Abstract
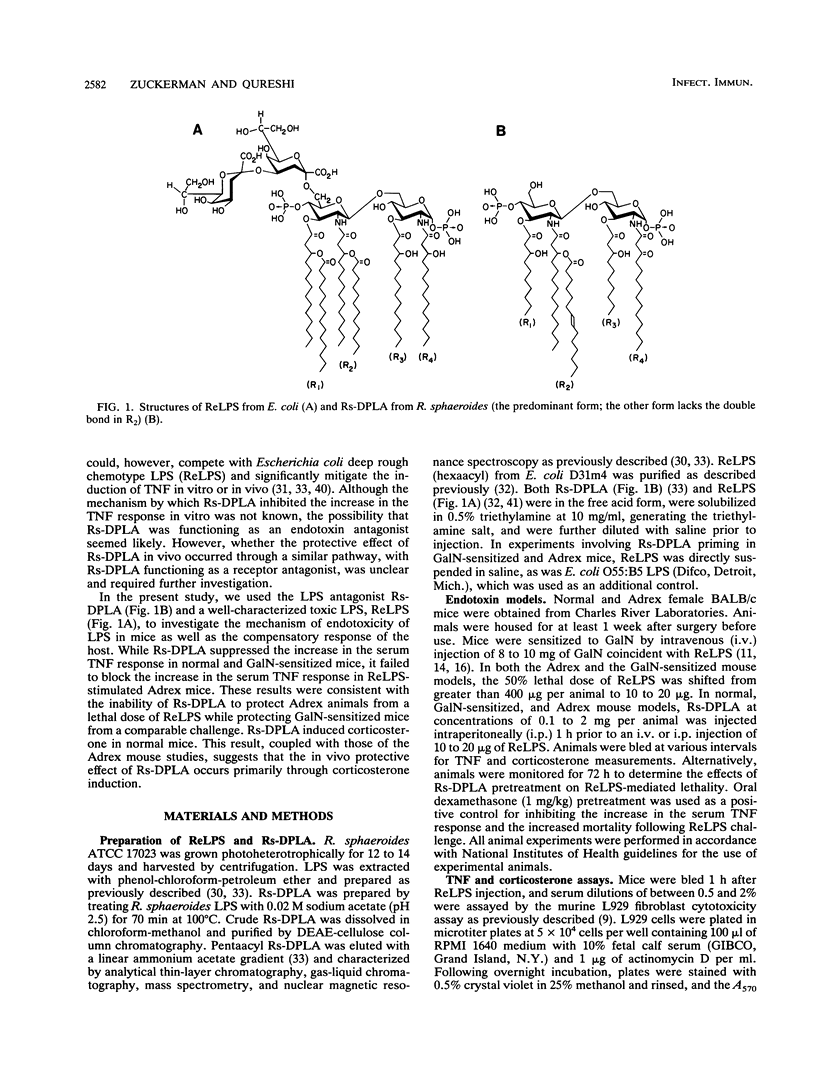
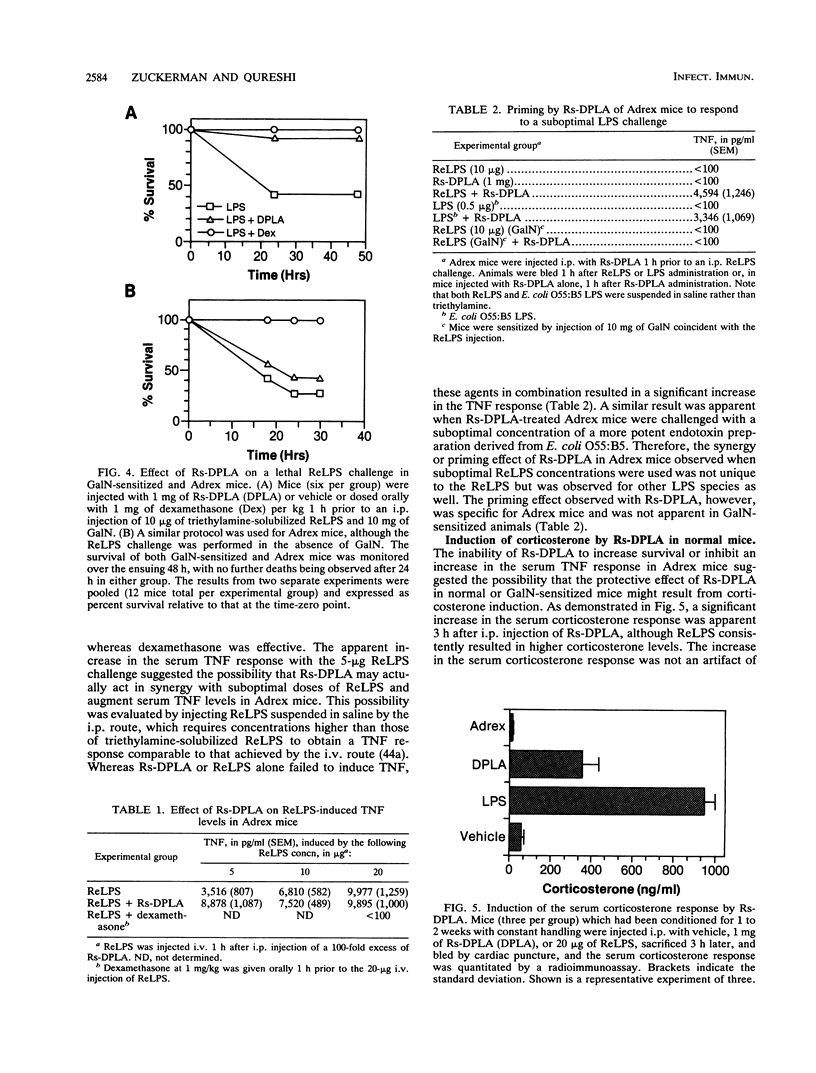
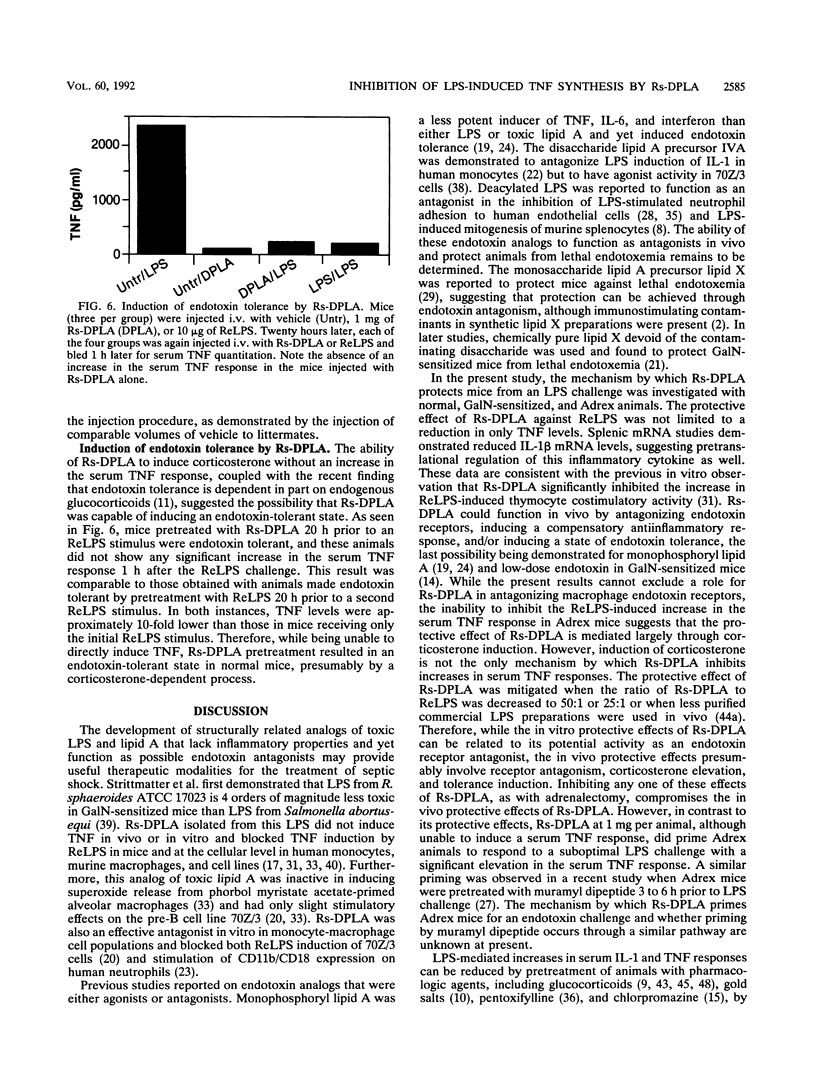
Diphosphoryl lipid A from the lipopolysaccharide (LPS) of Rhodobacter sphaeroides (Rs-DPLA) has been demonstrated to block in mice and guinea pigs the increase in the serum tumor necrosis factor (TNF) response induced by highly purified deep rough chemotype LPS from Escherichia coli D31m4 (ReLPS). The present study was designed to determine the role of corticosterone induction by Rs-DPLA and its effect on TNF regulation and survival in lethal endotoxin shock models and to evaluate the ability of Rs-DPLA to induce endotoxin tolerance. Administration of a 100-fold excess of Rs-DPLA 1 h prior to ReLPS administration inhibited the characteristic peak in serum TNF levels induced by LPS. Inhibition was apparent in normal and D-galactosamine (GalN)-sensitized mice and occurred at the pretranslational level, as splenic TNF and interleukin-1 beta mRNAs were present in lower amounts in LPS-stimulated mice pretreated with Rs-DPLA. Consistent with its effects in reducing serum TNF levels, Rs-DPLA pretreatment protected GalN-sensitized mice from a lethal ReLPS challenge. In contrast, Rs-DPLA did not inhibit the increase in the serum TNF response or protect against a lethal ReLPS challenge in parallel experiments with adrenalectomized (Adrex) mice, for which the 50% lethal dose of ReLPS was comparable to that for GalN-sensitized mice. Furthermore, Rs-DPLA appeared to prime Adrex animals and increase the magnitude of the serum TNF response to a suboptimal LPS stimulus. Priming by Rs-DPLA, however, was not observed in normal or GalN-sensitized mice. Although Rs-DPLA by itself was nontoxic and unable to elevate serum TNF levels in any of the models investigated, it did induce a significant increase in the serum corticosterone response and was capable of inducing endotoxin tolerance in normal mice. The inability of Rs-DPLA to protect Adrex mice from a lethal ReLPS stimulus or to inhibit the increase in the serum TNF response suggests that the protective effect of Rs-DPLA in normal or GalN-sensitized animals occurs through corticosterone induction. These results support the concept that endogenous glucocorticoids can modulate the endotoxic effects of LPS by inhibiting the synthesis of inflammatory cytokines.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aderka D., Le J. M., Vilcek J. IL-6 inhibits lipopolysaccharide-induced tumor necrosis factor production in cultured human monocytes, U937 cells, and in mice. J Immunol. 1989 Dec 1;143(11):3517–3523. [PubMed] [Google Scholar]
- Aschauer H., Grob A., Hildebrandt J., Schuetze E., Stuetz P. Highly purified lipid X is devoid of immunostimulatory activity. Isolation and characterization of immunostimulating contaminants in a batch of synthetic lipid X. J Biol Chem. 1990 Jun 5;265(16):9159–9164. [PubMed] [Google Scholar]
- Bertini R., Bianchi M., Ghezzi P. Adrenalectomy sensitizes mice to the lethal effects of interleukin 1 and tumor necrosis factor. J Exp Med. 1988 May 1;167(5):1708–1712. doi: 10.1084/jem.167.5.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler B., Milsark I. W., Cerami A. C. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985 Aug 30;229(4716):869–871. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- Decker K., Keppler D. Galactosamine hepatitis: key role of the nucleotide deficiency period in the pathogenesis of cell injury and cell death. Rev Physiol Biochem Pharmacol. 1974;(71):77–106. doi: 10.1007/BFb0027661. [DOI] [PubMed] [Google Scholar]
- Derijk R., Van Rooijen N., Tilders F. J., Besedovsky H. O., Del Rey A., Berkenbosch F. Selective depletion of macrophages prevents pituitary-adrenal activation in response to subpyrogenic, but not to pyrogenic, doses of bacterial endotoxin in rats. Endocrinology. 1991 Jul;129(1):330–338. doi: 10.1210/endo-129-1-330. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A. The proinflammatory cytokines interleukin-1 and tumor necrosis factor and treatment of the septic shock syndrome. J Infect Dis. 1991 Jun;163(6):1177–1184. doi: 10.1093/infdis/163.6.1177. [DOI] [PubMed] [Google Scholar]
- Erwin A. L., Mandrell R. E., Munford R. S. Enzymatically deacylated Neisseria lipopolysaccharide (LPS) inhibits murine splenocyte mitogenesis induced by LPS. Infect Immun. 1991 Jun;59(6):1881–1887. doi: 10.1128/iai.59.6.1881-1887.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans G. F., Snyder Y. M., Butler L. D., Zuckerman S. H. Differential expression of interleukin-1 and tumor necrosis factor in murine septic shock models. Circ Shock. 1989 Dec;29(4):279–290. [PubMed] [Google Scholar]
- Evans G. F., Zuckerman S. H. Glucocorticoid-dependent and -independent mechanisms involved in lipopolysaccharide tolerance. Eur J Immunol. 1991 Sep;21(9):1973–1979. doi: 10.1002/eji.1830210902. [DOI] [PubMed] [Google Scholar]
- Evans G. F., Zuckerman S. H. Pharmacologic modulation of TNF production by endotoxin stimulated macrophages: in vitro and in vivo effects of auranofin and other chrysotherapeutic compounds. Agents Actions. 1989 Mar;26(3-4):329–334. doi: 10.1007/BF01967297. [DOI] [PubMed] [Google Scholar]
- Flohé S., Heinrich P. C., Schneider J., Wendel A., Flohé L. Time course of IL-6 and TNF alpha release during endotoxin-induced endotoxin tolerance in rats. Biochem Pharmacol. 1991 Jun 1;41(11):1607–1614. doi: 10.1016/0006-2952(91)90161-w. [DOI] [PubMed] [Google Scholar]
- Fong Y., Tracey K. J., Moldawer L. L., Hesse D. G., Manogue K. B., Kenney J. S., Lee A. T., Kuo G. C., Allison A. C., Lowry S. F. Antibodies to cachectin/tumor necrosis factor reduce interleukin 1 beta and interleukin 6 appearance during lethal bacteremia. J Exp Med. 1989 Nov 1;170(5):1627–1633. doi: 10.1084/jem.170.5.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudenberg M. A., Galanos C. Induction of tolerance to lipopolysaccharide (LPS)-D-galactosamine lethality by pretreatment with LPS is mediated by macrophages. Infect Immun. 1988 May;56(5):1352–1357. doi: 10.1128/iai.56.5.1352-1357.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadina M., Bertini R., Mengozzi M., Zandalasini M., Mantovani A., Ghezzi P. Protective effect of chlorpromazine on endotoxin toxicity and TNF production in glucocorticoid-sensitive and glucocorticoid-resistant models of endotoxic shock. J Exp Med. 1991 Jun 1;173(6):1305–1310. doi: 10.1084/jem.173.6.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanos C., Freudenberg M. A., Reutter W. Galactosamine-induced sensitization to the lethal effects of endotoxin. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5939–5943. doi: 10.1073/pnas.76.11.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golenbock D. T., Hampton R. Y., Qureshi N., Takayama K., Raetz C. R. Lipid A-like molecules that antagonize the effects of endotoxins on human monocytes. J Biol Chem. 1991 Oct 15;266(29):19490–19498. [PubMed] [Google Scholar]
- Haslberger A., Sayers T., Reiter H., Chung J., Schütze E. Reduced release of TNF and PCA from macrophages of tolerant mice. Circ Shock. 1988 Oct;26(2):185–192. [PubMed] [Google Scholar]
- Henricson B. E., Benjamin W. R., Vogel S. N. Differential cytokine induction by doses of lipopolysaccharide and monophosphoryl lipid A that result in equivalent early endotoxin tolerance. Infect Immun. 1990 Aug;58(8):2429–2437. doi: 10.1128/iai.58.8.2429-2437.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland T. N., Qureshi N., Takayama K. Diphosphoryl lipid A derived from lipopolysaccharide (LPS) of Rhodopseudomonas sphaeroides inhibits activation of 70Z/3 cells by LPS. Infect Immun. 1991 Jan;59(1):131–136. doi: 10.1128/iai.59.1.131-136.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam C., Hildebrandt J., Schütze E., Rosenwirth B., Proctor R. A., Liehl E., Stütz P. Immunostimulatory, but not antiendotoxin, activity of lipid X is due to small amounts of contaminating N,O-acylated disaccharide-1-phosphate: in vitro and in vivo reevaluation of the biological activity of synthetic lipid X. Infect Immun. 1991 Jul;59(7):2351–2358. doi: 10.1128/iai.59.7.2351-2358.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loppnow H., Brade H., Dürrbaum I., Dinarello C. A., Kusumoto S., Rietschel E. T., Flad H. D. IL-1 induction-capacity of defined lipopolysaccharide partial structures. J Immunol. 1989 May 1;142(9):3229–3238. [PubMed] [Google Scholar]
- Lynn W. A., Raetz C. R., Qureshi N., Golenbock D. T. Lipopolysaccharide-induced stimulation of CD11b/CD18 expression on neutrophils. Evidence of specific receptor-based response and inhibition by lipid A-based antagonists. J Immunol. 1991 Nov 1;147(9):3072–3079. [PubMed] [Google Scholar]
- Madonna G. S., Peterson J. E., Ribi E. E., Vogel S. N. Early-phase endotoxin tolerance: induction by a detoxified lipid A derivative, monophosphoryl lipid A. Infect Immun. 1986 Apr;52(1):6–11. doi: 10.1128/iai.52.1.6-11.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengozzi M., Sironi M., Gadina M., Ghezzi P. Reversal of defective IL-6 production in lipopolysaccharide-tolerant mice by phorbol myristate acetate. J Immunol. 1991 Aug 1;147(3):899–902. [PubMed] [Google Scholar]
- Ohlsson K., Björk P., Bergenfeldt M., Hageman R., Thompson R. C. Interleukin-1 receptor antagonist reduces mortality from endotoxin shock. Nature. 1990 Dec 6;348(6301):550–552. doi: 10.1038/348550a0. [DOI] [PubMed] [Google Scholar]
- Parant M., Le Contel C., Parant F., Chedid L. Influence of endogenous glucocorticoid on endotoxin-induced production of circulating TNF-alpha. Lymphokine Cytokine Res. 1991 Aug;10(4):265–271. [PubMed] [Google Scholar]
- Pohlman T. H., Munford R. S., Harlan J. M. Deacylated lipopolysaccharide inhibits neutrophil adherence to endothelium induced by lipopolysaccharide in vitro. J Exp Med. 1987 May 1;165(5):1393–1402. doi: 10.1084/jem.165.5.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor R. A., Will J. A., Burhop K. E., Raetz C. R. Protection of mice against lethal endotoxemia by a lipid A precursor. Infect Immun. 1986 Jun;52(3):905–907. doi: 10.1128/iai.52.3.905-907.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi N., Honovich J. P., Hara H., Cotter R. J., Takayama K. Location of fatty acids in lipid A obtained from lipopolysaccharide of Rhodopseudomonas sphaeroides ATCC 17023. J Biol Chem. 1988 Apr 25;263(12):5502–5504. [PubMed] [Google Scholar]
- Qureshi N., Takayama K., Kurtz R. Diphosphoryl lipid A obtained from the nontoxic lipopolysaccharide of Rhodopseudomonas sphaeroides is an endotoxin antagonist in mice. Infect Immun. 1991 Jan;59(1):441–444. doi: 10.1128/iai.59.1.441-444.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi N., Takayama K., Mascagni P., Honovich J., Wong R., Cotter R. J. Complete structural determination of lipopolysaccharide obtained from deep rough mutant of Escherichia coli. Purification by high performance liquid chromatography and direct analysis by plasma desorption mass spectrometry. J Biol Chem. 1988 Aug 25;263(24):11971–11976. [PubMed] [Google Scholar]
- Qureshi N., Takayama K., Meyer K. C., Kirkland T. N., Bush C. A., Chen L., Wang R., Cotter R. J. Chemical reduction of 3-oxo and unsaturated groups in fatty acids of diphosphoryl lipid A from the lipopolysaccharide of Rhodopseudomonas sphaeroides. Comparison of biological properties before and after reduction. J Biol Chem. 1991 Apr 5;266(10):6532–6538. [PubMed] [Google Scholar]
- Qureshi N., Takayama K., Ribi E. Purification and structural determination of nontoxic lipid A obtained from the lipopolysaccharide of Salmonella typhimurium. J Biol Chem. 1982 Oct 10;257(19):11808–11815. [PubMed] [Google Scholar]
- Riedo F. X., Munford R. S., Campbell W. B., Reisch J. S., Chien K. R., Gerard R. D. Deacylated lipopolysaccharide inhibits plasminogen activator inhibitor-1, prostacyclin, and prostaglandin E2 induction by lipopolysaccharide but not by tumor necrosis factor-alpha. J Immunol. 1990 May 1;144(9):3506–3512. [PubMed] [Google Scholar]
- Schade U. F. Pentoxifylline increases survival in murine endotoxin shock and decreases formation of tumor necrosis factor. Circ Shock. 1990 Jun;31(2):171–181. [PubMed] [Google Scholar]
- Sheehan K. C., Ruddle N. H., Schreiber R. D. Generation and characterization of hamster monoclonal antibodies that neutralize murine tumor necrosis factors. J Immunol. 1989 Jun 1;142(11):3884–3893. [PubMed] [Google Scholar]
- Sibley C. H., Terry A., Raetz C. R. Induction of kappa light chain synthesis in 70Z/3 B lymphoma cells by chemically defined lipid A precursors. J Biol Chem. 1988 Apr 15;263(11):5098–5103. [PubMed] [Google Scholar]
- Strittmatter W., Weckesser J., Salimath P. V., Galanos C. Nontoxic lipopolysaccharide from Rhodopseudomonas sphaeroides ATCC 17023. J Bacteriol. 1983 Jul;155(1):153–158. doi: 10.1128/jb.155.1.153-158.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama K., Qureshi N., Beutler B., Kirkland T. N. Diphosphoryl lipid A from Rhodopseudomonas sphaeroides ATCC 17023 blocks induction of cachectin in macrophages by lipopolysaccharide. Infect Immun. 1989 Apr;57(4):1336–1338. doi: 10.1128/iai.57.4.1336-1338.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama K., Qureshi N., Mascagni P. Complete structure of lipid A obtained from the lipopolysaccharides of the heptoseless mutant of Salmonella typhimurium. J Biol Chem. 1983 Nov 10;258(21):12801–12803. [PubMed] [Google Scholar]
- Tracey K. J., Fong Y., Hesse D. G., Manogue K. R., Lee A. T., Kuo G. C., Lowry S. F., Cerami A. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987 Dec 17;330(6149):662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- Waage A. Production and clearance of tumor necrosis factor in rats exposed to endotoxin and dexamethasone. Clin Immunol Immunopathol. 1987 Dec;45(3):348–355. doi: 10.1016/0090-1229(87)90087-0. [DOI] [PubMed] [Google Scholar]
- Wichterman K. A., Baue A. E., Chaudry I. H. Sepsis and septic shock--a review of laboratory models and a proposal. J Surg Res. 1980 Aug;29(2):189–201. doi: 10.1016/0022-4804(80)90037-2. [DOI] [PubMed] [Google Scholar]
- Zuckerman S. H., Bendele A. M. Regulation of serum tumor necrosis factor in glucocorticoid-sensitive and -resistant rodent endotoxin shock models. Infect Immun. 1989 Oct;57(10):3009–3013. doi: 10.1128/iai.57.10.3009-3013.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman S. H., Evans G. F., Butler L. D. Endotoxin tolerance: independent regulation of interleukin-1 and tumor necrosis factor expression. Infect Immun. 1991 Aug;59(8):2774–2780. doi: 10.1128/iai.59.8.2774-2780.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman S. H., Evans G. F., Snyder Y. M., Roeder W. D. Endotoxin-macrophage interaction: post-translational regulation of tumor necrosis factor expression. J Immunol. 1989 Aug 15;143(4):1223–1227. [PubMed] [Google Scholar]
- Zuckerman S. H., Shellhaas J., Butler L. D. Differential regulation of lipopolysaccharide-induced interleukin 1 and tumor necrosis factor synthesis: effects of endogenous and exogenous glucocorticoids and the role of the pituitary-adrenal axis. Eur J Immunol. 1989 Feb;19(2):301–305. doi: 10.1002/eji.1830190213. [DOI] [PubMed] [Google Scholar]


