Abstract
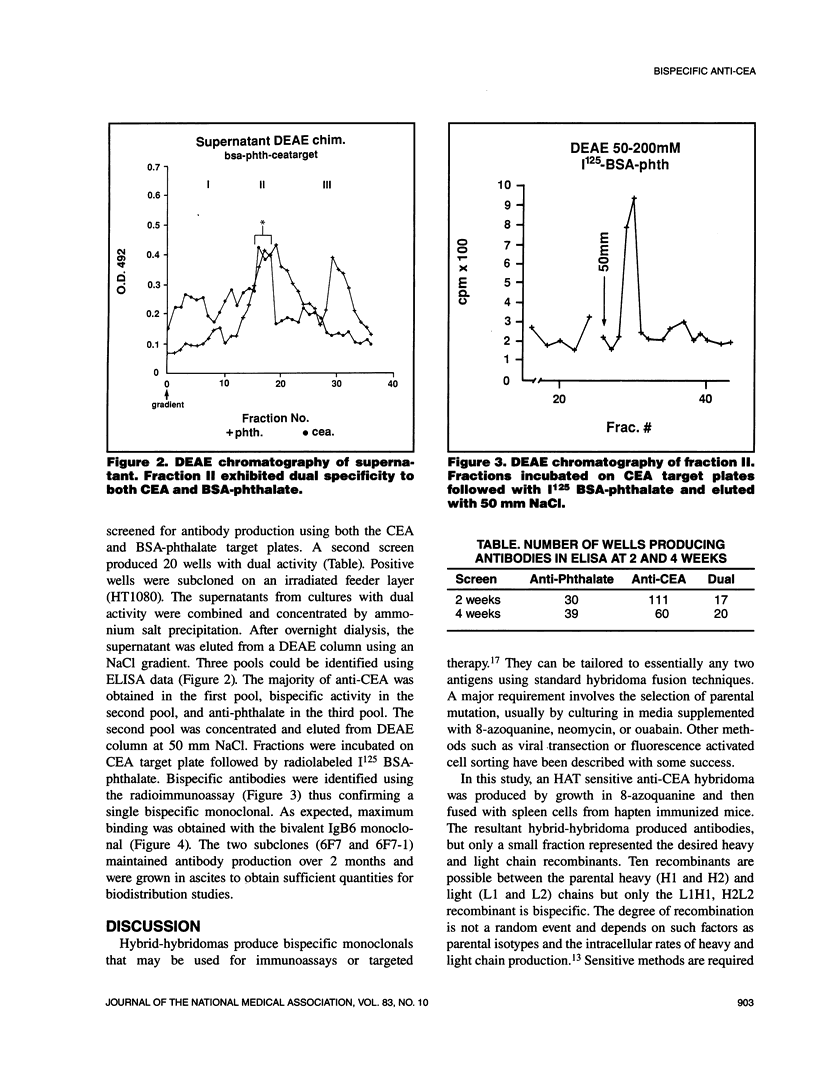
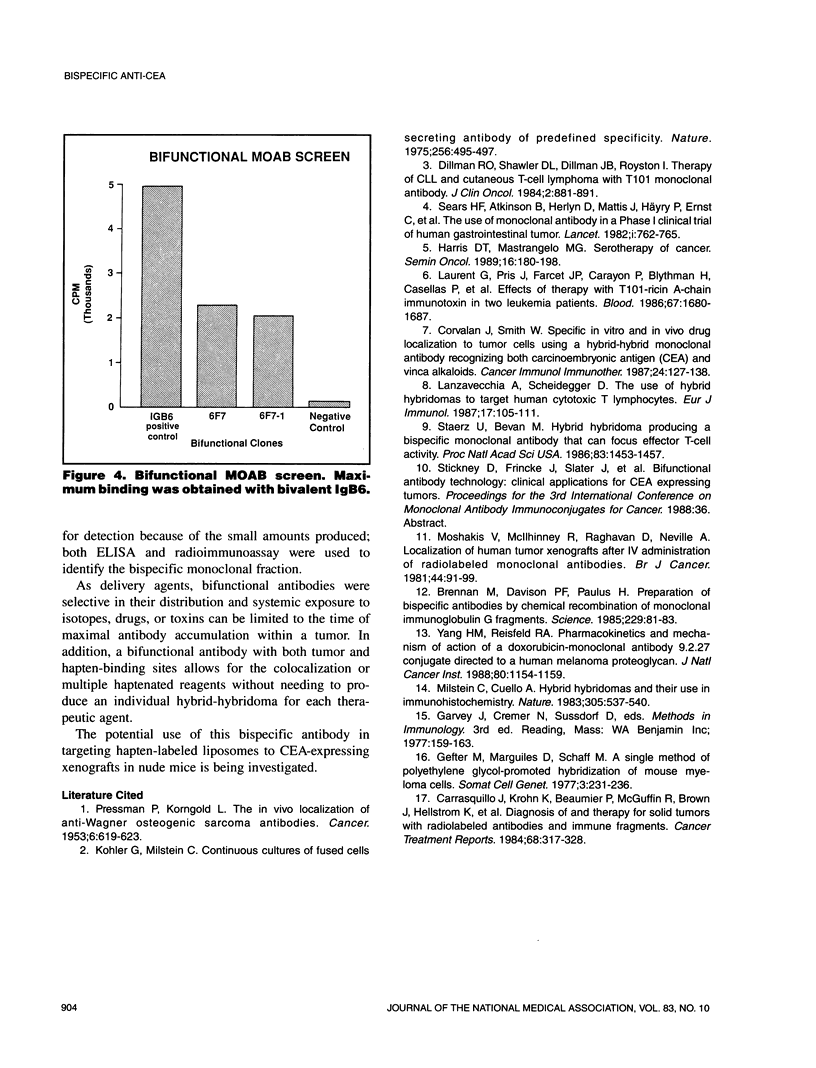
A standard hybridoma fusion technique was used to produce a monoclonal antibody capable of binding both carcinoembryonic antigen (CEA) and the hapten 4-amino-phthalate. A hypoxanthine-aminopterin-thymidine (HAT) sensitive anti-CEA hybridoma and KLH-phthalate immunized spleen cells were hybridized to yield clones producing bispecific monoclonal antibodies. The desired bispecific antibody was identified using both enzyme-linked immunosorbent assay (ELISA) and radio-immunoassay. The resultant hybrid-hybridoma or "tridoma" was subcloned and expanded to yield a stable population. Bifunctional antibody was then isolated from the various possible recombinants by ion exchange chromatography. This general method may be used to produce bispecific monoclonals against a wide variety of tumor associative antigens and reagents for immunodetection or treatment.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brennan M., Davison P. F., Paulus H. Preparation of bispecific antibodies by chemical recombination of monoclonal immunoglobulin G1 fragments. Science. 1985 Jul 5;229(4708):81–83. doi: 10.1126/science.3925553. [DOI] [PubMed] [Google Scholar]
- Carrasquillo J. A., Krohn K. A., Beaumier P., McGuffin R. W., Brown J. P., Hellström K. E., Hellström I., Larson S. M. Diagnosis of and therapy for solid tumors with radiolabeled antibodies and immune fragments. Cancer Treat Rep. 1984 Jan;68(1):317–328. [PubMed] [Google Scholar]
- Corvalan J. R., Smith W. Construction and characterisation of a hybrid-hybrid monoclonal antibody recognising both carcinoembryonic antigen (CEA) and vinca alkaloids. Cancer Immunol Immunother. 1987;24(2):127–132. doi: 10.1007/BF00205589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillman R. O., Shawler D. L., Dillman J. B., Royston I. Therapy of chronic lymphocytic leukemia and cutaneous T-cell lymphoma with T101 monoclonal antibody. J Clin Oncol. 1984 Aug;2(8):881–891. doi: 10.1200/JCO.1984.2.8.881. [DOI] [PubMed] [Google Scholar]
- Gefter M. L., Margulies D. H., Scharff M. D. A simple method for polyethylene glycol-promoted hybridization of mouse myeloma cells. Somatic Cell Genet. 1977 Mar;3(2):231–236. doi: 10.1007/BF01551818. [DOI] [PubMed] [Google Scholar]
- Harris D. T., Mastrangelo M. J. Serotherapy of cancer. Semin Oncol. 1989 Jun;16(3):180–198. [PubMed] [Google Scholar]
- Lanzavecchia A., Scheidegger D. The use of hybrid hybridomas to target human cytotoxic T lymphocytes. Eur J Immunol. 1987 Jan;17(1):105–111. doi: 10.1002/eji.1830170118. [DOI] [PubMed] [Google Scholar]
- Laurent G., Pris J., Farcet J. P., Carayon P., Blythman H., Casellas P., Poncelet P., Jansen F. K. Effects of therapy with T101 ricin A-chain immunotoxin in two leukemia patients. Blood. 1986 Jun;67(6):1680–1687. [PubMed] [Google Scholar]
- Milstein C., Cuello A. C. Hybrid hybridomas and their use in immunohistochemistry. Nature. 1983 Oct 6;305(5934):537–540. doi: 10.1038/305537a0. [DOI] [PubMed] [Google Scholar]
- Moshakis V., McIlhinney R. A., Raghavan D., Neville A. M. Localization of human tumour xenografts after i.v. administration of radiolabeled monoclonal antibodies. Br J Cancer. 1981 Jul;44(1):91–99. doi: 10.1038/bjc.1981.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears H. F., Atkinson B., Mattis J., Ernst C., Herlyn D., Steplewski Z., Häyry P., Koprowski H. Phase-I clinical trial of monoclonal antibody in treatment of gastrointestinal tumours. Lancet. 1982 Apr 3;1(8275):762–765. doi: 10.1016/s0140-6736(82)91811-6. [DOI] [PubMed] [Google Scholar]
- Staerz U. D., Bevan M. J. Hybrid hybridoma producing a bispecific monoclonal antibody that can focus effector T-cell activity. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1453–1457. doi: 10.1073/pnas.83.5.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]


