Abstract
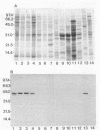
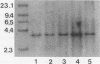
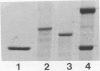
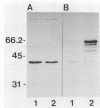
A gene encoding a protein antigen from Mycobacterium tuberculosis with a molecular weight of 40,000 has been sequenced. On the basis of sequence homology and functional analyses, we demonstrated that the protein is an L-alanine dehydrogenase (EC 1.4.1.1). The enzyme was demonstrated in M. tuberculosis and Mycobacterium marinum but not in Mycobacterium bovis BCG. The enzyme may play a role in cell wall synthesis because L-alanine is an important constituent of the peptidoglycan layer. Although no consensus signal sequence was identified, we found evidence which suggests that the enzyme is secreted across the cell membrane. The enzyme was characterized and purified by chromatography, thus enabling further studies of its role in virulence and interaction with the immune system of M. tuberculosis-infected individuals.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen A. B., Ljungqvist L., Olsen M. Evidence that protein antigen b of Mycobacterium tuberculosis is involved in phosphate metabolism. J Gen Microbiol. 1990 Mar;136(3):477–480. doi: 10.1099/00221287-136-3-477. [DOI] [PubMed] [Google Scholar]
- Andersen A. B., Worsaae A., Chaparas S. D. Isolation and characterization of recombinant lambda gt11 bacteriophages expressing eight different mycobacterial antigens of potential immunological relevance. Infect Immun. 1988 May;56(5):1344–1351. doi: 10.1128/iai.56.5.1344-1351.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen A. B., Yuan Z. L., Hasløv K., Vergmann B., Bennedsen J. Interspecies reactivity of five monoclonal antibodies to Mycobacterium tuberculosis as examined by immunoblotting and enzyme-linked immunosorbent assay. J Clin Microbiol. 1986 Mar;23(3):446–451. doi: 10.1128/jcm.23.3.446-451.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen P., Askgaard D., Ljungqvist L., Bennedsen J., Heron I. Proteins released from Mycobacterium tuberculosis during growth. Infect Immun. 1991 Jun;59(6):1905–1910. doi: 10.1128/iai.59.6.1905-1910.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Crawford J. T., Bates J. H. Isolation of plasmids from mycobacteria. Infect Immun. 1979 Jun;24(3):979–981. doi: 10.1128/iai.24.3.979-981.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDMAN D. S. Enzyme systems in the mycobacteria. VII. Purification, properties and mechanism of action of the alanine dehydrogenase. Biochim Biophys Acta. 1959 Aug;34:527–539. doi: 10.1016/0006-3002(59)90305-1. [DOI] [PubMed] [Google Scholar]
- Husson R. N., James B. E., Young R. A. Gene replacement and expression of foreign DNA in mycobacteria. J Bacteriol. 1990 Feb;172(2):519–524. doi: 10.1128/jb.172.2.519-524.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki K., Tanizawa K., Badet B., Walsh C. T., Tanaka H., Soda K. Thermostable alanine racemase from Bacillus stearothermophilus: molecular cloning of the gene, enzyme purification, and characterization. Biochemistry. 1986 Jun 3;25(11):3268–3274. doi: 10.1021/bi00359a028. [DOI] [PubMed] [Google Scholar]
- Kuroda S., Tanizawa K., Sakamoto Y., Tanaka H., Soda K. Alanine dehydrogenases from two Bacillus species with distinct thermostabilities: molecular cloning, DNA and protein sequence determination, and structural comparison with other NAD(P)(+)-dependent dehydrogenases. Biochemistry. 1990 Jan 30;29(4):1009–1015. doi: 10.1021/bi00456a025. [DOI] [PubMed] [Google Scholar]
- Ljungqvist L., Andersen A. B., Andersen P., Hasløv K., Worsaae A., Bennedsen J., Heron I. Affinity purification, biological characterization and serological evaluation of defined antigens from Mycobacterium tuberculosis. Trop Med Parasitol. 1990 Sep;41(3):333–335. [PubMed] [Google Scholar]
- Ljungqvist L., Worsaae A., Heron I. Antibody responses against Mycobacterium tuberculosis in 11 strains of inbred mice: novel monoclonal antibody specificities generated by fusions, using spleens from BALB.B10 and CBA/J mice. Infect Immun. 1988 Aug;56(8):1994–1998. doi: 10.1128/iai.56.8.1994-1998.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata S., Misono H., Nagasaki S., Esaki N., Tanaka H., Soda K. Thermostable alanine dehydrogenase of Bacillus sp. DSM730: gene cloning, purification, and characterization. Biochimie. 1989 Apr;71(4):559–563. doi: 10.1016/0300-9084(89)90187-9. [DOI] [PubMed] [Google Scholar]
- Oliver D. Protein secretion in Escherichia coli. Annu Rev Microbiol. 1985;39:615–648. doi: 10.1146/annurev.mi.39.100185.003151. [DOI] [PubMed] [Google Scholar]
- Porumb H., Vancea D., Mureşan L., Presecan E., Lascu I., Petrescu I., Porumb T., Pop R., Bârzu O. Structural and catalytic properties of L-alanine dehydrogenase from Bacillus cereus. J Biol Chem. 1987 Apr 5;262(10):4610–4615. [PubMed] [Google Scholar]
- Shinnick T. M. The 65-kilodalton antigen of Mycobacterium tuberculosis. J Bacteriol. 1987 Mar;169(3):1080–1088. doi: 10.1128/jb.169.3.1080-1088.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thangaraj H. S., Lamb F. I., Davis E. O., Jenner P. J., Jeyakumar L. H., Colston M. J. Identification, sequencing, and expression of Mycobacterium leprae superoxide dismutase, a major antigen. Infect Immun. 1990 Jun;58(6):1937–1942. doi: 10.1128/iai.58.6.1937-1942.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsaae A., Ljungqvist L., Heron I. Monoclonal antibodies produced in BALB.B10 mice define new antigenic determinants in culture filtrate preparations of Mycobacterium tuberculosis. J Clin Microbiol. 1988 Dec;26(12):2608–2614. doi: 10.1128/jcm.26.12.2608-2614.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]