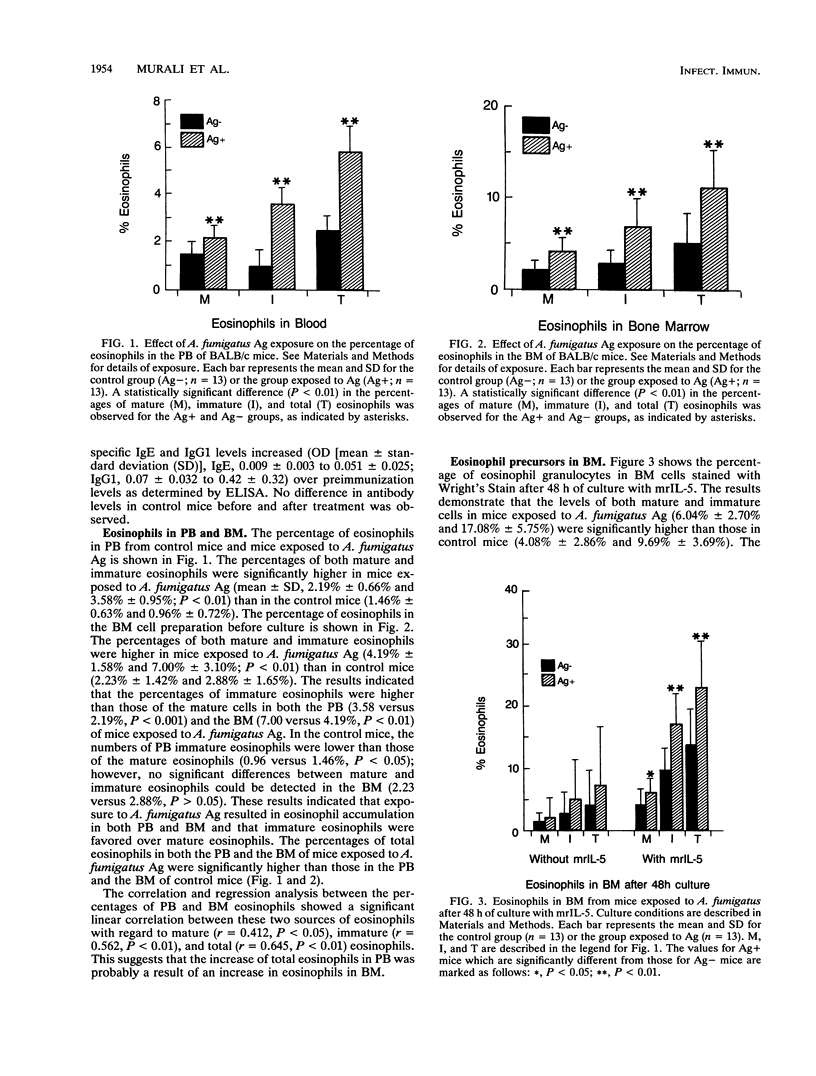
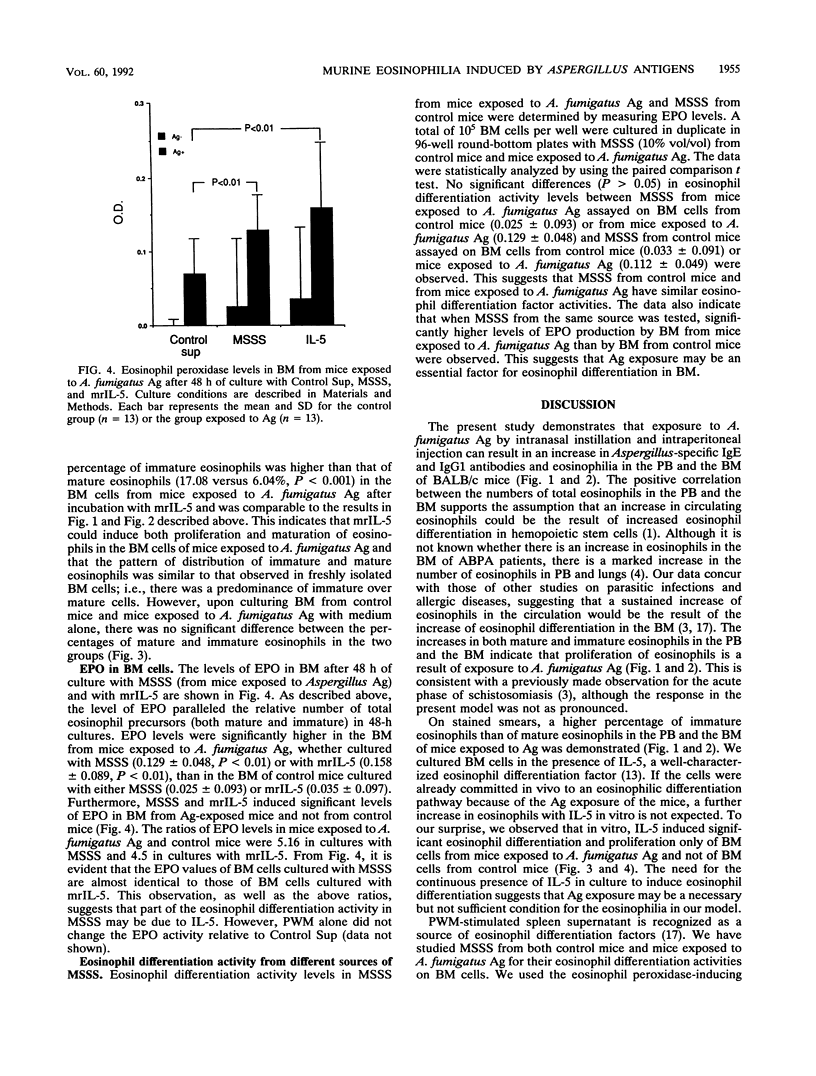
Eosinophilia is a prominent feature of the cellular response in allergic and parasitic diseases. Allergic bronchopulmonary aspergillosis due to colonization of the lungs of some asthmatics with Aspergillus fumigatus is characterized by high levels of serum immunoglobulin E and peripheral blood (PB) and lung eosinophilia. This study investigates the role of eosinophils in the pathogenesis of allergic bronchopulmonary aspergillosis by using a mouse model. BALB/c mice were immunized intranasally and intraperitoneally with A. fumigatus antigens (Ag), and the eosinophils in PB and bone marrow (BM) were enumerated. Eosinophilopoiesis in BM cultures was studied in the presence of murine recombinant interleukin-5 (mrIL-5) and supernatants from pokeweed mitogen-stimulated spleen cells as the source of eosinophil differentiation factors. Eosinophils were quantitated by direct counting and by estimating eosinophil peroxidase activity. The results indicate that the percentage of eosinophils in the PB (5.77 +/- 1.17) and the BM (11.19 +/- 4.31) of mice exposed to A. fumigatus Ag was higher than in controls (PB, 2.42 +/- 0.76; BM, 5.12 +/- 2.79; P less than 0.01 for both). Similarly, a significant increase in eosinophils was observed in the BM population from mice exposed to A. fumigatus Ag compared with that in controls when cultured with murine recombinant interleukin-5 (23.13 +/- 7.14 versus 13.77 +/- 5.79, P less than 0.01), indicating that the mice exposed to A. fumigatus Ag had significantly greater numbers of eosinophil precursors in their BM. This study demonstrates that A. fumigatus Ag may be involved in the in vivo commitment of stem cells in the eosinophil differentiation pathway.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basten A., Boyer M. H., Beeson P. B. Mechanism of eosinophilia. I. Factors affecting the eosinophil response of rats to Trichinella spiralis. J Exp Med. 1970 Jun 1;131(6):1271–1287. doi: 10.1084/jem.131.6.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffman H. F., Koëter G. H., van der Heide S., de Monchy J. G., Kloprogge E., de Vries K. Cellular and humoral observations in a patient with allergic bronchopulmonary aspergillosis during a nonasthmatic exacerbation. J Allergy Clin Immunol. 1989 Apr;83(4):829–838. doi: 10.1016/0091-6749(89)90022-5. [DOI] [PubMed] [Google Scholar]
- Kurup V. P., Choi H., Resnick A., Kalbfleisch J., Fink J. N. Immunopathological response of C57BL/6 and C3H/HeN mice to Aspergillus fumigatus antigens. Int Arch Allergy Appl Immunol. 1990;91(2):145–154. doi: 10.1159/000235106. [DOI] [PubMed] [Google Scholar]
- Kurup V. P., Fink J. N., Scribner G. H., Falk M. J. Antigenic variability of Aspergillus fumigatus strains. Microbios. 1977;19(77-78):191–204. [PubMed] [Google Scholar]
- Kurup V. P. Murine monoclonal antibodies binding to the specific antigens of Aspergillus fumigatus associated with allergic bronchopulmonary aspergillosis. J Clin Lab Anal. 1989;3(2):116–121. doi: 10.1002/jcla.1860030209. [DOI] [PubMed] [Google Scholar]
- Kurup V. P., Ramasamy M., Greenberger P. A., Fink J. N. Isolation and characterization of a relevant Aspergillus fumigatus antigen with IgG- and IgE-binding activity. Int Arch Allergy Appl Immunol. 1988;86(2):176–182. doi: 10.1159/000234568. [DOI] [PubMed] [Google Scholar]
- Kurup V. P., Resnick A., Scribner G. H., Gunasekaran M., Fink J. N. Enzyme profile and immunochemical characterization of Aspergillus fumigatus antigens. J Allergy Clin Immunol. 1986 Dec;78(6):1166–1173. doi: 10.1016/0091-6749(86)90267-8. [DOI] [PubMed] [Google Scholar]
- Kurup V. P., Sheth N. K. Experimental aspergillosis in rabbits. Comp Immunol Microbiol Infect Dis. 1981;4(2):161–174. doi: 10.1016/0147-9571(81)90002-3. [DOI] [PubMed] [Google Scholar]
- Locksley R. M., Heinzel F. P., Sadick M. D., Holaday B. J., Gardner K. D., Jr Murine cutaneous leishmaniasis: susceptibility correlates with differential expansion of helper T-cell subsets. Ann Inst Pasteur Immunol. 1987 Sep-Oct;138(5):744–749. doi: 10.1016/s0769-2625(87)80030-2. [DOI] [PubMed] [Google Scholar]
- Mahajan V. M., Dayal Y., Patra C. P., Bhatia I. M. Experimental aspergillosis in monkeys. Sabouraudia. 1978 Sep;16(3):199–201. doi: 10.1080/00362177885380261. [DOI] [PubMed] [Google Scholar]
- Sanderson C. J. Interleukin-5: an eosinophil growth and activation factor. Dev Biol Stand. 1988;69:23–29. [PubMed] [Google Scholar]
- Sandhu D. K., Sandhu R. S., Damodaran V. N., Randhawa H. S. Effect of cortisone on bronchopulmonary aspergillosis in mice exposed to spores of various Aspergillus species. Sabouraudia. 1970 May;8(1):32–38. [PubMed] [Google Scholar]
- Shaffer P. J., Medoff G., Kobayashi G. S. Demonstration of antigenemia by radioimmunoassay in rabbits experimentally infected with Aspergillus. J Infect Dis. 1979 Mar;139(3):313–319. doi: 10.1093/infdis/139.3.313. [DOI] [PubMed] [Google Scholar]
- Sonoda Y., Arai N., Ogawa M. Humoral regulation of eosinophilopoiesis in vitro: analysis of the targets of interleukin-3, granulocyte/macrophage colony-stimulating factor (GM-CSF), and interleukin-5. Leukemia. 1989 Jan;3(1):14–18. [PubMed] [Google Scholar]
- Strath M., Sanderson C. J. Detection of eosinophil differentiation factor and its relationship to eosinophilia in Mesocestoides corti-infected mice. Exp Hematol. 1986 Jan;14(1):16–20. [PubMed] [Google Scholar]
- Strath M., Warren D. J., Sanderson C. J. Detection of eosinophils using an eosinophil peroxidase assay. Its use as an assay for eosinophil differentiation factors. J Immunol Methods. 1985 Nov 7;83(2):209–215. doi: 10.1016/0022-1759(85)90242-x. [DOI] [PubMed] [Google Scholar]
- Venge P. The human eosinophil in inflammation. Agents Actions. 1990 Jan;29(1-2):122–126. doi: 10.1007/BF01964739. [DOI] [PubMed] [Google Scholar]
- el-Cheikh M. C., Borojevic R. Extramedullar proliferation of eosinophil granulocytes in chronic schistosomiasis mansoni is mediated by a factor secreted by inflammatory macrophages. Infect Immun. 1990 Mar;58(3):816–821. doi: 10.1128/iai.58.3.816-821.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]


