Abstract
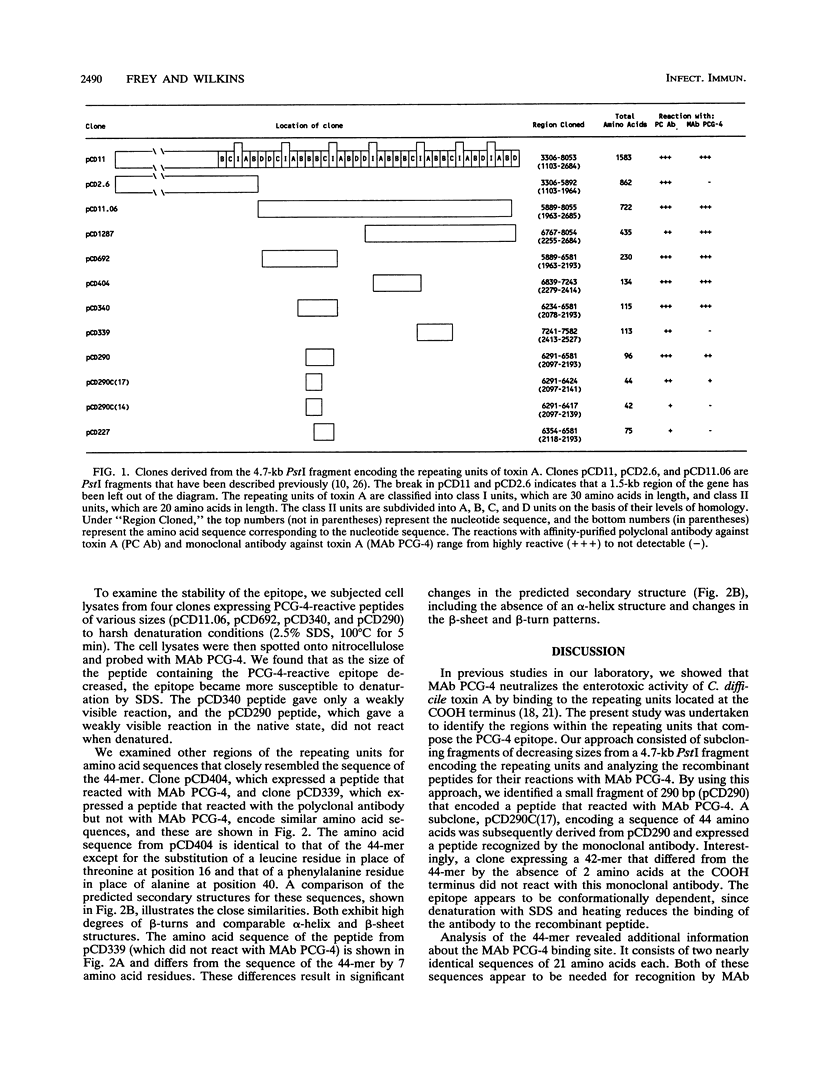
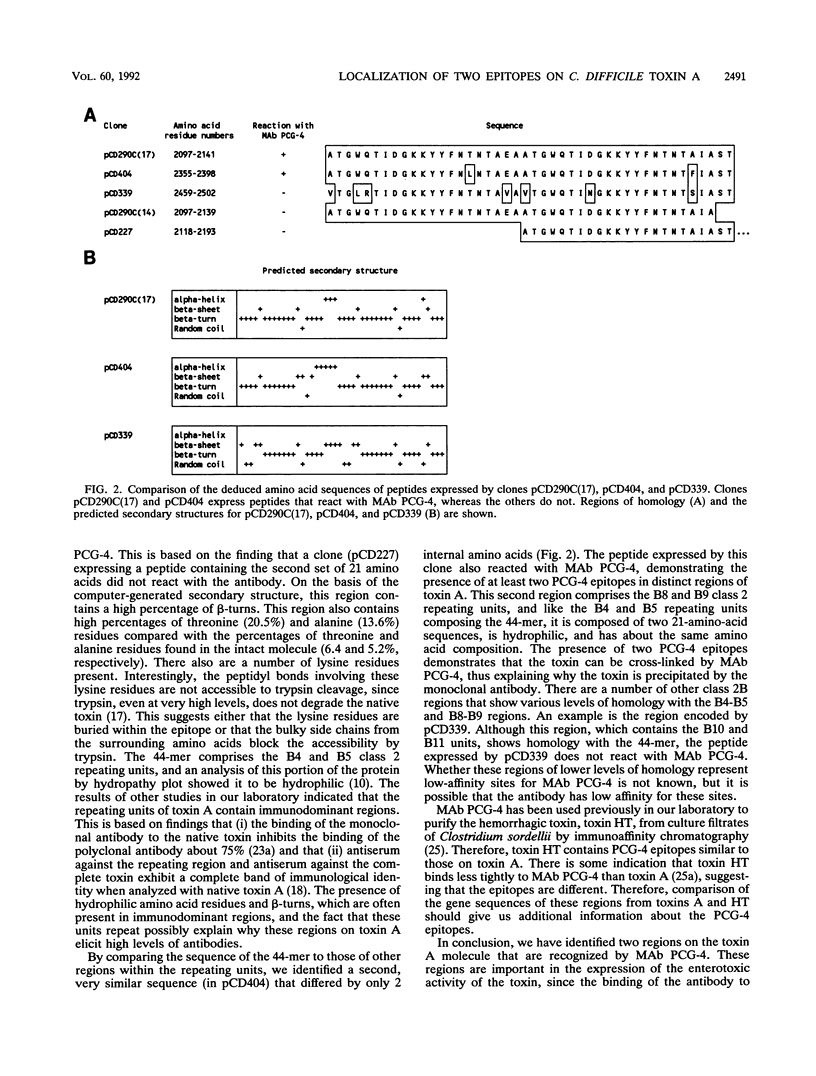
The toxin A gene of Clostridium difficile contains a 2.5-kb region encoding a series of contiguous repeating units located at the COOH terminus of the molecule. We previously showed that the monoclonal antibody (MAb) PCG-4, which neutralizes the enterotoxic activity of toxin A, binds to epitopes located within these repeating units. In the present study, we subcloned a series of fragments from this portion of the gene. The recombinant peptides expressed from the gene fragments were examined for reactivity with MAb PCG-4 to identify the epitopes involved in binding. Our results showed that MAb PCG-4 recognizes epitopes in amino acid residues 2097 through 2141 and amino acid residues 2355 through 2398.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banno Y., Kobayashi T., Kono H., Watanabe K., Ueno K., Nozawa Y. Biochemical characterization and biologic actions of two toxins (D-1 and D-2) from Clostridium difficile. Rev Infect Dis. 1984 Mar-Apr;6 (Suppl 1):S11–S20. doi: 10.1093/clinids/6.supplement_1.s11. [DOI] [PubMed] [Google Scholar]
- Barroso L. A., Wang S. Z., Phelps C. J., Johnson J. L., Wilkins T. D. Nucleotide sequence of Clostridium difficile toxin B gene. Nucleic Acids Res. 1990 Jul 11;18(13):4004–4004. doi: 10.1093/nar/18.13.4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett J. G., Chang T. W., Gurwith M., Gorbach S. L., Onderdonk A. B. Antibiotic-associated pseudomembranous colitis due to toxin-producing clostridia. N Engl J Med. 1978 Mar 9;298(10):531–534. doi: 10.1056/NEJM197803092981003. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C. A rapid alkaline extraction method for the isolation of plasmid DNA. Methods Enzymol. 1983;100:243–255. doi: 10.1016/0076-6879(83)00059-2. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- Dove C. H., Wang S. Z., Price S. B., Phelps C. J., Lyerly D. M., Wilkins T. D., Johnson J. L. Molecular characterization of the Clostridium difficile toxin A gene. Infect Immun. 1990 Feb;58(2):480–488. doi: 10.1128/iai.58.2.480-488.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George R. H., Symonds J. M., Dimock F., Brown J. D., Arabi Y., Shinagawa N., Keighley M. R., Alexander-Williams J., Burdon D. W. Identification of Clostridium difficile as a cause of pseudomembranous colitis. Br Med J. 1978 Mar 18;1(6114):695–695. doi: 10.1136/bmj.1.6114.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- He M., Beechey R. B., Kaderbhai M. A. A procedure for electro-elution of DNA from agarose gels. Biotechniques. 1988 Sep;6(8):728–730. [PubMed] [Google Scholar]
- Helfman D. M., Feramisco J. R., Fiddes J. C., Thomas G. P., Hughes S. H. Identification of clones that encode chicken tropomyosin by direct immunological screening of a cDNA expression library. Proc Natl Acad Sci U S A. 1983 Jan;80(1):31–35. doi: 10.1073/pnas.80.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krivan H. C., Clark G. F., Smith D. F., Wilkins T. D. Cell surface binding site for Clostridium difficile enterotoxin: evidence for a glycoconjugate containing the sequence Gal alpha 1-3Gal beta 1-4GlcNAc. Infect Immun. 1986 Sep;53(3):573–581. doi: 10.1128/iai.53.3.573-581.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson H. E., Price A. B., Honour P., Borriello S. P. Clostridium difficile and the aetiology of pseudomembranous colitis. Lancet. 1978 May 20;1(8073):1063–1066. doi: 10.1016/s0140-6736(78)90912-1. [DOI] [PubMed] [Google Scholar]
- Libby J. M., Jortner B. S., Wilkins T. D. Effects of the two toxins of Clostridium difficile in antibiotic-associated cecitis in hamsters. Infect Immun. 1982 May;36(2):822–829. doi: 10.1128/iai.36.2.822-829.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyerly D. M., Krivan H. C., Wilkins T. D. Clostridium difficile: its disease and toxins. Clin Microbiol Rev. 1988 Jan;1(1):1–18. doi: 10.1128/cmr.1.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyerly D. M., Lockwood D. E., Richardson S. H., Wilkins T. D. Biological activities of toxins A and B of Clostridium difficile. Infect Immun. 1982 Mar;35(3):1147–1150. doi: 10.1128/iai.35.3.1147-1150.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyerly D. M., Phelps C. J., Toth J., Wilkins T. D. Characterization of toxins A and B of Clostridium difficile with monoclonal antibodies. Infect Immun. 1986 Oct;54(1):70–76. doi: 10.1128/iai.54.1.70-76.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyerly D. M., Phelps C. J., Wilkins T. D. Monoclonal and specific polyclonal antibodies for immunoassay of Clostridium difficile toxin A. J Clin Microbiol. 1985 Jan;21(1):12–14. doi: 10.1128/jcm.21.1.12-14.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyerly D. M., Sullivan N. M., Wilkins T. D. Enzyme-linked immunosorbent assay for Clostridium difficile toxin A. J Clin Microbiol. 1983 Jan;17(1):72–78. doi: 10.1128/jcm.17.1.72-78.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez R. D., Wilkins T. D. Purification and characterization of Clostridium sordellii hemorrhagic toxin and cross-reactivity with Clostridium difficile toxin A (enterotoxin). Infect Immun. 1988 May;56(5):1215–1221. doi: 10.1128/iai.56.5.1215-1221.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan N. M., Pellett S., Wilkins T. D. Purification and characterization of toxins A and B of Clostridium difficile. Infect Immun. 1982 Mar;35(3):1032–1040. doi: 10.1128/iai.35.3.1032-1040.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor N. S., Thorne G. M., Bartlett J. G. Comparison of two toxins produced by Clostridium difficile. Infect Immun. 1981 Dec;34(3):1036–1043. doi: 10.1128/iai.34.3.1036-1043.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]


