Abstract
Genetic analysis of a set of six Mycobacterium tuberculosis strains differing in virulence for the guinea pig revealed an altered restriction enzyme fragmentation pattern associated with the superoxide dismutase (SOD) gene in a low-virulence, isoniazid-resistant strain. In addition, it was found that the SOD enzyme produced by the isoniazid-resistant strain differed in its electrophoretic mobility from the SOD of other M. tuberculosis strains. Detailed analysis of these strain-specific differences showed that the restriction fragment length polymorphism resulted from the presence of a copy of a repetitive element 552 bp upstream of the SOD gene and that the anomalous electrophoretic mobility arose from a single nucleotide change, resulting in replacement of an aspartic acid residue by histidine in the SOD enzyme of the isoniazid-resistant strain. Possible relationships between genetic changes and strain-dependent differences in virulence are discussed.
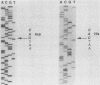
Full text
PDF


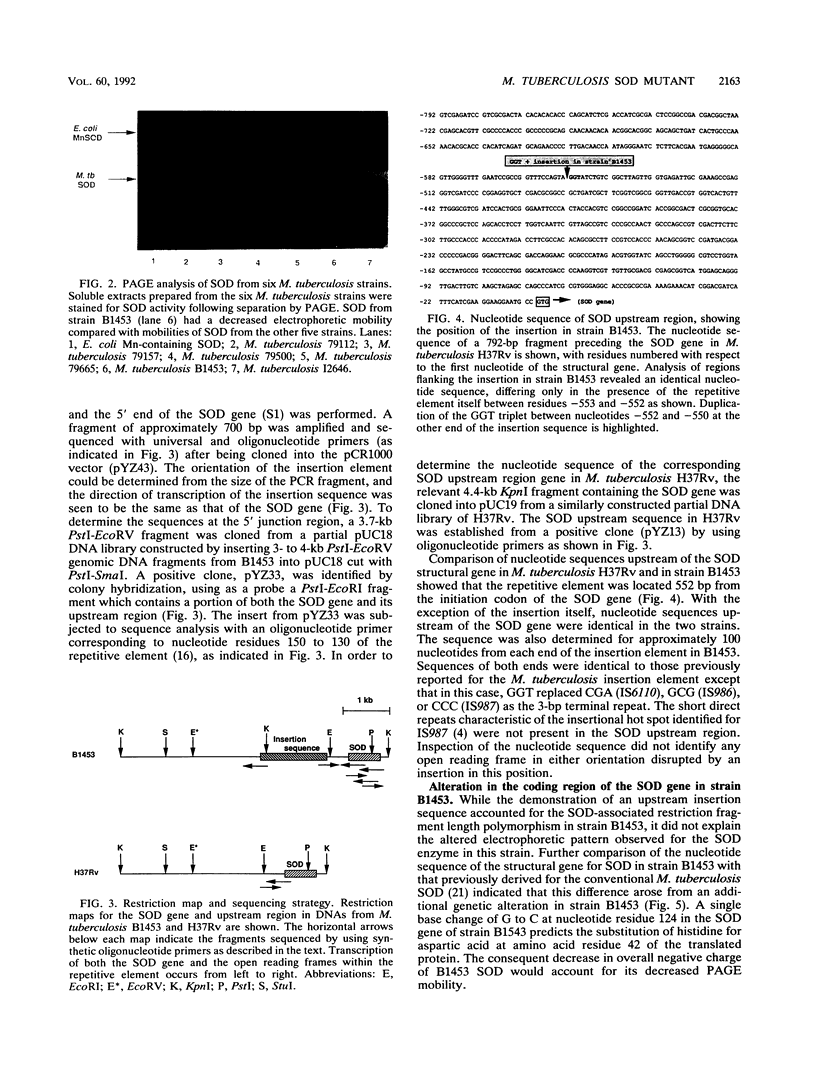


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cave M. D., Eisenach K. D., McDermott P. F., Bates J. H., Crawford J. T. IS6110: conservation of sequence in the Mycobacterium tuberculosis complex and its utilization in DNA fingerprinting. Mol Cell Probes. 1991 Feb;5(1):73–80. doi: 10.1016/0890-8508(91)90040-q. [DOI] [PubMed] [Google Scholar]
- Goren M. B., Brokl O., Schaefer W. B. Lipids of putative relevance to virulence in Mycobacterium tuberculosis: phthiocerol dimycocerosate and the attenuation indicator lipid. Infect Immun. 1974 Jan;9(1):150–158. doi: 10.1128/iai.9.1.150-158.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans P. W., van Soolingen D., Dale J. W., Schuitema A. R., McAdam R. A., Catty D., van Embden J. D. Insertion element IS986 from Mycobacterium tuberculosis: a useful tool for diagnosis and epidemiology of tuberculosis. J Clin Microbiol. 1990 Sep;28(9):2051–2058. doi: 10.1128/jcm.28.9.2051-2058.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackett P. S., Aber V. R., Lowrie D. B. Virulence and resistance to superoxide, low pH and hydrogen peroxide among strains of Mycobacterium tuberculosis. J Gen Microbiol. 1978 Jan;104(1):37–45. doi: 10.1099/00221287-104-1-37. [DOI] [PubMed] [Google Scholar]
- MIDDLEBROOK G. Isoniazid-resistance and catalase activity of tubercle bacilli; a preliminary report. Am Rev Tuberc. 1954 Mar;69(3):471–472. doi: 10.1164/art.1954.69.3.471. [DOI] [PubMed] [Google Scholar]
- MITCHISON D. A., SELKON J. B., LLOYD J. VIRULENCE IN THE GUINEA-PIG, SUSCEPTIBILITY TO HYDROGEN PEROXIDE, AND CATALASE ACTIVITY OF ISONIAZID-SENSITIVE TUBERCLE BACILLI FROM SOUTH INDIAN AND BRITISH PATIENTS. J Pathol Bacteriol. 1963 Oct;86:377–386. doi: 10.1002/path.1700860213. [DOI] [PubMed] [Google Scholar]
- Mazurek G. H., Cave M. D., Eisenach K. D., Wallace R. J., Jr, Bates J. H., Crawford J. T. Chromosomal DNA fingerprint patterns produced with IS6110 as strain-specific markers for epidemiologic study of tuberculosis. J Clin Microbiol. 1991 Sep;29(9):2030–2033. doi: 10.1128/jcm.29.9.2030-2033.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdam R. A., Hermans P. W., van Soolingen D., Zainuddin Z. F., Catty D., van Embden J. D., Dale J. W. Characterization of a Mycobacterium tuberculosis insertion sequence belonging to the IS3 family. Mol Microbiol. 1990 Sep;4(9):1607–1613. doi: 10.1111/j.1365-2958.1990.tb02073.x. [DOI] [PubMed] [Google Scholar]
- Prère M. F., Chandler M., Fayet O. Transposition in Shigella dysenteriae: isolation and analysis of IS911, a new member of the IS3 group of insertion sequences. J Bacteriol. 1990 Jul;172(7):4090–4099. doi: 10.1128/jb.172.7.4090-4099.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Thierry D., Brisson-Noël A., Vincent-Lévy-Frébault V., Nguyen S., Guesdon J. L., Gicquel B. Characterization of a Mycobacterium tuberculosis insertion sequence, IS6110, and its application in diagnosis. J Clin Microbiol. 1990 Dec;28(12):2668–2673. doi: 10.1128/jcm.28.12.2668-2673.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierry D., Cave M. D., Eisenach K. D., Crawford J. T., Bates J. H., Gicquel B., Guesdon J. L. IS6110, an IS-like element of Mycobacterium tuberculosis complex. Nucleic Acids Res. 1990 Jan 11;18(1):188–188. doi: 10.1093/nar/18.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne L. G., Diaz G. A., Doubek J. R. Acquired isoniazid resistance and catalase activity of myobacteria. Am Rev Respir Dis. 1968 May;97(5):909–913. doi: 10.1164/arrd.1968.97.5.909. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zainuddin Z. F., Dale J. W. Polymorphic repetitive DNA sequences in Mycobacterium tuberculosis detected with a gene probe from a Mycobacterium fortuitum plasmid. J Gen Microbiol. 1989 Sep;135(9):2347–2355. doi: 10.1099/00221287-135-9-2347. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Lathigra R., Garbe T., Catty D., Young D. Genetic analysis of superoxide dismutase, the 23 kilodalton antigen of Mycobacterium tuberculosis. Mol Microbiol. 1991 Feb;5(2):381–391. doi: 10.1111/j.1365-2958.1991.tb02120.x. [DOI] [PubMed] [Google Scholar]
- van Soolingen D., Hermans P. W., de Haas P. E., Soll D. R., van Embden J. D. Occurrence and stability of insertion sequences in Mycobacterium tuberculosis complex strains: evaluation of an insertion sequence-dependent DNA polymorphism as a tool in the epidemiology of tuberculosis. J Clin Microbiol. 1991 Nov;29(11):2578–2586. doi: 10.1128/jcm.29.11.2578-2586.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]