Abstract
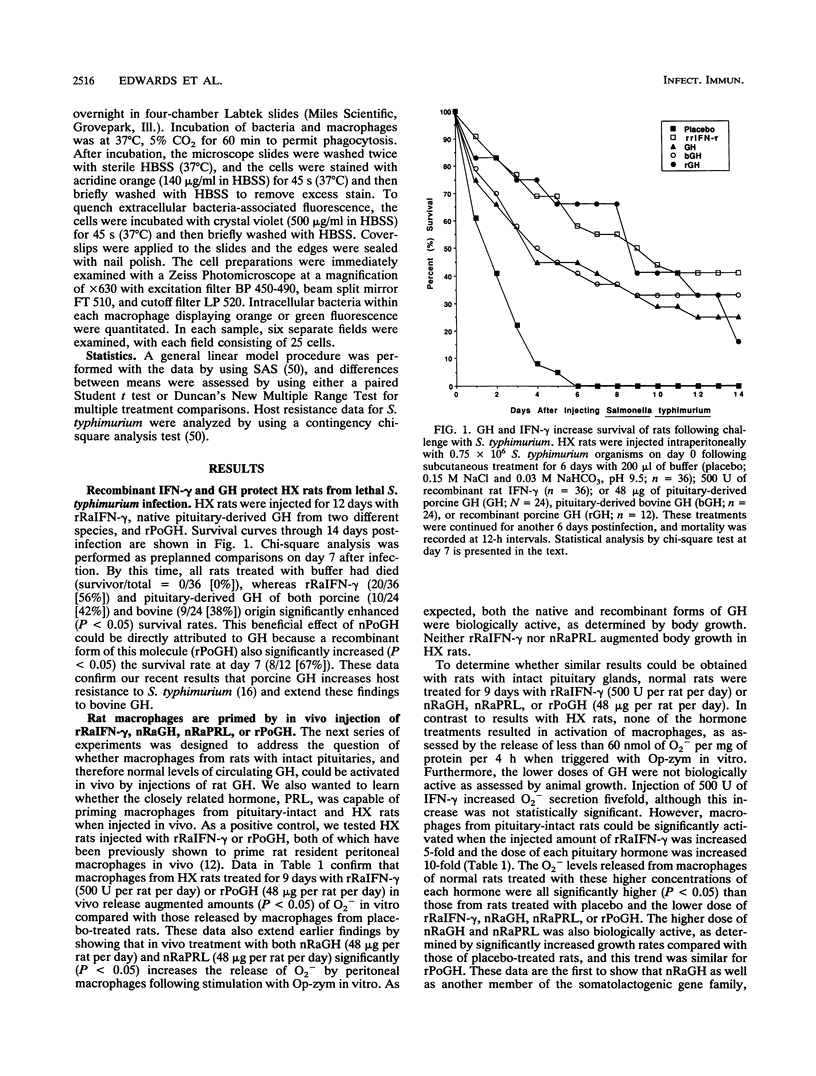
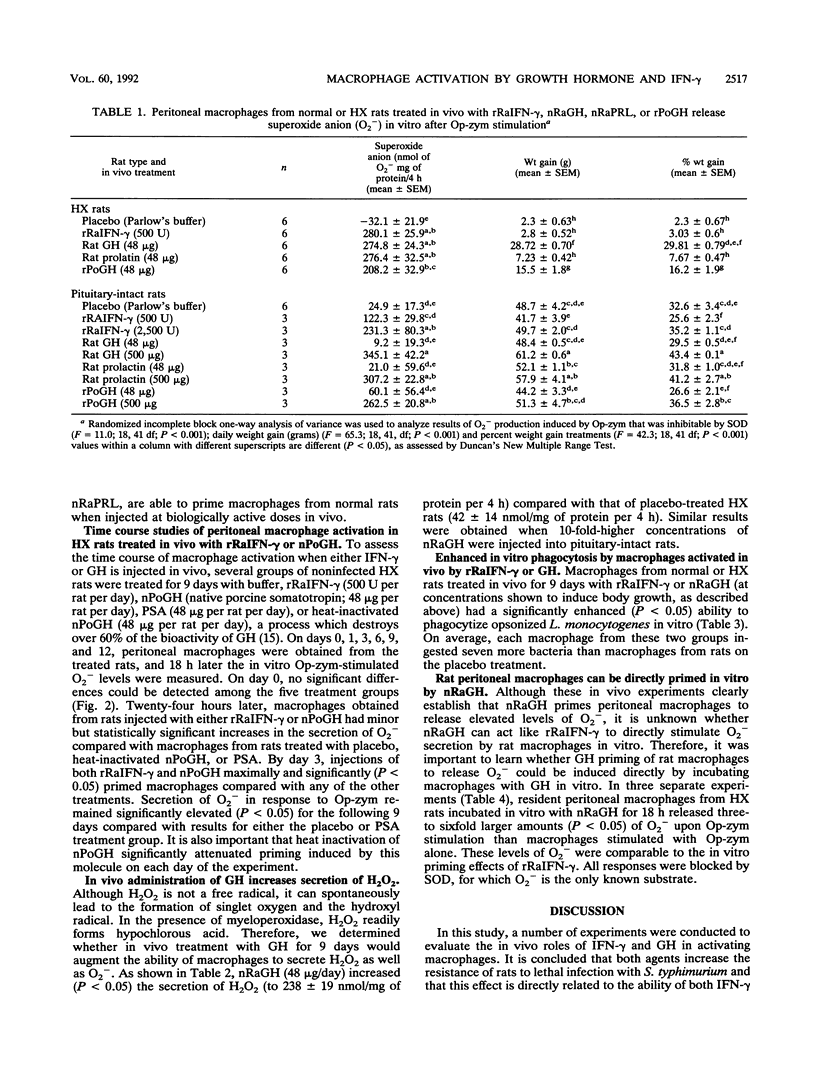
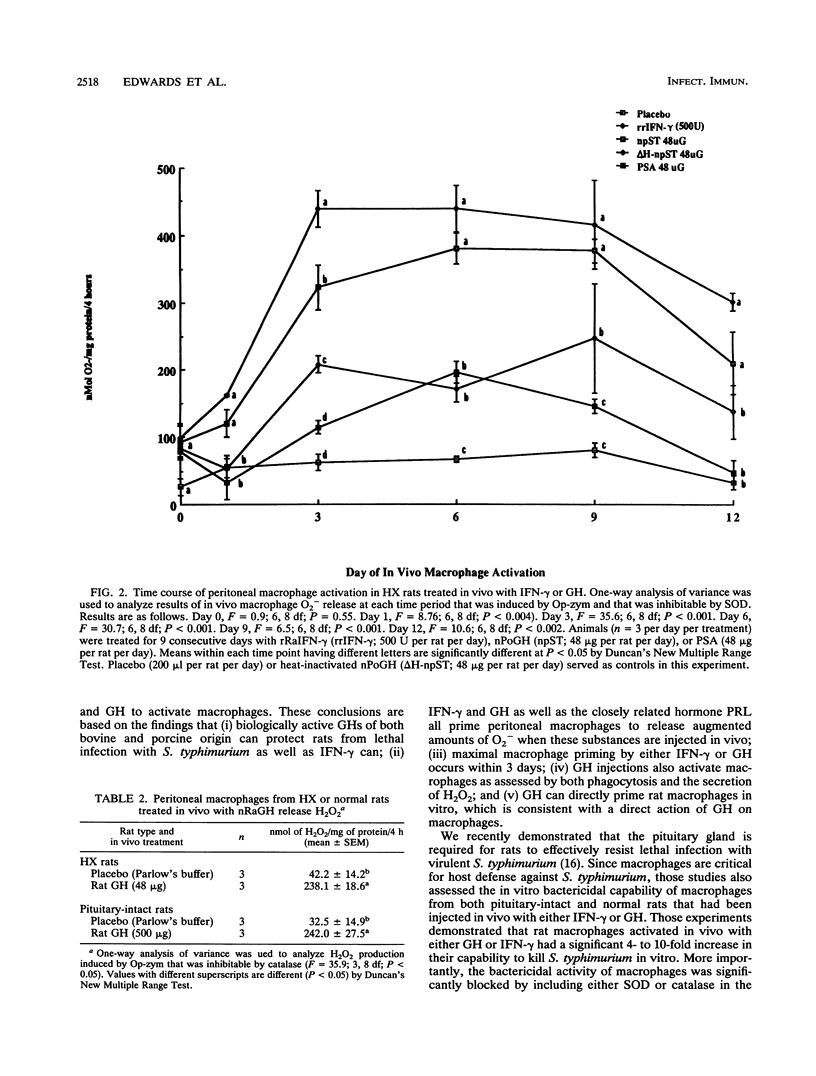
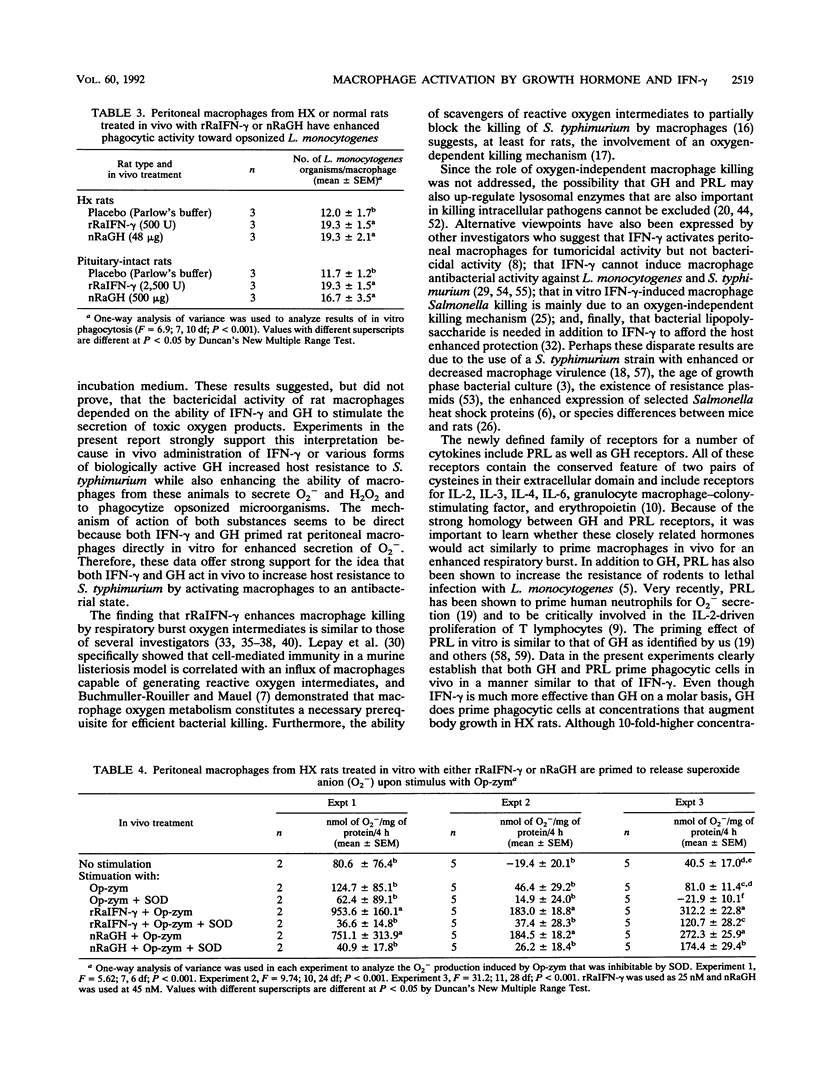
Purified and recombinant forms of growth hormone (GH) as well as of recombinant rat gamma interferon (IFN-gamma) enhance the survival of rats deprived of endogenous pituitary GH secretion by hypophysectomy (HX rats) and infected with virulent Salmonella typhimurium. Macrophages obtained from rats with intact pituitaries (pituitary-intact rats) or HX rats that were treated in vivo with either GH or the closely related hormone prolactin released elevated (P less than 0.05) levels of superoxide anion (O2-) after in vitro opsonized-zymosan stimulation compared with those from placebo-treated animals. These levels of O2- release were similar in magnitude to those of macrophages from rats treated in vivo with IFN-gamma. In time course in vivo macrophage activation studies, both IFN-gamma and GH significantly increased O2- secretion within 24 h, with maximal secretion occurring at day 3. Macrophages obtained from pituitary-intact and HX rats injected in vivo with GH also released elevated (P less than 0.05) levels of hydrogen peroxide (H2O2) and displayed enhanced (P less than 0.01) phagocytic activity toward opsonized Listeria monocytogenes in vitro. The mechanism of action of GH in vivo is likely to be a direct one because resident peritoneal macrophages from rats could be primed in vitro for enhanced secretion of O2- following triggering of these cells with opsonized zymosan. These data show that in vivo administration of two closely related pituitary hormones, GH and prolactin, can effectively prime macrophages, which is consistent with the hypothesis that GH mediates resistance to S. typhimurium by a direct stimulatory action on macrophages.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O., Hamilton T. A. Molecular transductional mechanisms by which IFN gamma and other signals regulate macrophage development. Immunol Rev. 1987 Jun;97:5–27. doi: 10.1111/j.1600-065x.1987.tb00514.x. [DOI] [PubMed] [Google Scholar]
- Adams D. O., Hamilton T. A. The cell biology of macrophage activation. Annu Rev Immunol. 1984;2:283–318. doi: 10.1146/annurev.iy.02.040184.001435. [DOI] [PubMed] [Google Scholar]
- Benjamin W. H., Jr, Posey B. S., Briles D. E. Effects of in vitro growth phase on the pathogenesis of Salmonella typhimurium in mice. J Gen Microbiol. 1986 May;132(5):1283–1295. doi: 10.1099/00221287-132-5-1283. [DOI] [PubMed] [Google Scholar]
- Bernton E. W., Meltzer M. S., Holaday J. W. Suppression of macrophage activation and T-lymphocyte function in hypoprolactinemic mice. Science. 1988 Jan 22;239(4838):401–404. doi: 10.1126/science.3122324. [DOI] [PubMed] [Google Scholar]
- Buchmeier N. A., Heffron F. Induction of Salmonella stress proteins upon infection of macrophages. Science. 1990 May 11;248(4956):730–732. doi: 10.1126/science.1970672. [DOI] [PubMed] [Google Scholar]
- Buchmüller-Rouiller Y., Mauël J. Correlation between enhanced oxidative metabolism and leishmanicidal activity in activated macrophages from healer and nonhealer mouse strains. J Immunol. 1986 May 15;136(10):3884–3890. [PubMed] [Google Scholar]
- Campbell P. A., Canono B. P., Cook J. L. Mouse macrophages stimulated by recombinant gamma interferon to kill tumor cells are not bactericidal for the facultative intracellular bacterium Listeria monocytogenes. Infect Immun. 1988 May;56(5):1371–1375. doi: 10.1128/iai.56.5.1371-1375.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevenger C. V., Altmann S. W., Prystowsky M. B. Requirement of nuclear prolactin for interleukin-2--stimulated proliferation of T lymphocytes. Science. 1991 Jul 5;253(5015):77–79. doi: 10.1126/science.2063207. [DOI] [PubMed] [Google Scholar]
- Cosman D., Lyman S. D., Idzerda R. L., Beckmann M. P., Park L. S., Goodwin R. G., March C. J. A new cytokine receptor superfamily. Trends Biochem Sci. 1990 Jul;15(7):265–270. doi: 10.1016/0968-0004(90)90051-c. [DOI] [PubMed] [Google Scholar]
- Edwards C. K., 3rd, Ghiasuddin S. M., Schepper J. M., Yunger L. M., Kelley K. W. A newly defined property of somatotropin: priming of macrophages for production of superoxide anion. Science. 1988 Feb 12;239(4841 Pt 1):769–771. doi: 10.1126/science.2829357. [DOI] [PubMed] [Google Scholar]
- Edwards C. K., 3rd, Hedegaard H. B., Zlotnik A., Gangadharam P. R., Johnston R. B., Jr, Pabst M. J. Chronic infection due to Mycobacterium intracellulare in mice: association with macrophage release of prostaglandin E2 and reversal by injection of indomethacin, muramyl dipeptide, or interferon-gamma. J Immunol. 1986 Mar 1;136(5):1820–1827. [PubMed] [Google Scholar]
- Edwards C. K., 3rd, Jacobs M. J., Myers-Keith P., Yunger L. M. Murine macrophage activation with resorcyclic acid lactones (RALs): comparison with diethylstilbestrol and 17 beta-estradiol. Immunopharmacology. 1989 Mar-Apr;17(2):107–118. doi: 10.1016/0162-3109(89)90056-8. [DOI] [PubMed] [Google Scholar]
- Edwards C. K., 3rd, Lorence R. M., Dunham D. M., Arkins S., Yunger L. M., Greager J. A., Walter R. J., Dantzer R., Kelley K. W. Hypophysectomy inhibits the synthesis of tumor necrosis factor alpha by rat macrophages: partial restoration by exogenous growth hormone or interferon gamma. Endocrinology. 1991 Feb;128(2):989–986. doi: 10.1210/endo-128-2-989. [DOI] [PubMed] [Google Scholar]
- Edwards C. K., 3rd, Yunger L. M., Lorence R. M., Dantzer R., Kelley K. W. The pituitary gland is required for protection against lethal effects of Salmonella typhimurium. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2274–2277. doi: 10.1073/pnas.88.6.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsbach P., Weiss J. A reevaluation of the roles of the O2-dependent and O2-independent microbicidal systems of phagocytes. Rev Infect Dis. 1983 Sep-Oct;5(5):843–853. doi: 10.1093/clinids/5.5.843. [DOI] [PubMed] [Google Scholar]
- Fields P. I., Swanson R. V., Haidaris C. G., Heffron F. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5189–5193. doi: 10.1073/pnas.83.14.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y. K., Arkins S., Fuh G., Cunningham B. C., Wells J. A., Fong S., Cronin M. J., Dantzer R., Kelley K. W. Growth hormone augments superoxide anion secretion of human neutrophils by binding to the prolactin receptor. J Clin Invest. 1992 Feb;89(2):451–457. doi: 10.1172/JCI115605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabay J. E., Heiple J. M., Cohn Z. A., Nathan C. F. Subcellular location and properties of bactericidal factors from human neutrophils. J Exp Med. 1986 Nov 1;164(5):1407–1421. doi: 10.1084/jem.164.5.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangadharam P. R., Edwards C. K., 3rd Release of superoxide anion from resident and activated mouse peritoneal macrophages infected with Mycobacterium intracellulare. Am Rev Respir Dis. 1984 Nov;130(5):834–838. doi: 10.1164/arrd.1984.130.5.834. [DOI] [PubMed] [Google Scholar]
- Goldberg M., Belkowski L. S., Bloom B. R. Regulation of macrophage function by interferon-gamma. Somatic cell genetic approaches in murine macrophage cell lines to mechanisms of growth inhibition, the oxidative burst, and expression of the chronic granulomatous disease gene. J Clin Invest. 1990 Feb;85(2):563–569. doi: 10.1172/JCI114473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould C. L., Sonnenfeld G. Effect of treatment with interferon-gamma and concanavalin A on the course of infection of mice with Salmonella typhimurium strain LT-2. J Interferon Res. 1987 Jun;7(3):255–260. doi: 10.1089/jir.1987.7.255. [DOI] [PubMed] [Google Scholar]
- Jesaitis A. J., Buescher E. S., Harrison D., Quinn M. T., Parkos C. A., Livesey S., Linner J. Ultrastructural localization of cytochrome b in the membranes of resting and phagocytosing human granulocytes. J Clin Invest. 1990 Mar;85(3):821–835. doi: 10.1172/JCI114509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagaya K., Watanabe K., Fukazawa Y. Capacity of recombinant gamma interferon to activate macrophages for Salmonella-killing activity. Infect Immun. 1989 Feb;57(2):609–615. doi: 10.1128/iai.57.2.609-615.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampschmidt R. F., Pulliam L. A. Stimulation of antimicrobial activity in the rat with leukocytic endogenous mediator. J Reticuloendothel Soc. 1975 Mar;17(3):162–169. [PubMed] [Google Scholar]
- Kelley K. W., Brief S., Westly H. J., Novakofski J., Bechtel P. J., Simon J., Walker E. B. GH3 pituitary adenoma cells can reverse thymic aging in rats. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5663–5667. doi: 10.1073/pnas.83.15.5663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley K. W. Growth hormone, lymphocytes and macrophages. Biochem Pharmacol. 1989 Mar 1;38(5):705–713. doi: 10.1016/0006-2952(89)90222-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Langermans J. A., van der Hulst M. E., Nibbering P. H., van Furth R. Activation of mouse peritoneal macrophages during infection with Salmonella typhimurium does not result in enhanced intracellular killing. J Immunol. 1990 Jun 1;144(11):4340–4346. [PubMed] [Google Scholar]
- Lepay D. A., Steinman R. M., Nathan C. F., Murray H. W., Cohn Z. A. Liver macrophages in murine listeriosis. Cell-mediated immunity is correlated with an influx of macrophages capable of generating reactive oxygen intermediates. J Exp Med. 1985 Jun 1;161(6):1503–1512. doi: 10.1084/jem.161.6.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura H., Onozuka K., Terada Y., Nakano Y., Nakano M. Effect of murine recombinant interferon-gamma in the protection of mice against Salmonella. Int J Immunopharmacol. 1990;12(1):49–56. doi: 10.1016/0192-0561(90)90067-w. [DOI] [PubMed] [Google Scholar]
- Murray H. W., Cohn Z. A. Macrophage oxygen-dependent antimicrobial activity. I. Susceptibility of Toxoplasma gondii to oxygen intermediates. J Exp Med. 1979 Oct 1;150(4):938–949. doi: 10.1084/jem.150.4.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Cohn Z. A. Macrophage oxygen-dependent antimicrobial activity. III. Enhanced oxidative metabolism as an expression of macrophage activation. J Exp Med. 1980 Dec 1;152(6):1596–1609. doi: 10.1084/jem.152.6.1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W. Interaction of Leishmania with a macrophage cell line. Correlation between intracellular killing and the generation of oxygen intermediates. J Exp Med. 1981 Jun 1;153(6):1690–1695. doi: 10.1084/jem.153.6.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W. Interferon-gamma, the activated macrophage, and host defense against microbial challenge. Ann Intern Med. 1988 Apr;108(4):595–608. doi: 10.7326/0003-4819-108-4-595. [DOI] [PubMed] [Google Scholar]
- Murray H. W., Juangbhanich C. W., Nathan C. F., Cohn Z. A. Macrophage oxygen-dependent antimicrobial activity. II. The role of oxygen intermediates. J Exp Med. 1979 Oct 1;150(4):950–964. doi: 10.1084/jem.150.4.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Nathan C. F., Cohn Z. A. Macrophage oxygen-dependent antimicrobial activity. IV. Role of endogenous scavengers of oxygen intermediates. J Exp Med. 1980 Dec 1;152(6):1610–1624. doi: 10.1084/jem.152.6.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Tsunawaki S. Secretion of toxic oxygen products by macrophages: regulatory cytokines and their effects on the oxidase. Ciba Found Symp. 1986;118:211–230. doi: 10.1002/9780470720998.ch14. [DOI] [PubMed] [Google Scholar]
- Nathan C., Nogueira N., Juangbhanich C., Ellis J., Cohn Z. Activation of macrophages in vivo and in vitro. Correlation between hydrogen peroxide release and killing of Trypanosoma cruzi. J Exp Med. 1979 May 1;149(5):1056–1068. doi: 10.1084/jem.149.5.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newburger P. E., Ezekowitz R. A., Whitney C., Wright J., Orkin S. H. Induction of phagocyte cytochrome b heavy chain gene expression by interferon gamma. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5215–5219. doi: 10.1073/pnas.85.14.5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niall H. D., Hogan M. L., Tregear G. W., Segre G. V., Hwang P., Friesen H. The chemistry of growth hormone and the lactogenic hormones. Recent Prog Horm Res. 1973;29:387–416. doi: 10.1016/b978-0-12-571129-6.50014-2. [DOI] [PubMed] [Google Scholar]
- Novotny M. J., Laughlin M. H., Adams H. R. Evidence for lack of importance of oxygen free radicals in Escherichia coli endotoxemia in dogs. Am J Physiol. 1988 May;254(5 Pt 2):H954–H962. doi: 10.1152/ajpheart.1988.254.5.H954. [DOI] [PubMed] [Google Scholar]
- Parlow A. F., Wilhelmi A. E., Reichert L. E., Jr Further studies on the fractionation of human pituitary glands. Endocrinology. 1965 Dec;77(6):1126–1134. doi: 10.1210/endo-77-6-1126. [DOI] [PubMed] [Google Scholar]
- Pick E., Keisari Y. A simple colorimetric method for the measurement of hydrogen peroxide produced by cells in culture. J Immunol Methods. 1980;38(1-2):161–170. doi: 10.1016/0022-1759(80)90340-3. [DOI] [PubMed] [Google Scholar]
- Pick E. Microassays for superoxide and hydrogen peroxide production and nitroblue tetrazolium reduction using an enzyme immunoassay microplate reader. Methods Enzymol. 1986;132:407–421. doi: 10.1016/s0076-6879(86)32026-3. [DOI] [PubMed] [Google Scholar]
- Pick E., Mizel D. Rapid microassays for the measurement of superoxide and hydrogen peroxide production by macrophages in culture using an automatic enzyme immunoassay reader. J Immunol Methods. 1981;46(2):211–226. doi: 10.1016/0022-1759(81)90138-1. [DOI] [PubMed] [Google Scholar]
- Scarborough D. E. Cytokine modulation of pituitary hormone secretion. Ann N Y Acad Sci. 1990;594:169–187. doi: 10.1111/j.1749-6632.1990.tb40477.x. [DOI] [PubMed] [Google Scholar]
- Scott P., James S., Sher A. The respiratory burst is not required for killing of intracellular and extracellular parasites by a lymphokine-activated macrophage cell line. Eur J Immunol. 1985 Jun;15(6):553–558. doi: 10.1002/eji.1830150605. [DOI] [PubMed] [Google Scholar]
- Vandenbosch J. L., Rabert D. K., Kurlandsky D. R., Jones G. W. Sequence analysis of rsk, a portion of the 95-kilobase plasmid of Salmonella typhimurium associated with resistance to the bactericidal activity of serum. Infect Immun. 1989 Mar;57(3):850–857. doi: 10.1128/iai.57.3.850-857.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein D. L., Carsiotis M., Lissner C. R., O'Brien A. D. Flagella help Salmonella typhimurium survive within murine macrophages. Infect Immun. 1984 Dec;46(3):819–825. doi: 10.1128/iai.46.3.819-825.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedermann C. J., Niedermühlbichler M., Beimpold H., Braunsteiner H. In vitro activation of neutrophils of the aged by recombinant human growth hormone. J Infect Dis. 1991 Nov;164(5):1017–1020. doi: 10.1093/infdis/164.5.1017. [DOI] [PubMed] [Google Scholar]
- Wiedermann C. J., Niedermühlbichler M., Geissler D., Beimpold H., Braunsteiner H. Priming of normal human neutrophils by recombinant human growth hormone. Br J Haematol. 1991 May;78(1):19–22. doi: 10.1111/j.1365-2141.1991.tb04376.x. [DOI] [PubMed] [Google Scholar]
- Zanetti M., Schmitt M., Lazary S. Bovine leukocyte phagocytosis and bacteria killing monitored by intracellular acridine orange fluorescence and extracellular fluorescence quenching. Vet Immunol Immunopathol. 1987 Nov;16(3-4):185–199. doi: 10.1016/0165-2427(87)90017-1. [DOI] [PubMed] [Google Scholar]
- van Dissel J. T., Stikkelbroeck J. J., Michel B. C., van den Barselaar M. T., Leijh P. C., van Furth R. Inability of recombinant interferon-gamma to activate the antibacterial activity of mouse peritoneal macrophages against Listeria monocytogenes and Salmonella typhimurium. J Immunol. 1987 Sep 1;139(5):1673–1678. [PubMed] [Google Scholar]
- van Dissel J. T., Stikkelbroeck J. J., van den Barselaar M. T., Sluiter W., Leijh P. C., van Furth R. Divergent changes in antimicrobial activity after immunologic activation of mouse peritoneal macrophages. J Immunol. 1987 Sep 1;139(5):1665–1672. [PubMed] [Google Scholar]


