Abstract
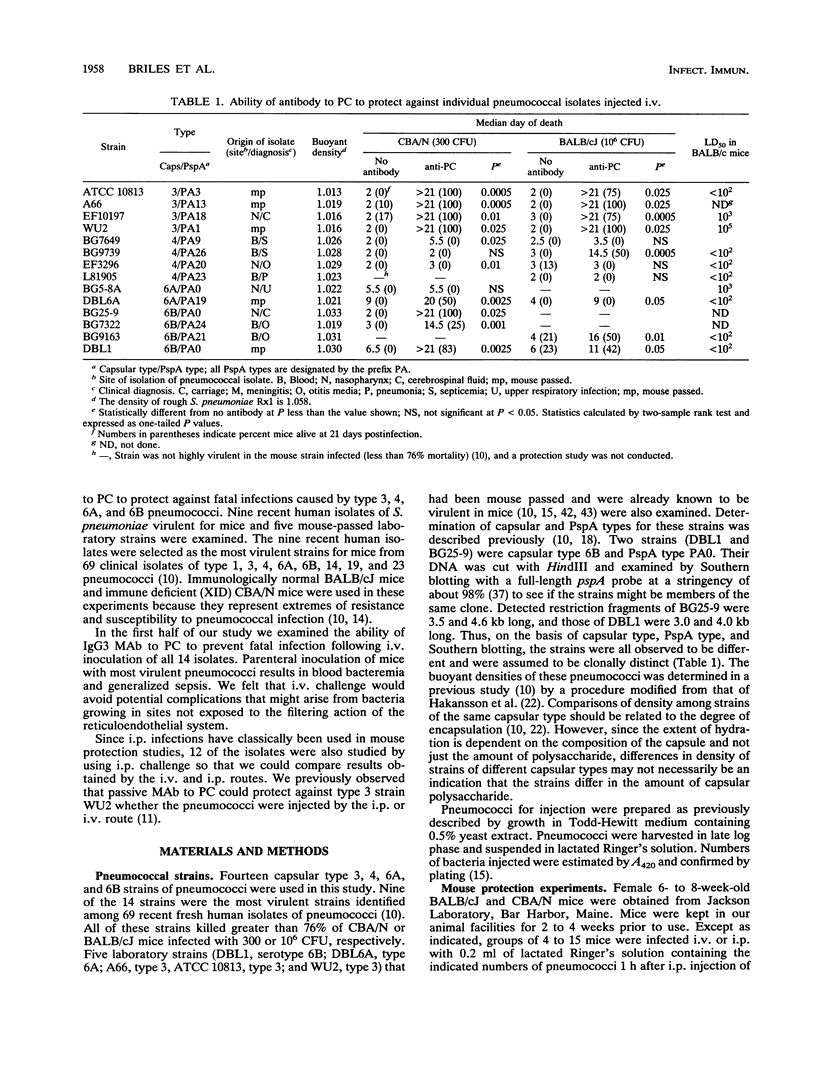
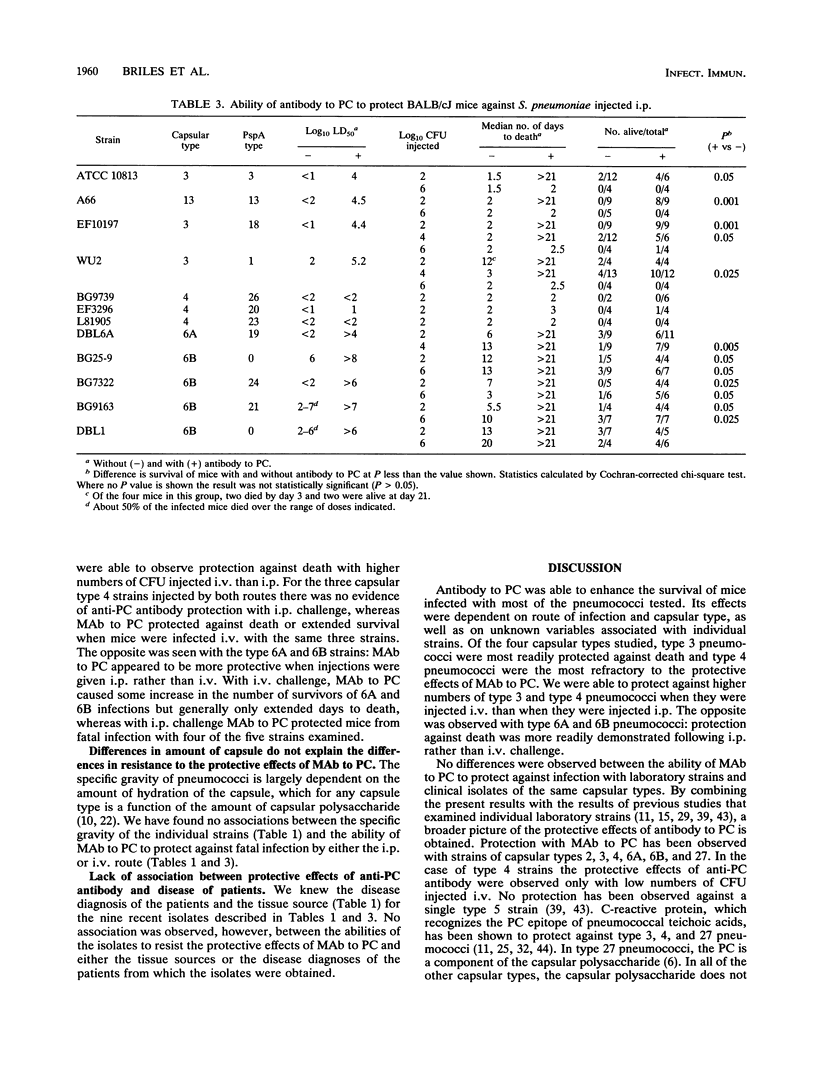
Previous studies have demonstrated that mouse antibodies to phosphocholine (PC) can protect mice against fatal infection caused by several, but not all, mouse-virulent laboratory strains of Streptococcus pneumoniae. Because the pneumococcal strains used in previous studies had been mouse passed and were propagated for many years outside of humans, it was not known whether antibody to PC would be able to protect mice against S. pneumoniae freshly isolated from humans. In the present study, we examined the ability of an immunoglobulin G (IgG) monoclonal antibody (MAb) to PC to protect against infections in mice caused by 14 pneumococcal strains of capsular types 3, 4, 6A, and 6B. Nine of these strains were selected as the most virulent strains for mice from a group of 69 fresh clinical isolates. Five were mouse-passed laboratory strains. Mouse IgG3 MAb to PC was able to exhibit protective effects (survival or increased time to death) against infection with virtually all of the strains injected intravenously and against infection with 70% of the strains injected intraperitoneally. The protective effects of antibody to PC appeared to be partially dependent on capsular type. MAb to PC was most effective against capsular type 3 strains and least effective against type 4 strains. With type 3 and type 4 strains, MAb to PC could frequently protect against larger numbers of CFU injected intravenously than intraperitoneally. For capsular type 6A and 6B strains the reverse was true.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AUSTRIAN R., GOLD J. PNEUMOCOCCAL BACTEREMIA WITH ESPECIAL REFERENCE TO BACTEREMIC PNEUMOCOCCAL PNEUMONIA. Ann Intern Med. 1964 May;60:759–776. doi: 10.7326/0003-4819-60-5-759. [DOI] [PubMed] [Google Scholar]
- Andres C. M., Maddalena A., Hudak S., Young N. M., Claflin J. L. Anti-phosphocholine hybridoma antibodies. II. Functional analysis of binding sites within three antibody families. J Exp Med. 1981 Nov 1;154(5):1584–1598. doi: 10.1084/jem.154.5.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au C. C., Eisenstein T. K. Nature of the cross-protective antigen in subcellular vaccines of Streptococcus pneumoniae. Infect Immun. 1981 Jan;31(1):160–168. doi: 10.1128/iai.31.1.160-168.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austrian R. Pneumococcal vaccine: development and prospects. Am J Med. 1979 Oct;67(4):547–549. doi: 10.1016/0002-9343(79)90222-5. [DOI] [PubMed] [Google Scholar]
- Berry A. M., Yother J., Briles D. E., Hansman D., Paton J. C. Reduced virulence of a defined pneumolysin-negative mutant of Streptococcus pneumoniae. Infect Immun. 1989 Jul;57(7):2037–2042. doi: 10.1128/iai.57.7.2037-2042.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briles D. E., Claflin J. L., Schroer K., Forman C. Mouse Igg3 antibodies are highly protective against infection with Streptococcus pneumoniae. Nature. 1981 Nov 5;294(5836):88–90. doi: 10.1038/294088a0. [DOI] [PubMed] [Google Scholar]
- Briles D. E., Crain M. J., Gray B. M., Forman C., Yother J. Strong association between capsular type and virulence for mice among human isolates of Streptococcus pneumoniae. Infect Immun. 1992 Jan;60(1):111–116. doi: 10.1128/iai.60.1.111-116.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briles D. E., Forman C., Horowitz J. C., Volanakis J. E., Benjamin W. H., Jr, McDaniel L. S., Eldridge J., Brooks J. Antipneumococcal effects of C-reactive protein and monoclonal antibodies to pneumococcal cell wall and capsular antigens. Infect Immun. 1989 May;57(5):1457–1464. doi: 10.1128/iai.57.5.1457-1464.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briles D. E., Forman C., Hudak S., Claflin J. L. The effects of idiotype on the ability of IgG1 anti-phosphorylcholine antibodies to protect mice from fatal infection with Streptococcus pneumoniae. Eur J Immunol. 1984 Nov;14(11):1027–1030. doi: 10.1002/eji.1830141112. [DOI] [PubMed] [Google Scholar]
- Briles D. E., Forman C., Hudak S., Claflin J. L. The effects of subclass on the ability of anti-phosphocholine antibodies to protect mice from fatal infection with Streptococcus pneumoniae. J Mol Cell Immunol. 1984;1(5):305–309. [PubMed] [Google Scholar]
- Briles D. E., Horowitz J., McDaniel L. S., Benjamin W. H., Jr, Claflin J. L., Booker C. L., Scott G., Forman C. Genetic control of the susceptibility to pneumococcal infection. Curr Top Microbiol Immunol. 1986;124:103–120. doi: 10.1007/978-3-642-70986-9_7. [DOI] [PubMed] [Google Scholar]
- Briles D. E., Nahm M., Schroer K., Davie J., Baker P., Kearney J., Barletta R. Antiphosphocholine antibodies found in normal mouse serum are protective against intravenous infection with type 3 streptococcus pneumoniae. J Exp Med. 1981 Mar 1;153(3):694–705. doi: 10.1084/jem.153.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briles E. B., Tomasz A. Pneumococcal Forssman antigen. A choline-containing lipoteichoic acid. J Biol Chem. 1973 Sep 25;248(18):6394–6397. [PubMed] [Google Scholar]
- Brundish D. E., Baddiley J. Pneumococcal C-substance, a ribitol teichoic acid containing choline phosphate. Biochem J. 1968 Dec;110(3):573–582. doi: 10.1042/bj1100573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crain M. J., Waltman W. D., 2nd, Turner J. S., Yother J., Talkington D. F., McDaniel L. S., Gray B. M., Briles D. E. Pneumococcal surface protein A (PspA) is serologically highly variable and is expressed by all clinically important capsular serotypes of Streptococcus pneumoniae. Infect Immun. 1990 Oct;58(10):3293–3299. doi: 10.1128/iai.58.10.3293-3299.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearhart P. J., Johnson N. D., Douglas R., Hood L. IgG antibodies to phosphorylcholine exhibit more diversity than their IgM counterparts. Nature. 1981 May 7;291(5810):29–34. doi: 10.1038/291029a0. [DOI] [PubMed] [Google Scholar]
- Henrichsen J., Sørensen C. H. The role of antibodies to pneumococcal C polysaccharide in otitis media. Pediatr Infect Dis J. 1989 Jan;8(1 Suppl):S26–S27. [PubMed] [Google Scholar]
- Horowitz J., Volanakis J. E., Briles D. E. Blood clearance of Streptococcus pneumoniae by C-reactive protein. J Immunol. 1987 Apr 15;138(8):2598–2603. [PubMed] [Google Scholar]
- Håkansson S., Holm S. E., Wagner M. Density profile of group B streptococci, type III, and its possible relation to enhanced virulence. J Clin Microbiol. 1987 Apr;25(4):714–718. doi: 10.1128/jcm.25.4.714-718.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. J., Banks S. D., Li J. P. Virulence, immunity, and vaccine related to Streptococcus pneumoniae. Crit Rev Microbiol. 1991;18(2):89–114. doi: 10.3109/10408419109113510. [DOI] [PubMed] [Google Scholar]
- Lock R. A., Paton J. C., Hansman D. Comparative efficacy of pneumococcal neuraminidase and pneumolysin as immunogens protective against Streptococcus pneumoniae. Microb Pathog. 1988 Dec;5(6):461–467. doi: 10.1016/0882-4010(88)90007-1. [DOI] [PubMed] [Google Scholar]
- McDaniel L. S., Benjamin W. H., Jr, Forman C., Briles D. E. Blood clearance by anti-phosphocholine antibodies as a mechanism of protection in experimental pneumococcal bacteremia. J Immunol. 1984 Dec;133(6):3308–3312. [PubMed] [Google Scholar]
- McDaniel L. S., Scott G., Kearney J. F., Briles D. E. Monoclonal antibodies against protease-sensitive pneumococcal antigens can protect mice from fatal infection with Streptococcus pneumoniae. J Exp Med. 1984 Aug 1;160(2):386–397. doi: 10.1084/jem.160.2.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel L. S., Waltman W. D., 2nd, Gray B., Briles D. E. A protective monoclonal antibody that reacts with a novel antigen of pneumococcal teichoic acid. Microb Pathog. 1987 Oct;3(4):249–260. doi: 10.1016/0882-4010(87)90058-1. [DOI] [PubMed] [Google Scholar]
- McDaniel L. S., Yother J., Vijayakumar M., McGarry L., Guild W. R., Briles D. E. Use of insertional inactivation to facilitate studies of biological properties of pneumococcal surface protein A (PspA). J Exp Med. 1987 Feb 1;165(2):381–394. doi: 10.1084/jem.165.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mold C., Nakayama S., Holzer T. J., Gewurz H., Du Clos T. W. C-reactive protein is protective against Streptococcus pneumoniae infection in mice. J Exp Med. 1981 Nov 1;154(5):1703–1708. doi: 10.1084/jem.154.5.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mufson M. A., Oley G., Hughey D. Pneumococcal disease in a medium-sized community in the United States. JAMA. 1982 Sep 24;248(12):1486–1489. [PubMed] [Google Scholar]
- Musher D. M., Chapman A. J., Goree A., Jonsson S., Briles D., Baughn R. E. Natural and vaccine-related immunity to Streptococcus pneumoniae. J Infect Dis. 1986 Aug;154(2):245–256. doi: 10.1093/infdis/154.2.245. [DOI] [PubMed] [Google Scholar]
- Paton J. C., Lock R. A., Lee C. J., Li J. P., Berry A. M., Mitchell T. J., Andrew P. W., Hansman D., Boulnois G. J. Purification and immunogenicity of genetically obtained pneumolysin toxoids and their conjugation to Streptococcus pneumoniae type 19F polysaccharide. Infect Immun. 1991 Jul;59(7):2297–2304. doi: 10.1128/iai.59.7.2297-2304.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proceedings of Local Branches of the Society of American Bacteriologists. Eastern Pennsylvaniaand New York City Branches. J Bacteriol. 1937 Mar;33(3):335–337. doi: 10.1128/jb.33.3.335-337.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro E. D., Berg A. T., Austrian R., Schroeder D., Parcells V., Margolis A., Adair R. K., Clemens J. D. The protective efficacy of polyvalent pneumococcal polysaccharide vaccine. N Engl J Med. 1991 Nov 21;325(21):1453–1460. doi: 10.1056/NEJM199111213252101. [DOI] [PubMed] [Google Scholar]
- Skov Sørensen U. B., Blom J., Birch-Andersen A., Henrichsen J. Ultrastructural localization of capsules, cell wall polysaccharide, cell wall proteins, and F antigen in pneumococci. Infect Immun. 1988 Aug;56(8):1890–1896. doi: 10.1128/iai.56.8.1890-1896.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szu S. C., Clarke S., Robbins J. B. Protection against pneumococcal infection in mice conferred by phosphocholine-binding antibodies: specificity of the phosphocholine binding and relation to several types. Infect Immun. 1983 Feb;39(2):993–999. doi: 10.1128/iai.39.2.993-999.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szu S. C., Schneerson R., Robbins J. B. Rabbit antibodies to the cell wall polysaccharide of Streptococcus pneumoniae fail to protect mice from lethal challenge with encapsulated pneumococci. Infect Immun. 1986 Nov;54(2):448–455. doi: 10.1128/iai.54.2.448-455.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vakil M., Briles D. E., Kearney J. F. Antigen-independent selection of T15 idiotype during B-cell ontogeny in mice. Dev Immunol. 1991;1(3):203–212. doi: 10.1155/1991/45352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallick S., Claflin J. L., Briles D. E. Resistance to Streptococcus pneumoniae is induced by a phosphocholine-protein conjugate. J Immunol. 1983 Jun;130(6):2871–2875. [PubMed] [Google Scholar]
- Yother J., Forman C., Gray B. M., Briles D. E. Protection of mice from infection with Streptococcus pneumoniae by anti-phosphocholine antibody. Infect Immun. 1982 Apr;36(1):184–188. doi: 10.1128/iai.36.1.184-188.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yother J., Volanakis J. E., Briles D. E. Human C-reactive protein is protective against fatal Streptococcus pneumoniae infection in mice. J Immunol. 1982 May;128(5):2374–2376. [PubMed] [Google Scholar]


