Abstract
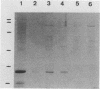
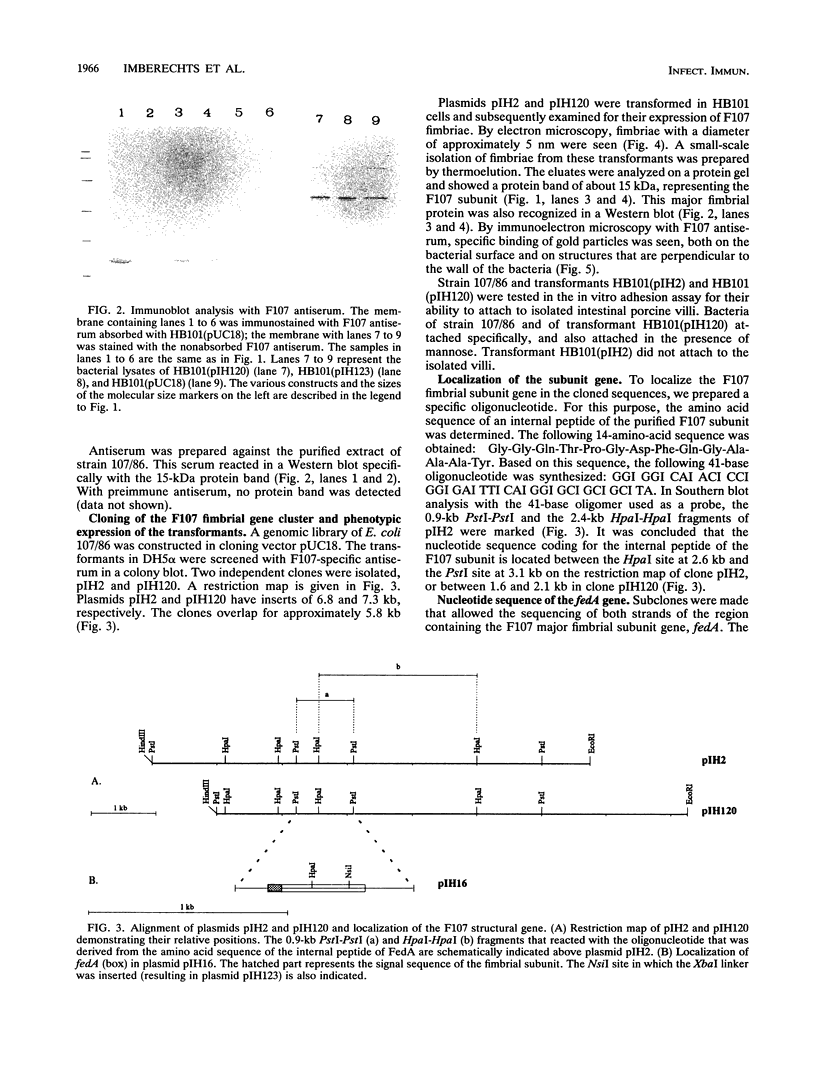
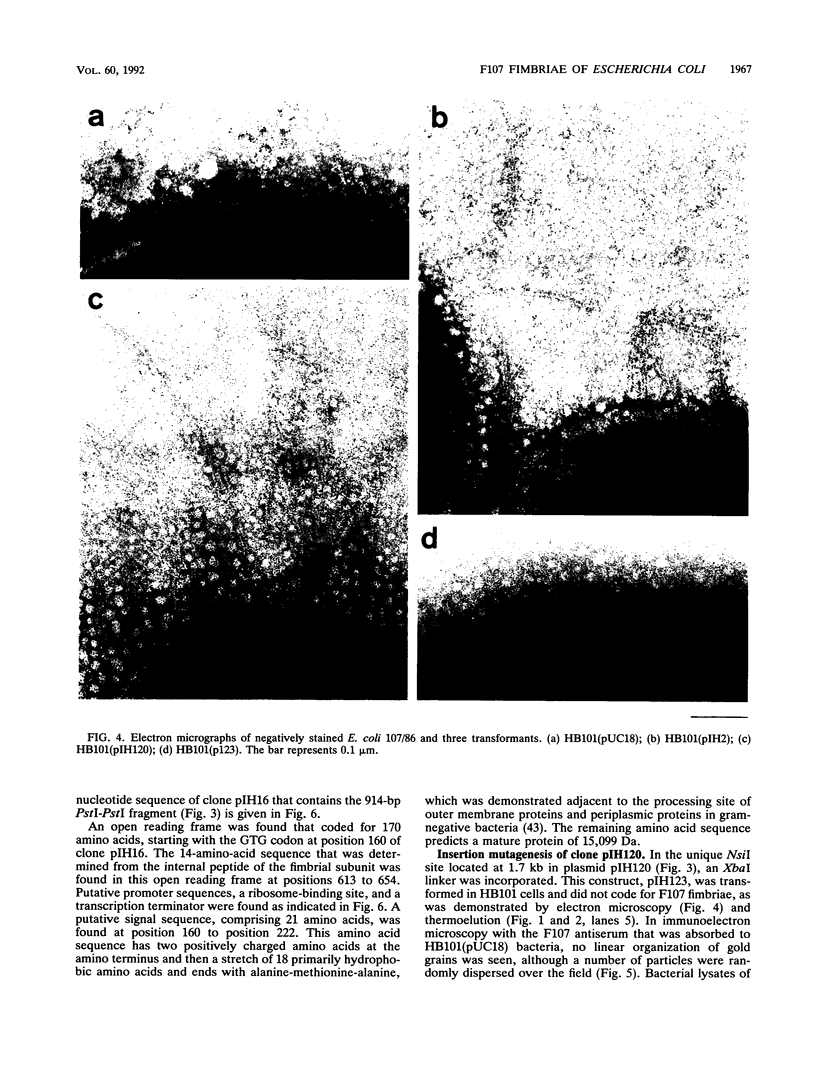
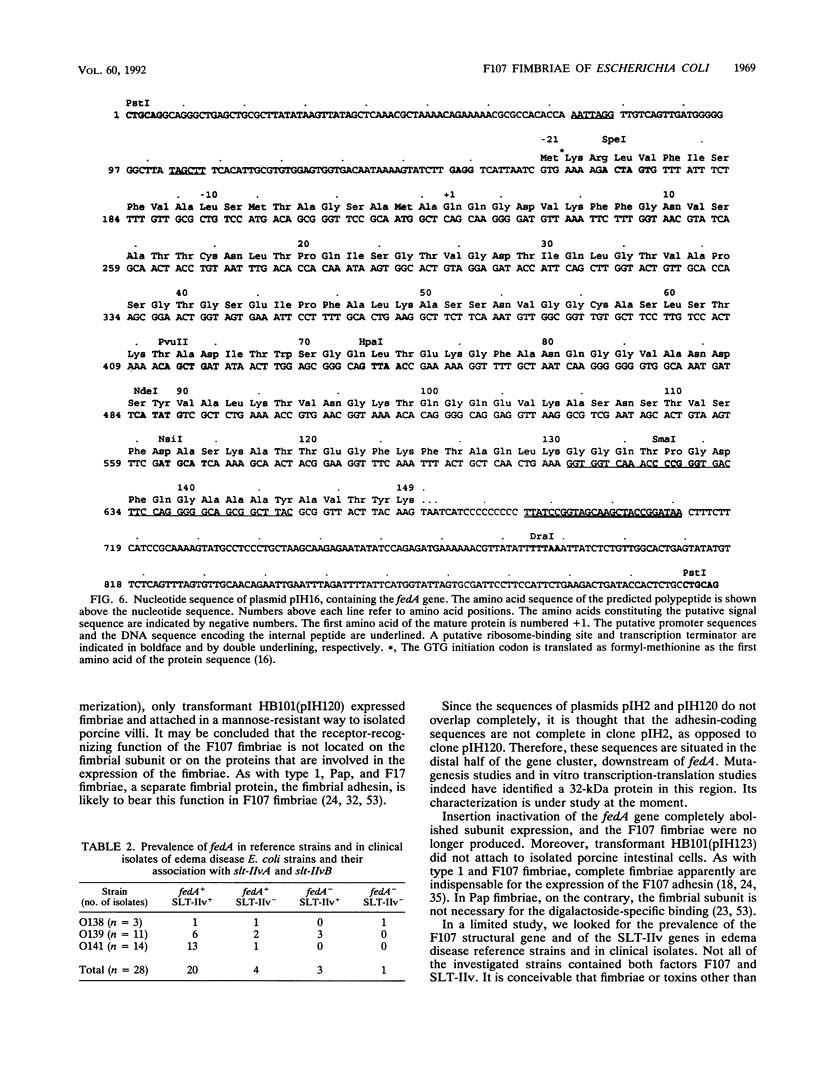
F107 fimbriae were isolated and purified from edema disease strain 107/86 of Escherichia coli. Plasmid pIH120 was constructed, which contains the gene cluster that codes for adhesive F107 fimbriae. The major fimbrial subunit gene, fedA, was sequenced. An open reading frame that codes for a protein with 170 amino acids, including a 21-amino-acid signal peptide, was found. The protein without the signal sequence has a calculated molecular mass of 15,099 Da. Construction of a nonsense mutation in the open reading frame of fedA abolished both fimbrial expression and the capacity to adhere to isolated porcine intestinal villi. In a screening of 28 reference edema disease strains and isolates from clinically ill piglets, fedA was detected in 24 cases (85.7%). In 20 (83.3%) of these 24 strains, fedA was found in association with Shiga-like toxin II variant genes, coding for the toxin that is characteristic for edema disease strains of E. coli. The fimbrial subunit gene was not detected in enterotoxigenic E. coli strains. Because of the capacity of E. coli HB101(pIH120) transformants to adhere to isolated porcine intestinal villi, the high prevalence of fedA in edema disease strains, and the high correlation with the Shiga-like toxin II variant toxin-encoding genes, we suggest that F107 fimbriae are an important virulence factor in edema disease strains of E. coli.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauw G., Rasmussen H. H., van den Bulcke M., van Damme J., Puype M., Gesser B., Celis J. E., Vandekerckhove J. Two-dimensional gel electrophoresis, protein electroblotting and microsequencing: a direct link between proteins and genes. Electrophoresis. 1990 Jul;11(7):528–536. doi: 10.1002/elps.1150110703. [DOI] [PubMed] [Google Scholar]
- Bertschinger H. U., Bachmann M., Mettler C., Pospischil A., Schraner E. M., Stamm M., Sydler T., Wild P. Adhesive fimbriae produced in vivo by Escherichia coli O139:K12(B):H1 associated with enterotoxaemia in pigs. Vet Microbiol. 1990 Nov;25(2-3):267–281. doi: 10.1016/0378-1135(90)90083-8. [DOI] [PubMed] [Google Scholar]
- Bertschinger H. U., Pohlenz J. Bacterial colonization and morphology of the intestine in porcine Escherichia coli enterotoxemia (edema disease). Vet Pathol. 1983 Jan;20(1):99–110. doi: 10.1177/030098588302000111. [DOI] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Båga M., Normark S., Hardy J., O'Hanley P., Lark D., Olsson O., Schoolnik G., Falkow S. Nucleotide sequence of the papA gene encoding the Pap pilus subunit of human uropathogenic Escherichia coli. J Bacteriol. 1984 Jan;157(1):330–333. doi: 10.1128/jb.157.1.330-333.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clugston R. E., Nielsen N. O. Experimental edema disease of swine (E. coli enterotoxemia). I. Dectection and preparation of an active principle. Can J Comp Med. 1974 Jan;38(1):22–28. [PMC free article] [PubMed] [Google Scholar]
- Clugston R. E., Nielsen N. O., Smith D. L. Experimental edema disease of swine (E. coli enterotoxemia). 3. Pathology and pathogenesis. Can J Comp Med. 1974 Jan;38(1):34–43. [PMC free article] [PubMed] [Google Scholar]
- Dhaese P., De Greve H., Decraemer H., Schell J., Van Montagu M. Rapid mapping of transposon insertion and deletion mutations in the large Ti-plasmids of Agrobacterium tumefaciens. Nucleic Acids Res. 1979 Dec 11;7(7):1837–1849. doi: 10.1093/nar/7.7.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrescu L. New biological effect of edema disease principle (Escherichia coli-neurotoxin) and its use as an in vitro for this toxin. Am J Vet Res. 1983 Jan;44(1):31–34. [PubMed] [Google Scholar]
- Gold L. Posttranscriptional regulatory mechanisms in Escherichia coli. Annu Rev Biochem. 1988;57:199–233. doi: 10.1146/annurev.bi.57.070188.001215. [DOI] [PubMed] [Google Scholar]
- Gyles C. L., De Grandis S. A., MacKenzie C., Brunton J. L. Cloning and nucleotide sequence analysis of the genes determining verocytotoxin production in a porcine edema disease isolate of Escherichia coli. Microb Pathog. 1988 Dec;5(6):419–426. doi: 10.1016/0882-4010(88)90003-4. [DOI] [PubMed] [Google Scholar]
- Hanson M. S., Brinton C. C., Jr Identification and characterization of E. coli type-1 pilus tip adhesion protein. Nature. 1988 Mar 17;332(6161):265–268. doi: 10.1038/332265a0. [DOI] [PubMed] [Google Scholar]
- Klemm P. The fimA gene encoding the type-1 fimbrial subunit of Escherichia coli. Nucleotide sequence and primary structure of the protein. Eur J Biochem. 1984 Sep 3;143(2):395–399. doi: 10.1111/j.1432-1033.1984.tb08386.x. [DOI] [PubMed] [Google Scholar]
- Korhonen T. K., Nurmiaho E. L., Ranta H., Edén C. S. New Method for isolation of immunologically pure pili from Escherichia coli. Infect Immun. 1980 Feb;27(2):569–575. doi: 10.1128/iai.27.2.569-575.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lindberg F. P., Lund B., Normark S. Genes of pyelonephritogenic E. coli required for digalactoside-specific agglutination of human cells. EMBO J. 1984 May;3(5):1167–1173. doi: 10.1002/j.1460-2075.1984.tb01946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lintermans P. F., Bertels A., Schlicker C., Deboeck F., Charlier G., Pohl P., Norgren M., Normark S., van Montagu M., De Greve H. Identification, characterization, and nucleotide sequence of the F17-G gene, which determines receptor binding of Escherichia coli F17 fimbriae. J Bacteriol. 1991 Jun;173(11):3366–3373. doi: 10.1128/jb.173.11.3366-3373.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lintermans P. F., Pohl P., Bertels A., Charlier G., Vandekerckhove J., Van Damme J., Schoup J., Schlicker C., Korhonen T., De Greve H. Characterization and purification of the F17 adhesin on the surface of bovine enteropathogenic and septicemic Escherichia coli. Am J Vet Res. 1988 Nov;49(11):1794–1799. [PubMed] [Google Scholar]
- Lintermans P., Pohl P., Deboeck F., Bertels A., Schlicker C., Vandekerckhove J., Van Damme J., Van Montagu M., De Greve H. Isolation and nucleotide sequence of the F17-A gene encoding the structural protein of the F17 fimbriae in bovine enterotoxigenic Escherichia coli. Infect Immun. 1988 Jun;56(6):1475–1484. doi: 10.1128/iai.56.6.1475-1484.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod D. L., Gyles C. L. Purification and characterization of an Escherichia coli Shiga-like toxin II variant. Infect Immun. 1990 May;58(5):1232–1239. doi: 10.1128/iai.58.5.1232-1239.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod D. L., Gyles C. L., Valdivieso-Garcia A., Clarke R. C. Physicochemical and biological properties of purified Escherichia coli Shiga-like toxin II variant. Infect Immun. 1991 Apr;59(4):1300–1306. doi: 10.1128/iai.59.4.1300-1306.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod D. L., Gyles C. L., Wilcock B. P. Reproduction of edema disease of swine with purified Shiga-like toxin-II variant. Vet Pathol. 1991 Jan;28(1):66–73. doi: 10.1177/030098589102800109. [DOI] [PubMed] [Google Scholar]
- Methiyapun S., Pohlenz J. F., Bertschinger H. U. Ultrastructure of the intestinal mucosa in pigs experimentally inoculated with an edema disease-producing strain of Escherichia coli (0139:K12:H1). Vet Pathol. 1984 Sep;21(5):516–520. doi: 10.1177/030098588402100511. [DOI] [PubMed] [Google Scholar]
- Minion F. C., Abraham S. N., Beachey E. H., Goguen J. D. The genetic determinant of adhesive function in type 1 fimbriae of Escherichia coli is distinct from the gene encoding the fimbrial subunit. J Bacteriol. 1986 Mar;165(3):1033–1036. doi: 10.1128/jb.165.3.1033-1036.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moch T., Hoschützky H., Hacker J., Kröncke K. D., Jann K. Isolation and characterization of the alpha-sialyl-beta-2,3-galactosyl-specific adhesin from fimbriated Escherichia coli. Proc Natl Acad Sci U S A. 1987 May;84(10):3462–3466. doi: 10.1073/pnas.84.10.3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooi F. R., van Buuren M., Koopman G., Roosendaal B., de Graaf F. K. K88ab gene of Escherichia coli encodes a fimbria-like protein distinct from the K88ab fimbrial adhesin. J Bacteriol. 1984 Aug;159(2):482–487. doi: 10.1128/jb.159.2.482-487.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon H. W., Isaacson R. E., Pohlenz J. Mechanisms of association of enteropathogenic Escherichia coli with intestinal epithelium. Am J Clin Nutr. 1979 Jan;32(1):119–127. doi: 10.1093/ajcn/32.1.119. [DOI] [PubMed] [Google Scholar]
- Ofek I., Mirelman D., Sharon N. Adherence of Escherichia coli to human mucosal cells mediated by mannose receptors. Nature. 1977 Feb 17;265(5595):623–625. doi: 10.1038/265623a0. [DOI] [PubMed] [Google Scholar]
- Oliver D. Protein secretion in Escherichia coli. Annu Rev Microbiol. 1985;39:615–648. doi: 10.1146/annurev.mi.39.100185.003151. [DOI] [PubMed] [Google Scholar]
- Raleigh E. A., Murray N. E., Revel H., Blumenthal R. M., Westaway D., Reith A. D., Rigby P. W., Elhai J., Hanahan D. McrA and McrB restriction phenotypes of some E. coli strains and implications for gene cloning. Nucleic Acids Res. 1988 Feb 25;16(4):1563–1575. doi: 10.1093/nar/16.4.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salit I. E., Gotschlich E. C. Hemagglutination by purified type I Escherichia coli pili. J Exp Med. 1977 Nov 1;146(5):1169–1181. doi: 10.1084/jem.146.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. W., Halls S. The production of oedema disease and diarrhoea in weaned pigs by the oral administration of Escherichia coli: factors that influence the course of the experimental disease. J Med Microbiol. 1968 Aug;1(1):45–59. doi: 10.1099/00222615-1-1-45. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlin B. E., Norgren M., Båga M., Normark S. Adhesion to human cells by Escherichia coli lacking the major subunit of a digalactoside-specific pilus-adhesin. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1800–1804. doi: 10.1073/pnas.82.6.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein D. L., Jackson M. P., Samuel J. E., Holmes R. K., O'Brien A. D. Cloning and sequencing of a Shiga-like toxin type II variant from Escherichia coli strain responsible for edema disease of swine. J Bacteriol. 1988 Sep;170(9):4223–4230. doi: 10.1128/jb.170.9.4223-4230.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- de Graaf F. K., Klaasen P. Organization and expression of genes involved in the biosynthesis of 987P fimbriae. Mol Gen Genet. 1986 Jul;204(1):75–81. doi: 10.1007/BF00330190. [DOI] [PubMed] [Google Scholar]