Abstract
Among diverse factors regulating excitatory synaptic transmission, the abundance of postsynaptic glutamate receptors figures prominently in molecular memory and learning-related synaptic plasticity. To allow for both long-term maintenance of synaptic transmission and acute changes in synaptic strength, the relative rates of glutamate receptor insertion and removal must be tightly regulated. Interactions with scaffolding proteins control the targeting and signaling properties of glutamate receptors within the postsynaptic membrane. In addition, extrasynaptic receptor populations control the equilibrium of receptor exchange at synapses and activate distinct signaling pathways involved in plasticity. Here, we review recent findings that have shaped our current understanding of receptor mobility between synaptic and extrasynaptic compartments at glutamatergic synapses, focusing on AMPA and NMDA receptors. We also examine the cooperative relationship between intracellular trafficking and surface diffusion of glutamate receptors that underlies the expression of learning-related synaptic plasticity.
Introduction
Information storage and plasticity require that synapses carry out two opposing tasks: maintaining stable long-term synaptic connections while at the same time remaining plastic and allowing for rapid changes in synaptic strength. A major challenge has been to understand how the large array of synaptic proteins that govern these opposing processes are regulated to selectively establish, maintain, and modify the strength of synapses. Increasing evidence has shown that synaptic complexes once viewed as static are, in fact, highly dynamic structures that rapidly exchange receptors and scaffold proteins. At excitatory synapses in the mammalian brain, much work has focused on the proteins that regulate the stability and signaling properties of synapses on dendritic spines. These femtoliter-sized protrusions receive excitatory glutamatergic input from directly apposing presynaptic terminals (Bourne and Harris, 2008). Occupying the small portion of the spine membrane directly opposite the contacting presynaptic terminal is the postsynaptic density (PSD), an electron-dense protein network containing glutamate receptors and various membrane proteins anchored to cytoskeletal scaffolding molecules (Okabe, 2007; Sheng and Hoogenraad, 2007). Within the PSD, most rapid excitatory synaptic transmission occurs through two types of glutamate receptors, the N-methyl-D-aspartate (NMDA)-type receptor and the α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA)-type receptor. Homo- or heterotetrameric AMPA receptors are assembled from combinations of GluR1-4 subunits, while NMDA receptors are heterotetramers composed of two NR1 subunits, two NR2 subunits selected from among four gene products (NR2A-D), and occasionally an NR3 subunit (NR3A-B) (Kohr, 2006; Greger et al., 2007). The differential expression and trafficking of these glutamate receptor subunits directly affects their targeting to and retention within synaptic compartments and, thus, the magnitude of synaptic transmission (Kennedy and Ehlers, 2006; Derkach et al., 2007; Lau and Zukin, 2007; Shepherd and Huganir, 2007). The equally important topic of receptor trafficking at inhibitory synapses has been recently reviewed (Kneussel and Loebrich, 2007; Michels and Moss, 2007) and will not be discussed in detail.
Emanating from classic studies on the vertebrate neuromuscular junction (NMJ), the concept of the stable synapse was inferred from imaging studies of the nicotinic acetylcholine receptor (nAChR), which established that the half-life of nAChRs residing in the NMJ was many days (Berg and Hall, 1975) and that the total number of nAChRs per NMJ remains relatively constant throughout the lifetime of an animal (Pestronk et al., 1980). Furthermore, the concentration of junctional nAChRs was found to greatly exceed that of extrajunctional receptors (Fertuck and Salpeter, 1976), indicating that nAChRs are trapped and stabilized within synapses. Similar findings of apparently stable synapses were also made in the central nervous system, where glycine receptors are enriched within synapses (Triller et al., 1985; Seitanidou et al., 1988). The relatively high concentration of neurotransmitter receptors within the postsynaptic specialization and the interactions between these receptors and intracellular scaffold proteins both led to the predominant view that synaptic receptors are tightly fixed within the synapse. However, evidence for dynamic receptor populations and receptor exchange at synapses has long existed. Early studies of nAChR diffusion in the developing NMJ showed that nAChRs fluorescently labeled with α-bungarotoxin undergo spontaneous redistribution and could become integrated into newly assembled NMJs (Anderson and Cohen, 1977), providing clear evidence that extrajunctional receptors can aggregate into nascent NMJs on the plasma membrane. Building on these findings was the observation that functional nAChRs replace locally inactivated receptors through lateral diffusion (Young and Poo, 1983). The recovery of acetylcholine responses at the sites of local inactivation did not occur when receptor diffusion was perturbed by cross-linking surface receptors. In addition, fluorescently labeled surface nAChRs in developing muscle fibers can exist as either freely moving pools diffusely distributed in the plasma membrane or as relatively immobile pools contained within concentrated patches (Axelrod et al., 1978). Importantly, the relatively immobile nAChRs contained in patches could be dispersed by electrical stimulation (Axelrod et al., 1978), showing that receptor aggregation is directly affected by synaptic activity. Subsequently, time-lapse imaging in vivo found that nAChRs undergo rapid and reversible activity-dependent exchange through perijunctional domains (Akaaboune et al., 1999, 2002). Taken together, these important studies demonstrated that nAChRs exchange between distinct membrane microdomains through a process involving lateral diffusion in the plasma membrane.
Later work at central excitatory synapses would show that glutamate receptors are present in multiple locations throughout the cell, suggesting that glutamate receptors may also undergo lateral diffusion and exchange between membrane compartments. A powerful combination of imaging and electrophysiological studies identified functional glutamate receptor populations localized within synapses and in extrasynaptic domains. For example, immunohistochemical studies demonstrated synaptic and extrasynaptic glutamate receptor distribution throughout the dendritic arborization (Rogers et al., 1991; Blackstone et al., 1992; Petralia and Wenthold, 1992; Martin et al., 1993a, 1993b; Molnar et al., 1993, 1994; Aoki et al., 1994; Baude et al., 1994; Siegel et al., 1994; Kharazia et al., 1996a, 1996b; Perez-Otano et al., 2006). In addition, application of glutamate receptor agonists to membrane patches isolated from both dendritic and somatic regions of hippocampal neurons revealed AMPA receptor-mediated currents, implying that functional glutamate receptors exist outside of synapses (Jonas and Sakmann, 1992; Spruston et al., 1995; Andrasfalvy and Magee, 2001). Later work using subcellular immunogold labeling of NMDA and AMPA receptor subunits provided high-resolution electron microscopy (EM) visualization of receptors at extrasynaptic sites on spines, dendrites, somata, and within intracellular compartments (Baude et al., 1995; Kharazia et al., 1996a; He et al., 1998; Nusser et al., 1998; Petralia and Wenthold, 1999; Takumi et al., 1999b). In addition, EM studies revealed that synaptic glutamate receptors occupy distinct regions within the postsynaptic membrane (Baude et al., 1995; Kharazia et al., 1996a; Nusser et al., 1998; Takumi et al., 1999a; Racca et al., 2000; He et al., 2001; Perez-Otano et al., 2006; Masugi-Tokita et al., 2007). Taken together, these EM studies clearly demonstrated the existence of multiple receptor populations occupying diverse regions of the plasma membrane and intracellular membrane compartments. However, whether these glutamate receptor populations represented static pools or were able to exchange between synaptic, extrasynaptic, and intracellular compartments would not be entirely appreciated until the development of highly sensitive optical and biochemical techniques for tracking receptors.
Techniques to Track Glutamate Receptor Mobility
The total pool of extrasynaptic glutamate receptors available to enter into synapses is ultimately established by the relative rates of synthesis, degradation, endocytosis, and exocytosis. Before discussing the cooperative relationship between intracellular trafficking and surface diffusion of glutamate receptors (see below), we first describe the methods used to track receptor exchange and lateral mobility at the postsynaptic membrane and the factors that influence surface mobility of glutamate receptors.
As a result of thermal agitation, all receptors are naturally mobile within a lipid membrane and undergo random Brownian motion. However, in a typical cell membrane, receptor mobility is strongly influenced by physical obstacles and reversible biochemical interactions (Kusumi et al., 2005). To better understand how these barriers affect receptor diffusion, a variety of methods have been developed to optically track glutamate receptor movement, yielding important insight into the physical interactions and local environment of a receptor (Triller and Choquet, 2005; Groc et al., 2007b). One method involves tagging heterologously expressed receptors with variants of green fluorescent protein (GFP) and measuring fluorescent recovery after photobleaching (FRAP). By measuring FRAP, it is possible to quantify the proportion of receptors that are exchangeable in a given area, based on the extent of fluorescence recovery, which in turn provides important information on the bulk dynamics of a receptor population. Furthermore, this technique makes it possible to calculate the apparent diffusion coefficient of the mobile and exchangeable receptor population (Reits and Neefjes, 2001; Chen et al., 2006), although this is necessarily an averaged value that can be highly sensitive to the availability of exchangeable pools of unbleached molecules, the specific geometry of the bleached area, and the properties of the interface with neighboring structures. Importantly, a slow rate of fluorescence recovery of a labeled receptor can indicate the presence of many different physical barriers, such as reversible chemical interactions, temporary cytoskeletal corrals and pickets, or restricted membrane geometry (Choquet and Triller, 2003). It is also possible to specifically track the surface population of glutamate receptors using the pH-sensitive GFP variant superecliptic pHluorin (SEP), which fluoresces at the neutral pH of the extracellular space but is quenched within acidic internal compartments (Ashby et al., 2004, 2006; Kopec et al., 2006; Park et al., 2006; Yudowski et al., 2006; Yudowski et al., 2007; Heine et al., 2008). SEP-tagged receptors have also been effectively used to measure relative rates of endocytosis and exocytosis (Ashby et al., 2004; Bogdanov et al., 2006; Kopec et al., 2006; Park et al., 2006; Yudowski et al., 2006, 2007).
One drawback of measuring diffusion with tagged receptors is the requirement for expression of exogenous receptors, typically at levels where the stoichiometry, protein interactions, or intracellular trafficking may not accurately reflect endogenous receptors. For example, increasing the pool of extrasynaptic receptors could change the equilibrium of receptor exchange at synapses and influence multiple types of plasticity (Passafaro et al., 2001; Shi et al., 2001; Tovar and Westbrook, 2002; Ashby et al., 2004; Holcman and Triller, 2006; Oh et al., 2006; Ehlers et al., 2007; Yudowski et al., 2007). However, it should be noted that overexpression of AMPA receptor subunits does not increase AMPA excitatory postsynaptic currents (EPSCs) (Shi et al., 2001) or change the proportion of mobile GluR2-containing AMPA receptors (Tardin et al., 2003; Ashby et al., 2006). Finally, it is important to consider that the size of the illumination spot in FRAP experiments is typically larger than the synapse, which prohibits an unequivocal distinction between synaptic or extrasynaptic receptor exchange.
Single-particle tracking (SPT) is a powerful technique to track the movement of individual receptors in real time with high temporal and spatial resolution. Unlike FRAP (or the complementary technique of photoactivation using photoswitchable fluorescent proteins), which measures the bulk exchange of a population of molecules, SPT can be used to measure the diffusion of individual receptors, or at least that of individual probes bound to diffusing receptors. Fluorescently coupled antibodies directed against extracellular receptor epitopes allow the visualization and mapping of receptor trajectories. Diffusion coefficients can be derived by plotting the receptor mean square displacement (MSD) over time, which can also distinguish free versus confined diffusion. In the case of Brownian or free diffusion, the MSD plot over time appears linear, whereas for confined receptor movement the MSD plot will curve to a quasi-maximum (Qian et al., 1991; Kusumi et al., 1993; Saxton, 1993) (Figures 1A and 1B). As receptors move between different membrane microdomains, they can undergo alternating periods of free and confined diffusion. Several different types of physical barriers could confine receptor movements. In the case of receptor “corralling,” rapidly moving receptors will diffuse in a semiconfined space, an effect that does not necessarily lead to changes in the instantaneous diffusion coefficient. However, confined receptors that undergo a local decrease in the diffusion coefficient indicate the presence of either reversible biochemical interactions (e.g., receptor-PSD protein interactions) or nonchemical interactions arising from molecular crowding and collisions with other molecules in or near the membrane. SPT also allows quantification of receptor exchange rates between distinct compartments and, conversely, dwell times, providing important information on the equilibria between receptor populations, which define their statistical thermodynamic distribution within the plasma membrane.
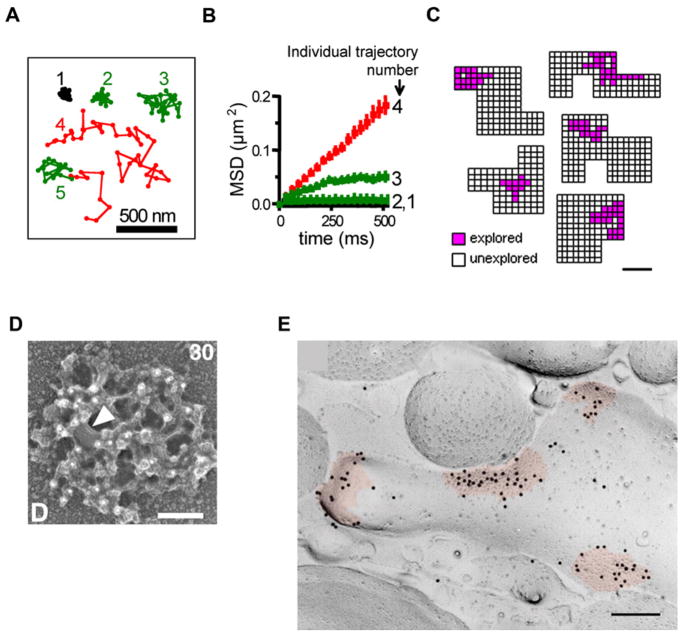
Figure 1. Nanoarchitecture of the PSD and Single-Particle Tracking of AMPA Receptors in Synaptic and Extrasynaptic Compartments.
(A) AMPA receptor trajectories within synaptic and extrasynaptic compartments. As a control, immobilized Cy5-anti-GluR2 was fixed onto a coverslip (1). Examples 2–5 are trajectories from tracking of single Cy5-anti-GluR2 bound to AMPA receptors on living dendrites. The trajectories recorded in synaptic and extrasynaptic regions are shown in green and red, respectively. Examples 2 and 3 remained within synaptic sites, example 4 remained in the extrasynaptic membrane, and example 5 began in the extrasynaptic region and entered into a synaptic site.
(B) Plots of the mean square displacement (MSD) versus time corresponding to the examples shown in (A). Trajectories 2 and 3 remaining in synaptic regions had varying degrees of confinement and were less mobile than trajectory 4. Error bars are equal to the SEM.
(A) and (B) are adapted from Tardin et al. (2003); reprinted with permission from Nature Publishing Group, copyright 2003.
(C) Individual GluR1-QDs are restricted to subdomains within active synapses. Five individual synaptic regions defined as a set of connected pixels are indicated. Individual pixels divided into 0.0016 μm2 subdomains were coded based on the presence (pink) or absence (white) of a GluR1-QD residing in that location at any time during the imaging period as defined by the centroid of a 2D Gaussian function fit to the GluR1-QD fluorescent signal. Scale bar, 200 nm. Adapted from Ehlers et al. (2007); reprinted with permission from Elsevier, copyright 2007.
(D) CaMKII immunogold labeling (white dots) on the cytoplasmic surface of a biochemically isolated PSD. Shown is the cytoplasmic surface of the PSD. A membrane patch is indicated by the arrowhead. Scale bar, 100 nm. Adapted from Petersen et al. (2003); reprinted with permission from the Society for Neuroscience, copyright 2003.
(E) AMPA receptor distribution at synapses (colored in red) in the molecular layer of the cerebellum shown by SDS-digested freeze-fracture replica labeling. Intra-membrane particles are shown on the E face of the PSD and contain dark immunogold particles for pan-AMPA receptors (GluR1-4). Scale bar, 100 nm. Adapted from Masugi-Tokita et al. (2007); reprinted with permission from the Society for Neuroscience, copyright 2007.
Initial SPT experiments in neurons utilized 500 nm antibody-coated latex beads that were directed against subunits of glycine receptors or AMPA receptors (Meier et al., 2001; Borgdorff and Choquet, 2002) and provided a first glimpse of individual glutamate receptor mobility in the extrasynaptic neuronal membrane. An improvement over latex bead tracking was the use of antibodies conjugated to organic dyes (e.g., Cy3, Cy5). The smaller antibody-receptor complex allows for optical tracking of receptor movement within more spatially restricted domains, such as the synaptic cleft. Such experimental paradigms have demonstrated that extrasynaptic AMPA receptors exchange laterally in and out of the PSD and are mobile within the PSD (Tardin et al., 2003). The major limitation to using single organic dyes is their rapid photobleaching, which prevents the acquisition of receptor trajectories over time periods longer than a few seconds.
The introduction of semiconductor quantum dots (QDs) has circumvented many of these limitations (Dahan et al., 2003). QDs are resistant to photobleaching (Michalet et al., 2005; Triller and Choquet, 2005), allowing tracking of labeled receptors for minutes. Importantly, the characteristic “blinking” of individual QDs enables the identification of single molecules. In addition, the high signal-to-noise ratio allows one to fit the fluorescent signal to a two-dimensional Gaussian function to identify the centroid of the object with a pointing accuracy typically between 5 and 50 nm. This pointing accuracy is below the diffraction limited resolution of the light microscope, which makes possible high-precision nanometer-scale spatial tracking of receptors within a compartment. This technique has been used to map areas explored by AMPA receptors within the PSD, demonstrating that individual receptors remain confined to nanometer-scale subdomains of the synapse (Ehlers et al., 2007) (Figure 1C).
There are several limitations to SPT of glutamate receptors. The labeling method itself may influence diffusive properties or restrict access to sterically confined membrane domains. For example, both the size of the antibody-receptor complex and the potential cross-linking of receptors could alter the rate of receptor endocytosis and their signaling properties. In addition, the mobility of attached probes (e.g., antibody-receptor complexes and QDs) could be slowed or impeded by extracellular obstacles and barriers. Such factors are especially important when tracking receptors within restricted synaptic compartments. Indeed, synaptic receptors tracked using organic-dye-conjugated antibodies have faster mobility than QD-conjugated antibodies (Groc et al., 2004). Nonetheless, QDs do enter into and exchange at synapses (Dahan et al., 2003). Moreover, in cultured hippocampal neurons, QDs loaded into synaptic vesicles are released during action potential stimuli and diffuse rapidly out of the synaptic cleft, indicating diffusional access to the synapse (Zhang et al., 2007). Recent advances in reducing the size of QDs and engineering small monovalent affinity ligands coupled to QDs should allow for better access to the synapse (Howarth et al., 2006).
Several techniques have also been devised to track the exchange of functional glutamate receptors at synapses. One method, known as electrophysiological tagging, involves expression of receptor subunits with altered channel properties. For example, in the case of certain AMPA receptor subunits, mutations that “tag” the expressed receptor by altering current rectification can be directly measured. After delivering stimuli that alter synaptic properties, it is then possible to identify newly inserted synaptic AMPA receptors with distinct channel properties relative to the original synaptic receptor population (Hayashi et al., 2000; Zhu et al., 2000; Shi et al., 2001; Barria and Malinow, 2002; Esteban et al., 2003). A second electrophysiological technique to track functional receptor movements utilizes pharmacological inactivation of synaptic receptors with use-dependent blockers (e.g., MK-801 for NMDA receptors), allowing for temporal measurements of endogenous receptor exchange at synapses (Tovar and Westbrook, 2002; Harris and Pettit, 2007; Zhao et al., 2008). Another method for functionally tracking native glutamate receptors has been the direct covalent attachment of a photoreactive antagonist, whose proximity to the ligand-binding site is determined by absorption of a UV photon (Adesnik et al., 2005; Nilsen and England, 2007). Two-photon uncaging of glutamate is yet another powerful electrophysiological technique for mapping glutamate receptor responses. This method has been used to measure the strength of responses along dendrites, which ultimately reveals the location and density of functional glutamate receptors (Pettit et al., 1997; Pettit and Augustine, 2000; Matsuzaki et al., 2001; Smith et al., 2003).
No single technique can reveal the entire complex behavior of a dynamic receptor population; each has caveats. It is only when SPT, FRAP, and electrophysiological techniques are combined and interpreted as a whole that a clear and more complete picture of glutamate receptor dynamics begins to emerge.
Ongoing Glutamate Receptor Exchange at Synapses
Several different techniques have demonstrated that glutamate receptors exchange between synaptic and extrasynaptic compartments. Quantification of the bulk movements of surface SEP-GluR2 AMPA receptors using FRAP revealed that ~50% of surface GluR2 in spines is exchangeable, with a recovery phase that plateaus within 10–15 min (Ashby et al., 2006). This fluorescence recovery of SEP-GluR2 in spines is likely a result of surface lateral diffusion rather than plasma membrane insertion, as incubation of neurons with anti-GFP antibodies, a treatment that alters lateral diffusion of SEP-labeled surface receptors, slowed the fluorescence recovery of SEP-GluR2 in spines (Ashby et al., 2006). A similar fluorescence recovery rate was obtained following photobleaching of spine-localized EYFP-GluR1 (Sharma et al., 2006). In addition, a recent FRAP study in intact hippocampal brain slices found that 30% of spine- and 60% of shaft-localized SEP-GluR2 is exchangeable (Heine et al., 2008), indicating that AMPA receptors are mobile in both dissociated culture and brain slices. Therefore, a major fraction of surface AMPA receptors in spines exist in a mobile population that undergoes exchange within minutes. Such rapid exchange of glutamate receptors at spines could provide the basis for acute changes in synaptic strength. Yet, the finding that approximately half the GluR2 AMPA receptors in spines do not exchange on this timescale indicates that a population of receptors is confined or immobile within synapses.
Both the exchange of mobile glutamate receptors and stabilization within synapses have been demonstrated by SPT experiments. Latex bead tracking of GluR2 revealed a wide range of diffusion coefficients for extrasynaptic AMPA receptors in hippocampal neurons, with a tendency for slower diffusion rates as neurons mature (Borgdorff and Choquet, 2002). Organic-dye-labeled antibodies subsequently allowed for the tracking of GluR2 in both synaptic and extrasynaptic compartments and for visualizing receptor exchange at synapses (Tardin et al., 2003). Similar to latex bead tracking, a wide range of diffusion coefficients exists for extrasynaptic GluR2. Within synaptic regions, two populations were detected, one apparently immobile pool and a second mobile population, consistent with the 50% mobile pool detected by FRAP methods (Ashby et al., 2006; Sharma et al., 2006). The MSD plot of GluR2 trajectories in synaptic and extrasynaptic domains revealed confined movements in synapses compared to primarily free Brownian diffusion in extrasynaptic domains (Figures 1A and 1B). Importantly, extrasynaptic GluR2 AMPA receptors can enter into synaptic regions (Figures 1A and 3A), providing evidence that extrasynaptic receptors can act as a readily available pool to supply synapses.
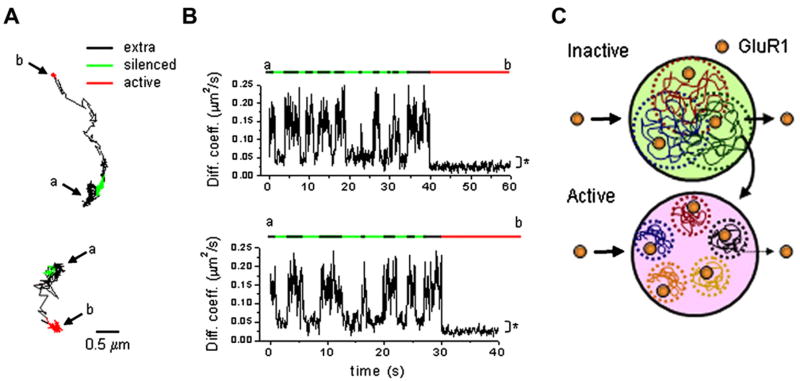
Figure 3. Active Synapses Capture GluR1-QDs by Diffusional Exchange.
(A) Diffusion of GluR1 from silenced to active synapses. Trajectories of GluR1-QDs originating in a silenced synapse (green), escaping from the synapse (black, extrasynaptic), and transiting to an active synapse (red). The trajectory starts at point (a) and ends at point (b).
(B) Plots of the instantaneous diffusion coefficient versus time for the examples in (A). Bars correspond to extrasynaptic (black), silenced synapses (green), and active synapses (red).
(C) A model for GluR1 lateral diffusion at active and inactive synapses viewed en face. Input-specific spontaneous synaptic activity reduces receptor mobility, limits exchange with the extrasynaptic membrane, and confines GluR1 within small subdomains of the postsynaptic membrane. This diffusional trap leads to GluR1 accumulation at active synapses.
(A) and (B) are adapted from Ehlers et al. (2007); reprinted with permission from Elsevier, copyright 2007.
The mobility of individual NMDA receptors has been directly visualized at synapses using a combination of FRAP, single dye molecule, and QD tracking. Under basal conditions, extrasynaptic NR1 has a median diffusion coefficient that is four times slower than extrasynaptic GluR2, while the median diffusion coefficient of synaptic NR1 is similar to synaptic GluR2 (Groc et al., 2004). In addition, the fluorescence recovery of synaptic EYFP-NR1 following photobleaching plateaus at 15% after 5 min versus 40% for EYFP-GluR1 (Sharma et al., 2006), indicating that the bulk mobile pool of NMDA receptors at synapses is reduced compared to that of AMPA receptors (Sharma et al., 2006), perhaps due to stronger or more abundant receptor-scaffold interactions.
Electrophysiological approaches have also shown that glutamate receptors undergo lateral diffusion and exchange. Lateral diffusion of native AMPA receptors has been demonstrated with the photoreactive AMPA receptor antagonist ANQX. Following irreversible AMPA receptor inactivation at the somatic plasma membrane by focal UV irradiation, AMPA-mediated currents recover within tens of seconds (Adesnik et al., 2005; Nilsen and England, 2007), indicating that functional extrasynaptic AMPA receptors are mobile in the plasma membrane. Using the irreversible NMDA receptor open-channel blocker MK-801 together with synaptic stimulation of young cultured hippocampal neurons to selectively inactivate synaptic NMDA receptors, initial studies found that 65% of NMDA receptors activated by synaptically released glutamate exchanged within 7 min (Tovar and Westbrook, 2002). The recovery from MK-801 block was not a result of receptor insertion into the plasma membrane, as blocking extrasynaptic receptors with coapplication of NMDA and MK-801 prevented synaptic recovery. Thus, recovery of synaptic NMDA responses following MK-801 block requires exchange from extrasynaptic sources, which are likely NR2B-containing NMDA receptors (Zhao et al., 2008). The time course of recovery measured from MK-801 block appears faster (65% in 7 min) than detected by FRAP methods (15% in 5 min) with the NR1 subunit (Sharma et al., 2006). Possible reasons for this difference could be that GFP-tagged NR1 exchanges more slowly than endogenous NR1 or that synaptically activated NMDA receptors make up the majority of exchangeable receptors. A similar electrophysiological approach in acute hippocampal slices found essentially no recovery of synaptic NMDA current after MK-801 synaptic blockade and subsequent washout in CA1 pyramidal neurons, suggesting that a stable pool of extrasynaptic NMDA receptors does not rapidly exchange with synaptic receptors (Harris and Pettit, 2007). The reason for this apparent discrepancy is not yet clear but could involve developmental differences between cell cultures and brain slices or the presence of astrocytes, secreted factors, or extracellular matrix in brain slices that restrict NMDA receptor mobility (Groc et al., 2007a). Thus, a variety of experimental approaches have documented distinct diffusional properties of glutamate receptors distinguished by receptor type, subunit composition, membrane localization, and developmental stage.
Spine and Dendrite Geometry Influence Glutamate Receptor Diffusion
Perhaps the most evident factor limiting glutamate receptor diffusion is the tortuous geometry of dendrites and dendritic spines. Biophysical models of receptor influx into spines predict that changes in spine neck length are an important mechanism to control receptor exchange between the spine head and the dendritic shaft and, therefore, synaptic receptor content (Holcman and Triller, 2006). Photobleaching experiments with SEP-tagged glutamate receptors also reveal a strong influence of spine neck geometry on glutamate receptor diffusion (Ashby et al., 2006). Following photobleaching, the recovery of SEP-GluR2 fluorescence in spines is much slower than flat surfaces, and the recovery in large mushroom spines (containing thin necks) is slower than stubby spines with thick necks (Ashby et al., 2006). Therefore, the spine neck acts as a barrier to glutamate receptor diffusion. However, other factors could contribute, as large mature spines may have stronger receptor-PSD interactions, and other mechanisms for maintaining or recapturing mobile glutamate receptors could conceivably covary with spine geometry. Nonetheless, spine neck geometry does influence the diffusion of membrane-associated proteins that are not associated with the PSD. In particular, the diffusion of membrane-bound GFP in dendritic spines is retarded relative to the dendritic shaft, suggesting that escape of membrane-associated proteins from spines is also influenced by spine neck diameter (Richards et al., 2004).
In addition to the effects of spine neck geometry on synaptic glutamate receptor exchange, the mere presence of spines would be expected to strongly influence receptor diffusion along the length of a dendrite. Before extrasynaptic glutamate receptors can enter into spines, they first must travel some distance along the dendritic membrane. As with freely diffusing cytosolic proteins (Santamaria et al., 2006), a pool of extrasynaptic receptors moving laterally along a dendrite is likely to encounter a “minefield” of dendritic spines operating as diffusional traps. Indeed, biophysical models of the behavior of receptors inserted into the somatic plasma membrane and diffusing from proximal to distal sites predict that spines will cause a steep decay profile in steady-state receptor concentration along the dendrite by acting as diffusional sinks (Bressloff and Earnshaw, 2007). Therefore, although a significant fraction of glutamate receptors may be inserted at the soma (Adesnik et al., 2005), it seems unlikely that receptors inserted into the soma could supply spiny dendrites several hundred microns away. Moreover, the somatic source model predicts a decreasing proximal-to-distal gradient of receptors. However, a reverse proximal-to-distal increasing gradient is observed for AMPA receptors in CA1 pyramidal neurons (Magee and Cook, 2000; Andrasfalvy and Magee, 2001; Smith et al., 2003). Such considerations highlight the important role of intracellular membrane trafficking coupled with receptor lateral diffusion for delivery of glutamate receptors to distal dendritic locations.
Nanoarchitecture of the PSD and Spatial Confinement of Glutamate Receptors
In addition to the effect of spine neck geometry on receptor mobility, the arrangement and composition of the PSD will directly control the establishment of diffusion barriers (corrals and pickets) and submembranous receptor-binding sites that in turn determine receptor dwell time and thus abundance within synapses. Indeed, electron microscope images of PSDs clearly indicate the presence of physical obstacles that could potentially lead to receptor confinement (Figures 1D and 1E). PSDs have a disc-like shape with a diameter of ~100–500 nm and a thickness of 30–50 nm (Carlin et al., 1980; Harris et al., 1992; Spacek and Harris, 1997; Valtschanoff and Weinberg, 2001). Serial-section EM reconstruction and freeze-fracture EM indicate that PSD surface area ranges from 0.008–0.54 μm2 (Harris and Stevens, 1989; Tanaka et al., 2005). This large range in PSD surface area corresponds to variations in spine size; indeed, PSD surface area positively correlates with spine head size (Harris and Stevens, 1989; Okabe, 2007). The variability in PSD surface area is an important factor to consider when analyzing single molecule trajectories or interpreting FRAP data, as large differences in PSD area could naturally lead to variation in the mobility and confinement of synaptic receptors.
Stoichiometry of Glutamate Receptors and PSD Scaffold Proteins
PSD size and stability are controlled by scaffolding proteins that assemble into this complex structure (Okabe, 2007; Sheng and Hoogenraad, 2007). The most abundant PSD proteins are involved in signal transduction and scaffold formation; for example, calcium/calmodulin-dependent protein kinase II (CaMKII)-α and -β make up 7.4% and 1.3% of the PSD mass (Peng et al., 2004; Cheng et al., 2006). These measurements are consistent with EM images of immunogold-labeled biochemically isolated PSDs, which reveal CaMKII holoenzyme towers studded over the cytosolic face of the PSD (Petersen et al., 2003; Gaertner et al., 2004) (Figure 1D). Also abundant are PDZ scaffold proteins that tether glutamate receptors to the PSD through reversible chemical interactions. Both mass spectrometry and immunogold EM estimate between 60 to 400 of these major scaffold molecules per PSD (e.g., PSD-95, GKAP/SAPAP, SAP97, shank, and homer) (Peng et al., 2004; Chen et al., 2005; Cheng et al., 2006). Importantly, estimates of the number of synaptic scaffolds by quantitative light microscopy using calibration of fluorescence signal from GFP-tagged molecules yielded comparable results, opening promising possibilities for similar quantifications in real time (Sugiyama et al., 2005).
The number of glutamate receptors per synapse appears to be less than PSD scaffold proteins; mass spectrometry, immunogold EM, and electrophysiological experiments estimate AMPA receptor numbers in the range of ~5–200 (Nusser et al., 1998; Matsuzaki et al., 2001; Smith et al., 2003; Peng et al., 2004; Tanaka et al., 2005; Cheng et al., 2006; Masugi-Tokita et al., 2007). Unlike AMPA receptors, NMDA receptor numbers are less variable and have a smaller positive correlation with spine head size (Takumi et al., 1999b; Racca et al., 2000). Ca2+ imaging studies estimate a very small number (~1–5) of NMDA receptors activated by synaptic stimulation at CA1 hippocampal synapses (Nimchinsky et al., 2004), suggesting that there are fewer NMDA receptors than AMPA receptors at mature synapses.
The overabundance of scaffold proteins relative to glutamate receptors is consistent with the availability of free receptor positions or “slots” for changes in synaptic strength (Lisman and Raghavachari, 2006). The presence and spatial arrangement of such slots may determine how receptors partition between synaptic and extrasynaptic domains. Although the nature of these slots is not known, both immunogold freeze-fracture replica labeling and single-particle QD imaging reveal that AMPA receptors are contained within intrasynaptic microdomains, suggesting that these nanoscale compartments may concentrate receptor-binding proteins (Ehlers et al., 2007; Masugi-Tokita et al., 2007) (Figures 1C and 1E). In addition, the placement of AMPA receptor microdomains relative to presynaptic release sites could be an important factor controlling synaptic strength (Raghavachari and Lisman, 2004).
Structural Organization of the PSD and Glutamate Receptor Exchange
In addition to differences in the absolute number of receptors at synapses, AMPA and NMDA receptors may display distinct spatial distributions within and outside of the PSD (Baude et al., 1995; Kharazia et al., 1996a; Nusser et al., 1998; Takumi et al., 1999a; Racca et al., 2000; He et al., 2001). For example, AMPA receptors appear either concentrated at the periphery or homogeneous throughout the PSD (Masugi-Tokita et al., 2007), while NMDA receptors are more compactly distributed at the center of the PSD (Kharazia et al., 1996a; Racca et al., 2000). These differences likely reflect distinct constraints on receptor diffusion consistent with FRAP experiments showing that GluR1 has a faster rate of fluorescence recovery compared to NR1 (Sharma et al., 2006). Differences in the distribution and mobility between receptor types could be a result of distinct binding partners of their cytosolic tails. NMDA receptors have large cytosolic tails of 600 amino acids or more, whereas AMPA receptor tails are from 50 to 100 amino acids in length; longer cytosolic tails could lead to both multiple and stronger scaffold interactions. Also suggesting stronger PSD interactions is the observation that NMDA receptors are insoluble following detergent extraction, whereas AMPA receptors remain soluble (Allison et al., 1998). Conversely, the difference in glutamate receptor intra-PSD distribution could result from differential interactions of extracellular domains. Indeed, extracellular domains of AMPA receptors associate with N-cadherin (Nuriya and Huganir, 2006; Saglietti et al., 2007; Silverman et al., 2007), which may localize to the edge of the PSD (Uchida et al., 1996; Saglietti et al., 2007). In addition, N-terminal extracellular domains of AMPA receptors bind to neuronal pentraxins (O’Brien et al., 1999, 2002; Mi et al., 2002; Xu et al., 2003; Sia et al., 2007), whose spatial distribution at synapses is not known but could contribute to altered receptor mobility or distribution.
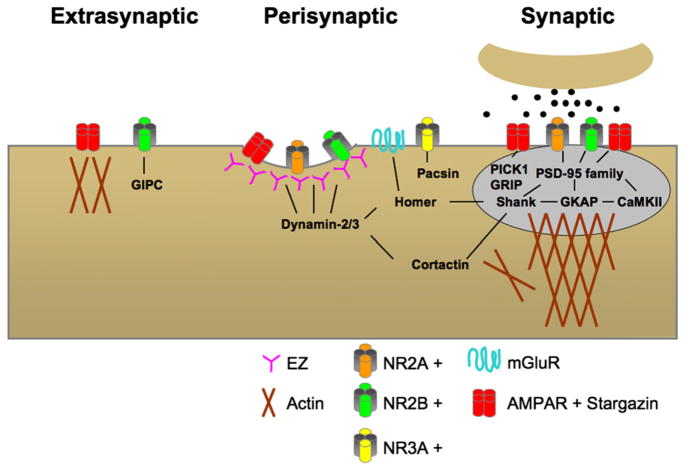
Beyond the border of the PSD is a loosely defined “perisynaptic” region, located within ~100–200 nm of the PSD edge. The perisynaptic region is compositionally (and likely functionally) distinct from more distant extrasynaptic domains and contains a cohort of glutamate receptor types, including group I metabotropic glutamate receptors (mGluRs) and NR3A-containing NMDA receptors, as well as the scaffold protein homer (Baude et al., 1993; Lujan et al., 1997; Tu et al., 1999; Perez-Otano et al., 2006) (Figure 2). The perisynaptic region may have functional significance in controlling glutamate receptor exchange. For example, SPT demonstrated that GluR2-containing AMPA receptors shift into the perisynaptic domain upon bath application of glutamate (Tardin et al., 2003), a manipulation that causes loss of synaptic AMPA receptors (Carroll et al., 1999b). Conversely, synapse-potentiating stimuli (bicuculline/glycine) cause a reduction in the number of perisynaptic receptors (Tardin et al., 2003). On the other hand, imaging of SEP-GluR2 showed a rapid loss of extrasynaptic AMPA receptors that precedes the loss of synaptic receptors following activation of NMDA receptors (Ashby et al., 2004). These data suggest that the perisynaptic region may be a transition zone or reservoir for receptor trafficking into or out of the PSD, a process that may be controlled by the cytoskeleton, scaffold density, and binding capacity of the PSD.
Figure 2. Glutamate Receptor Interactions in Synaptic, Perisynaptic, and Extrasynaptic Compartments.
Distinct protein networks engage AMPA receptors and NMDA receptors in synaptic, perisynaptic, and extrasynaptic compartments. Direct protein interactions are shown by connecting lines. See text for details.
Many unanswered questions remain as to what types of scaffold interactions regulate glutamate receptor exchange between synaptic and perisynaptic regions and how these interactions are regulated by synaptic activity. For AMPA receptors, extracellular interactions with N-cadherin may provide an important control over mobility in the perisynaptic region (Nuriya and Huganir, 2006; Saglietti et al., 2007). In addition to AMPA receptors, the perisynaptic localization and mobility of the group I mGluRs are also regulated by synaptic activity. Normally, mGluR5 undergoes either fast or slow mobility, corresponding to receptors existing in dispersed or clustered states, respectively (Serge et al., 2002). Addition of the group I selective mGluR agonist DHPG increases mGluR5 diffusion, which may occur through uncoupling of Gq from mGluR5, thus leading to the loss of underlying cytoskeletal interactions (Serge et al., 2002). Cytoskeletal tethering of mGluRs to the perisynaptic region may occur through homer binding (Figure 2), as mGluR5 confinement increases following homer-1b overexpression and confined mGluR5 receptors exist in clusters containing homer (Serge et al., 2002). Therefore, activity-dependent interactions between glutamate receptors and perisynaptic scaffold proteins provide an important control over glutamate receptor retention and transit through the perisynaptic zone.
Beyond the perisynaptic membrane is the “extrasynaptic” region, consisting of the remaining spine membrane, spine neck, dendrites, and soma (Figure 2). Although the precise dimensions are somewhat arbitrary, synaptic, perisynaptic, and extrasynaptic regions comprise compositionally and functionally distinct membrane domains, which lead to different diffusional behavior of receptors and activation of downstream signaling pathways. In the case of NMDA receptors, subunit composition may lead to differential targeting between these compartments. Specifically, NR2A-containing receptors are abundant at synapses, while NR2B-containing receptors are often more enriched in extrasynaptic domains, although both are found in synaptic and extrasynaptic domains (Watanabe et al., 1992; Williams et al., 1993; Sheng et al., 1994; Wang et al., 1995; Li et al., 1998; Tovar and Westbrook, 1999; Sans et al., 2000a; Barria and Malinow, 2002; Thomas et al., 2006). Extrasynaptic targeting of NMDA receptor subunits may occur through the NMDA receptor-binding protein GIPC, which associates with surface and intracellular receptors but is excluded from synapses (Yi et al., 2007) (Figure 2). Interestingly, nonconventional NMDA receptors containing NR3A are also enriched at the edge of the PSD and in perisynaptic domains (Perez-Otano et al., 2006) (Figure 2). Correspondingly, selective activation of synaptic versus extrasynaptic receptors may trigger distinct signaling cascades, thereby producing very different effects on synapse plasticity and neuronal survival (Sala et al., 2000; Sattler et al., 2000; Lu et al., 2001; Hardingham and Bading, 2002; Hardingham et al., 2002; Ehlers, 2003; Massey et al., 2004; Morishita et al., 2007; Sun and June Liu, 2007). Thus, microdomains at andnear the synapse mayact as distinct platforms for signaling.
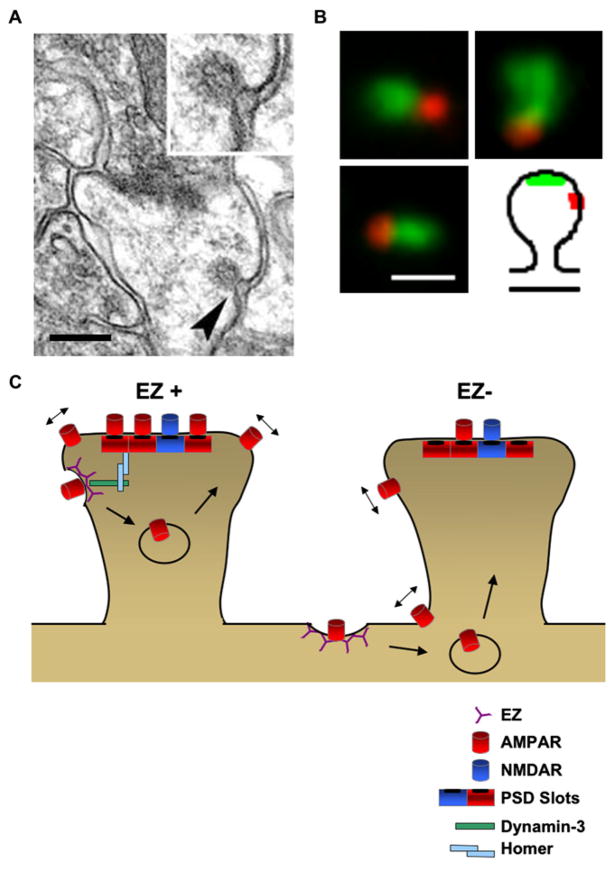
In addition to their lateral organization, the PSDs at glutamatergic synapses have a laminar organization that may also influence receptor exchange. Early direct freeze deep etch EM of excitatory synapses in the cochlear nucleus revealed a meshwork of 4 nm thick actin filaments in receptor-rich domains of the post-synaptic membrane attached to a subjacent lattice of 8–9 nm filaments (Gulley and Reese, 1981). This tighter filament lattice at the postsynaptic plasmalemma was proposed to limit receptor mobility. Immunogold labeling has revealed the laminar distance of specific PSD proteins from the postsynaptic membrane into the spine interior. The cytoplasmic C-terminal tail of AMPA and NMDA receptors define the first molecular layer between the postsynaptic membrane and the underlying scaffold proteins (Valtschanoff and Weinberg, 2001). In stepwise order, starting closest to the plasma membrane, GluR1, CaMKII, ProSAP2, VASP, cortactin, and actin extend from 17 to 110 nm into the spine cytosol (Rostaing et al., 2006). These findings comport with previous results showing that the tail of the NR2 subunit extends 12 nm into the cytosol, whereas GKAP, shank, and homer reside ~25 nm deep (Valtschanoff and Weinberg, 2001; Petralia et al., 2005). To date, little is known about how this laminar organization is established and regulated or how the architecture of these layers influences receptor stabilization. Interestingly, molecular exchange of the “deep” scaffold proteins GKAP and shank is sensitive to actin depolymerization, whereas the exchange of the membrane-associated scaffold PSD-95 is not (Allison et al., 1998; Kuriu et al., 2006). Recent electron tomography studies have identified vertical filaments containing PSD-95 as a major structural feature of the PSD (Chen et al., 2008). The precise vertical and horizontal arrangement of scaffolds could easily be envisioned to trap or tether glutamate receptors to defined sites within the PSD to regulate receptor exchange (Rostaing et al., 2006; Chen et al., 2008) (Figures 1D and 2).
Glutamate Receptor Microdomains within the PSD
Both EM images and single-particle tracking experiments support restricted receptor movements within the postsynaptic membrane. For example, the mobility of individual AMPA receptors is confined within subregions of the synaptic membrane (Ehlers et al., 2007). Super-resolution analysis of receptor diffusion within the postsynapse demonstrated that only 20% of the PSD area in active synapses is accessible to individual QD-labeled GluR1 AMPA receptors (Ehlers et al., 2007) (Figure 1C). In contrast, 80% of the PSD is accessible to single diffusing GluR1 receptors at inactive synapses, suggesting that there are activity-dependent barriers to receptor diffusion within synapses. The confinement of GluR1 to subregions inside active synapses could be due to the increased presence of scaffold binding sites (or “slots”) acting as receptor traps, to altered arrangement of the scaffold network, to changes in underlying cytoskeletal organization, to altered affinities of receptor-scaffold interactions, or some combination of these factors. The limited access of diffusing GluR1 receptors to most of the PSD area is fully consistent with EM studies showing the presence of proteinaceous clumps and voids in isolated PSDs (Petersen et al., 2003) (Figure 1D). CaMKII holoenzymes form large towers on the cytosolic face of the PSD membrane, separated by deep valleys, which could conceivably act as channels for receptor lateral diffusion within the synaptic membrane or may serve to define receptor diffusion boundaries. Below the membrane, the core of the PSD is dominated by vertically oriented filaments containing PSD-95, which contact NMDA receptors and AMPA receptors (Chen et al., 2008). Further supporting subdomain confinement of AMPA receptors in the PSD is the nonhomogeneous clumped distribution of AMPA receptors viewed en face in freeze-fracture electron micrographs (Masugi-Tokita et al., 2007) (Figure 1E). Although the biophysical nature of these PSD subdomains is not clear, the three-dimensional architecture of the PSD likely has strong effects on receptor mobility. One attractive possibility is that constriction and expansion of diffusional channels or scaffold barriers could control receptor entry, intra-PSD mobility, and stabilization. Indeed, CaMKII undergoes translocation into spines following NMDA receptor stimulation (Shen and Meyer, 1999), an event that could trigger rearrangement of PSD structure.
Due to their low affinity for glutamate and differences in activation and desensitization kinetics, the packing density and spatial arrangement of AMPA receptors within the PSD could be an important contributor to synaptic strength, independent of absolute receptor number. Modeling studies indicate that AMPA receptor density within the PSD is more important than the overall size of the PSD (Franks et al., 2003; Raghavachari and Lisman, 2004; Lisman and Raghavachari, 2006). In cultured hippocampal neurons, the variability in miniature excitatory postsynaptic current (mEPSC) amplitude is proportional to the quantity of neurotransmitter released (McAllister and Stevens, 2000), indicating that AMPA receptors are not saturated by quantal release. It thus follows that alterations in the density of AMPA receptors and their positioning relative to glutamate release sites could dramatically alter the postsynaptic response. Indeed, local diffusion can replace desensitized AMPA receptors and thereby fine tune synaptic transmission (Heine et al., 2008). Determining the impact of receptor packing density and subsynaptic confinement remains a difficult experimental problem. It will be important in the future to understand how receptor lateral diffusion is regulated to organize receptors into assembled clusters. In addition, increased filling of receptor slots could produce increased molecular crowding and retention of glutamate receptors through non-chemical interactions, which may be especially important for persistent trapping of newly mobilized receptors during synaptic potentiation. The long-term persistence or depletion of ensembles of synaptic receptors may provide a basis for enduring information storage at the single-synapse level (Shouval and Kalantzis, 2005).
Glutamate Receptor Interactions in the PSD
In addition to their confinement by physical barriers established by the overall architecture of the PSD, a wide array of receptor-interacting proteins have been identified that control the stability and residence time of glutamate receptors within the postsynaptic membrane. With the notable exception of PSD-95 family members, NMDA receptors and AMPA receptor/TARP complexes bind to different sets of synaptic scaffold proteins (Figure 2). For NR3A-containing NMDA receptors, targeting to perisynaptic regions requires an interaction with syndapin-1/PACSIN-1 (Perez-Otano et al., 2006). For NR3A-lacking NMDA receptors, synaptic targeting requires binding of the NR2 subunit C-terminal tails to members of the PSD-95 family of proteins (Niethammer et al., 1996) (Figure 2). This family of proteins includes PSD-93/chapsyn-110, PSD-95, SAP-97/hDlg, and SAP-102 (reviewed in Lau and Zukin, 2007), and all contain PDZ domains. Notably, genetic deletion of NR2 C-terminal tails leads to a deficit in synaptic targeting (Mori et al., 1998; Steigerwald et al., 2000; Mohrmann et al., 2002). A significant portion of this targeting defect is likely due to disrupted binding to PDZ scaffolds like PSD-95, because selective mutation of the PDZ-binding domain in NR2B prevents synaptic incorporation (Prybylowski et al., 2005).
In addition to binding NR2 subunits of NMDA receptors, PSD-95 family members bind to AMPA receptors via their auxiliary TARP subunits (Chen et al., 2000; Chetkovich et al., 2002; Schnell et al., 2002; Dakoji et al., 2003; Tomita et al., 2004). The canonical TARP protein stargazin/γ2 (and likely the other TARPs) functions at multiple levels of the secretory pathway to control surface expression of AMPA receptors (Chen et al., 2000, 2003; Schnell et al., 2002; Cuadra et al., 2004; Vandenberghe et al., 2005; Matsuda et al., 2008). Although TARP-dependent trafficking to the extrasynaptic membrane does not require PSD-95 binding, this interaction is required for subsequent stabilization of AMPA receptor/TARP to the synapse (Schnell et al., 2002). This was directly supported by single-particle tracking experiments in hippocampal neurons revealing that stargazin-dependent interactions between AMPA receptors and PSD-95 control receptor diffusion and trapping at the PSD (Bats et al., 2007). Both GluR1- and GluR2-labeled QDs have reduced mobility at PSD-95-labeled synaptic sites compared to regions outside of PSD-95 clusters. Confinement of AMPA receptors within PSD-95 puncta involves stargazin, as overexpression of a stargazin mutant that cannot interact with PSD-95 increases the mobile fraction and decreases the dwell time of receptors within synapses (Bats et al., 2007). QD tracking of HA-tagged stargazin also revealed reduced mobility at PSD-95 clusters (Bats et al., 2007). Therefore, TARPs and AMPA receptors diffuse together in synaptic and extrasynaptic sites, and binding of TARPs to PSD-95 stabilizes synaptic AMPA receptors.
In addition to binding PSD-95 family members via TARPs, a distinct set of PDZ proteins bind AMPA receptor subunits directly. Both protein interacting with C kinase (PICK1) (Xia et al., 1999) and glutamate receptor interacting protein/AMPA receptor binding protein (GRIP/ABP) (Dong et al., 1997; Srivastava et al., 1998) are PDZ-domain-containing proteins that bind to the C terminus of GluR2 AMPA receptors and regulate their targeting to synapses (Figure 2) (Xia et al., 1999; Chung et al., 2000; Daw et al., 2000; Osten et al., 2000; Kim et al., 2001; Seidenman et al., 2003; Hanley and Henley, 2005). PICK1 and GRIP bind the same extreme C-terminal domain of GluR2, although their binding is differentially regulated by phosphorylation of GluR2 at serine 880 (Hirbec et al., 2003; Seidenman et al., 2003; Lu and Ziff, 2005; Lin and Huganir, 2007). These distinct binding properties of PICK1 and GRIP/ABP are important for AMPA receptor removal during long-term depression (LTD) (Xia et al., 2000; Kim et al., 2001; Chung et al., 2003; Steinberg et al., 2006) and for rapid plasticity of Ca2+-permeable AMPA receptors at cerebellar stellate cell synapses (Liu and Cull-Candy, 2000; Gardner et al., 2005; Liu and Cull-Candy, 2005). Activation of Ca2+-permeable AMPA receptors (which lack GluR2) can lead to incorporation of GluR2-containing AMPA receptors that have a smaller single-channel conductance, a linear current-voltage relationship, and are impermeable to Ca2+ (Isaac et al., 2007). PICK1 is also important for expression of long-term potentiation (LTP), and overexpression of PICK1 occludes LTP by increasing levels of synaptic GluR2-lacking AMPA receptors (Terashima et al., 2008). Although the precise trafficking functions of PICK1 are not completely resolved, PICK1 contains a BAR domain that binds phospholipids on curved membranes (Peter et al., 2004; Jin et al., 2006) and thus may participate in targeting GluR2 AMPA receptors to microdomains of membrane curvature. PICK1 also binds actin and the Arp2/3 complex and may regulate the assembly of actin at sites of GluR2 endocytosis (Rocca et al., 2008).
Interestingly, studies in mice lacking GluR2 and/or GluR3 indicate that the GluR2 C terminus is not required for synaptic insertion (Panicker et al., 2008), and GluR2/3-lacking AMPA receptors are endocytosed in a manner indistinguishable from GluR2-containing AMPA receptors in wild-type neurons (Biou et al., 2008), although targeted in vivo mutations in the GluR2 PDZ-binding domain eliminate cerebellar LTD (Steinberg et al., 2006). Taken together, these findings support an emerging concept that AMPA receptor-binding proteins have subunit preferences that allow precise, and perhaps redundant, control over synaptic stabilization and membrane trafficking of distinct AMPA receptor subtypes.
Postsynaptic scaffold proteins are themselves dynamic. Not all PSD scaffold proteins have a similar retention time within the PSD, and it is likely that networks of protein interactions will affect scaffold stability and their ability to retain receptors. Recent studies have measured exchange kinetics and dynamics of PSD proteins (Friedman et al., 2000; Bresler et al., 2004; Gray et al., 2006; Kuriu et al., 2006; Sharma et al., 2006; Tsuriel et al., 2006). When the exchange kinetics of different PSD proteins were compared by FRAP, CaMKII had the greatest mobile pool (~82%), followed by GluR1 (~56%), PSD-95 (~45%), and NR1 (~38%) (Sharma et al., 2006). Another study compared the stability of four different postsynaptic scaffold proteins—PSD-95, GKAP, shank, and homer-1c/Zip45—and found that each protein had distinct exchange rates in the PSD as measured by FRAP (Kuriu et al., 2006). PSD-95 had the least mobile population (~13%), followed by GKAP (~36%), shank (~35%), and homer-1c/Zip45 (~62%). Given that PSD-95 is relatively stable in the PSD (Kuriu et al., 2006; Sharma et al., 2006) and occupies a position immediately subjacent to the postsynaptic membrane (Valtschanoff and Weinberg, 2001), one might have thought that loss of PSD-95 would have a dramatic effect on the retention of less stable PSD proteins. However, disruption of PSD-95 did not affect the dynamics of GKAP, shank, or Zip45 (Kuriu et al., 2006), suggesting that multiple interactions and internal scaffold redundancy control the exchange of individual scaffold molecules. On the other hand, acute pharmacological disruption of F-actin rapidly eliminates the dynamic fraction of GKAP, shank, and homer-1c/Zip45 (Kuriu et al., 2006). Although it is generally accepted that increased expression of core PSD scaffolds including PSD-95 and shank increase synaptic AMPA receptor content (El-Husseini et al., 2000; Sala et al., 2001; Stein et al., 2003; Ehrlich and Malinow, 2004; Beique et al., 2006; Ehrlich et al., 2007), it remains to be determined how ongoing exchange of PSD scaffolds impacts glutamate receptor dynamics, which protein interactions are facilitated or impaired during scaffold exchange, and whether scaffold exchange regulates or reflects PSD nanocompartmentalization.
The Cortical Actin Cytoskeleton Controls Receptor Diffusion
In all eukaryotic cells, the membrane-associated cortical F-actin cytoskeleton is a key determinant of membrane protein diffusion by providing points of direct cytoskeletal anchoring and organizing membrane microdomains (Kusumi et al., 2005). In spines, depolymerization of F-actin increases the exchange of membrane-anchored GFP (Richards et al., 2004), suggesting that the submembranous actin cytoskeleton constrains lateral diffusion. In addition, the actin cytoskeleton determines spine morphology (Fischer et al., 1998), which in turn influences receptor diffusion (Ashby et al., 2006; Holcman and Triller, 2006). Actin dynamics are tightly tuned by synaptic activity and may confer a rapid mechanism to control receptor diffusion (Star et al., 2002; Okamoto et al., 2004, 2007). At inhibitory synapses, SPT studies show that acute depolymerization of F-actin increases glycine receptor exchange between synaptic and extrasynaptic compartments, leading to a decreased dwell time within inhibitory synapses and ultimately a loss of synaptic receptors (Charrier et al., 2006). There is evidence that the actin cytoskeleton tethers glutamate receptors at both synaptic and nonsynaptic sites. For example, depolymerization of F-actin causes a 40% decrease in the number of synaptic AMPA and NMDA receptor clusters (Allison et al., 2000). Nonsynaptic NMDA receptor clusters persist, while nonsynaptic AMPA receptor clusters disperse following F-actin depolymerization (Allison et al., 2000), indicating that extrasynaptic AMPA receptor clustering is controlled by interactions with the actin cytoskeleton (Figure 2). Interestingly, F-actin filaments can directly contact the PSD (Landis and Reese, 1983; Rostaing et al., 2006) and associate with diverse protein elements of the PSD (Okabe, 2007; Sheng and Hoogenraad, 2007), including scaffold complexes containing shank and cortactin (Okabe, 2007; Sheng and Hoogenraad, 2007) (Figure 2). Likewise, pharmacological disruption of F-actin eliminates the dynamic fraction of several core PSD scaffold proteins, including GKAP and shank, which associate with AMPA and NMDA receptors (Kuriu et al., 2006). Submicron variation in actin organization may account for differences in glutamate receptor mobility in synaptic, perisynaptic, and extrasynaptic membrane microdomains.
Glutamate Receptor Mobility and Synaptic Activity
Activity Level Controls Glutamate Receptor Movement
Synaptic activity can influence glutamate receptor diffusion through control of membrane trafficking, spine morphology, cytoskeletal organization, and the affinity of interactions with PSD scaffold proteins. Indeed, chemically induced LTP and LTD, as well as chronic modifications of synaptic activity influence surface AMPA receptor diffusion (Borgdorff and Choquet, 2002; Tardin et al., 2003; Ashby et al., 2004; Groc et al., 2004; Sharma et al., 2006; Ehlers et al., 2007). Besides the direct influence on receptor diffusion consequent to plasticity-associated changes of spine morphology (Ashby et al., 2006), LTP-inducing stimuli reduce the diffusion of soluble molecules between spines and shafts, indicating local activity-dependent diffusional coupling of the spine neck with the dendritic shaft (Bloodgood and Sabatini, 2005).
AMPA receptor diffusion is highly sensitive to local changes in Ca2+. For example, GluR2 pauses reversibly near synapses, and local elevation of dendritic Ca2+ by ionophore uncaging decreases GluR2 extrasynaptic mobility by 80%, while buffering intracellular Ca2+ with BAPTA increases GluR2 mobility (Borgdorff and Choquet, 2002). Surprisingly, GluR2 mobility is also arrested following Ca2+ uncaging in young neurons (DIV1–4) lacking synapses (Borgdorff and Choquet, 2002), indicating that decreased receptor movement is not merely a result of spine or PSD entrapment. Therefore, extrasynaptic AMPA receptors can be confined by Ca2+-dependent scaffolding interactions or remodeling of the submembranous cytoskeleton. In addition, increases in Ca2+ levels triggered by physiological synaptic stimulation decrease the mobility of GluR1- and GluR2-containing AMPA receptors, an effect that has important implications for short-term plasticity. For example, the decreased mobility of AMPA receptors following synaptic stimulation is associated with increased paired-pulse depression (PPD) (Heine et al., 2008). Although the recovery from fast synaptic depression is widely believed to be dominated by a presynaptic component (Zucker and Regehr, 2002), a recent study has demonstrated that lateral exchange of desensitized synaptic AMPA receptors for naive functional AMPA receptors also contributes to recovery from PPD (Heine et al., 2008). Conditions that reduce surface AMPA receptor mobility (e.g., antibody cross-linking, receptor clustering, or increases in Ca2+) prevent the normal recovery from PPD by preventing exchange with desensitized receptors (Heine et al., 2008). These findings highlight the important role of AMPA receptor diffusion and synaptic exchange on a rapid millisecond timescale. Moreover, these data suggest that subtle changes in the mobility and dwell time of synaptic AMPA receptors may have a dramatic impact on the exchange of desensitized receptors and expression of short-term synaptic plasticity.
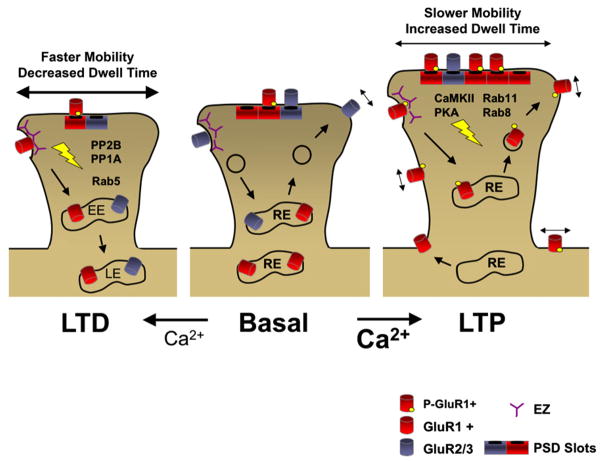
Changes in AMPA receptor mobility are also important for long-term forms of synaptic plasticity. In synaptic compartments, diffusion of Cy-5-labeled synaptic GluR2 subunits is accelerated by bath application of glutamate (Tardin et al., 2003). This effect is similar to population dynamics of SEP-GluR2, as NMDA receptor-induced chemical LTD causes a loss of synaptic GluR2 AMPA receptors, which is preceded by endocytosis of extrasynaptic GluR2 AMPA receptors (Ashby et al., 2004). These findings suggest a model whereby LTD expression involves destabilization of scaffold interactions, followed by increased mobility of synaptic AMPA receptors, and finally receptor release into the extrasynaptic compartment for endocytosis.
Given that chemically induced LTD correlates with an increased mobility of synaptic AMPA receptors, does LTP decrease AMPA receptor mobility at synapses? Such a mechanism could provide a basis for the net increase in synaptic AMPA receptors during LTP (Shi et al., 1999; Hayashi et al., 2000; Lu et al., 2001; Kopec et al., 2006; Sharma et al., 2006). Within 5 min after chemical LTP induction, the percentage of mobile synaptic GluR2 AMPA receptors increases but returns to baseline levels after 40 min, suggesting that newly incorporated synaptic GluR2 receptors are mobile but soon become stabilized within synapses (Tardin et al., 2003). These results are consistent with FRAP experiments showing that chemical LTP stimuli increase the proportion of exchangeable EYFP-GluR1 in spines but decrease the rate of recovery following photobleaching (Sharma et al., 2006), suggesting that, while the number of mobile GluR1 AMPA receptors increases, synaptic receptor exchange decreases, ultimately allowing for synaptic trapping of AMPA receptors. In addition, SPT analysis revealed that the percentage of perisynaptic GluR2 receptors is reduced following chemical LTP induction (Tardin et al., 2003), suggesting that the perisynaptic pool of AMPA receptors is depleted as receptors enter into the PSD (Tardin et al., 2003). Still to be resolved are differences in rapid activity-dependent diffusional behavior of AMPA receptors containing GluR1 and GluR2. Nonetheless, these results suggest that the perisynaptic region maintains tight control over the synaptic exchange of AMPA receptors in response to activity.
Exchange of AMPA receptors at synapses during long-term plasticity could conceivably occur by altering AMPA receptor-TARP interactions or TARP-PSD-95 interactions, and phosphoregulation could provide the basis for these reversible interactions. For example, serine 295 phosphorylation of PSD-95 is associated with increased synaptic targeting of AMPA receptors, while dephosphorylation of serine 295 in PSD-95 is important for synaptic depression (Kim et al., 2007). In addition, protein kinase A (PKA)-mediated phosphorylation of the stargazin C terminus prevents its interaction with PSD-95 (Chetkovich et al., 2002), suggesting that surface mobility of AMPA receptors during LTD could be increased by PKAphosphorylation ofstargazin. Stargazin phosphorylation is controlled by additional kinases and phosphatases, whose activities are dependent on NMDA receptor activation (Tomita et al., 2005). For example, phosphorylation of TARPs by CaM-KII and protein kinase C (PKC) is important for LTP and synaptic targeting of AMPA receptors, while dephosphorylation by PP1/PP2B is important for LTD, indicating that the phosphorylation state of TARPs allows for bidirectional plasticity by controlling AMPA receptor exchange at synapses (Tomita et al., 2005). Phosphorylation of AMPA receptors may also facilitate their delivery to the plasma membrane, thereby increasing the pool of extrasynaptic receptors and thus altering the equilibrium of synaptic receptor exchange. For example, chemical LTP induction increases the phosphorylation ofserine 845 onGluR1 (Lee etal., 2000), which accelerates receptor recycling (Ehlers, 2000) and increases the extrasynaptic pool of GluR1 (Oh et al., 2006).
In contrast to AMPA receptors, the mobility of NMDA receptors in synaptic and extrasynaptic compartments is unaffected by inhibiting network activity (TTX) or by KCl depolarization (Groc et al., 2004), consistent with FRAP data showing that NR1 dynamics are unaffected by chemical LTP induction (Sharma et al., 2006). However, activation of PKC with phorbol ester dramatically increased the diffusion coefficient of both extrasynaptic NR1 (12-fold) and synaptic (5-fold) receptors (Groc et al., 2004). These findings demonstrate that NMDA receptor mobility is more restricted than that of AMPA receptors, both in synaptic and extrasynaptic compartments, and is not significantly affected by synaptic activity, perhaps reflecting stronger scaffold interactions than AMPA receptors. In addition, the difference in mobility between NMDA and AMPA receptors could result from the more peripheral localization of AMPA receptors in synapses relative to NMDA receptors, as detected by immunogold labeling (Kharazia et al., 1996a; Tanaka et al., 2005; Masugi-Tokita et al., 2007).
Input-Specific Activity and Local Control of AMPA Receptor Diffusion
In addition to acute cell-wide manipulations of neural activity, the long-term activity state of a single synapse influences local receptor abundance and exchange (Harms et al., 2005; Ehlers et al., 2007; Hou et al., 2008a). Single-molecule imaging of GluR1 AMPA receptors using QDs has shown that active synapses more efficiently trap AMPA receptors than nearby inactive synapses rendered silent by expression of the tetanus toxin light chain (Ehlers et al., 2007). The median diffusion coefficient of GluR1 was reduced at active synapses as compared to inactive synapses, and the dwell time of GluR1 was significantly longer at active synapses (Ehlers et al., 2007) (Figures 3A and 3B). At inactive synapses GluR1 explores a much greater area of the postsynaptic membrane compared to nearby active synapses, although GluR1 at both types of synapses exhibited confined movement (Ehlers et al., 2007) (Figures 3C). During a 1 min time course, 20% of GluR1 exited active synapses, and 75% of GluR1 exited silent synapses and, in certain instances, GluR1 receptors that exited silent synapses entered into active synapses and became immediately trapped (Ehlers et al., 2007) (Figures 3A and 3B). Interestingly, acute blockade of glutamate receptors and action potentials did not alter GluR1 diffusion at either active or silenced synapses, indicating that activity-dependent changes in local receptor diffusional properties require prolonged differences in spontaneous synaptic activity and thus could represent an enduring biophysical modification. More broadly, these observations support a “capitalistic synapse” model whereby excitatory synapses compete for a limiting pool of diffusible GluR1 AMPA receptors based on local levels of vesicular release from presynaptic terminals, suggesting a diffusional basis for input-specific strengthening of synapses (Figure 3C).
In addition to AMPA receptors, PSD scaffold proteins also exist as a limited pool that is selectively retained in larger synapses. Two-photon imaging of GFP-tagged PSD-95 in vivo showed that a subset of PSD-95 puncta are stable for days, whereas a separate population turns over rapidly and is exchanged with nearby spines (Gray et al., 2006). Large PSDs capture more PSD-95 than smaller PSDs, and changes in PSD size correlate with PSD-95 retention time. Therefore, in a situation analogous to GluR1 AMPA receptor trapping by active synapses (Ehlers et al., 2007), neighboring PSDs also seem to compete for a limited pool of PSD-95. In addition to PSD-95 exchange, the scaffold protein ProSAP2/shank3 also undergoes activity-regulated exchange between neighboring synapses on a timescale of minutes (Tsuriel et al., 2006). In a separate study, pharmacological enhancement of neuronal activity increased GKAP content at synapses but, conversely, caused loss of shank and homer-1c/Zip45 (Kuriu et al., 2006). The ability of active synapses to capture AMPA receptors (Ehlers et al., 2007) and large synapses to sequester PSD scaffold proteins (Isaac, 2003; Perez-Otano and Ehlers, 2004; Petralia et al., 2005; Gray et al., 2006), suggests that stable differences in synaptic strength are maintained by dynamic equilibria between local pools of exchangeable synaptic proteins, the size and dynamics of which are determined by local synaptic activity.
Glutamate Receptor Mobility for Synapse Maturation and Development
During brain development, excitatory synapses throughout the CNS undergo stereotyped changes in molecular and physiological properties. Such changes result in part from alterations in glutamate receptor trafficking, subunit expression, and changes in postsynaptic scaffolding proteins that alter receptor residence time (Isaac, 2003; Perez-Otano and Ehlers, 2004; Petralia et al., 2005). In the case of AMPA receptors at CA1 hippocampal synapses, GluR4 is expressed early during neuronal maturation and recruited to synapses displaying spontaneous activity (Zhu et al., 2000). Synaptic trapping of GluR4 involves an extracellular binding to pentraxins (Sia et al., 2007), a process that may be important for recruitment of AMPA receptors to the postsynaptic specialization during synaptogenesis. In the case of NMDA receptors, NR2B-containing NMDA receptors are expressed early and are later replaced at synapses by NR2A-containing receptors, which eventually predominate at synapses (Carmignoto and Vicini, 1992; Watanabe et al., 1992; Williams et al., 1993; Sheng et al., 1994; Wang et al., 1995; Li et al., 1998; Rumbaugh and Vicini, 1999; Tovar and Westbrook, 1999; Sans et al., 2000b; Barria and Malinow, 2002). The basis for these developmental subunit changes at synapses may result from a differential expression of scaffolding proteins, such as SAP102 and PSD-95 (Sans et al., 2000a), as the C-terminal domains of NR2 subunits possess distinct binding preferences and interact with distinct sets of scaffold proteins and signaling molecules (Kornau et al., 1995; Niethammer et al., 1996; Sans et al., 2000a). As NR2 subunit expression regulates NMDA receptor targeting and trafficking during development (Mori et al., 1998; Steigerwald et al., 2000; Roche et al., 2001; Mohrmann et al., 2002; Lavezzari et al., 2004; Prybylowski et al., 2005), one might expect subunit-specific differences in NMDA receptor mobility. Indeed, QD tracking of NR2A- versus NR2B-containing NMDA receptors has demonstrated slower mobility of surface NR2A (Groc et al., 2006, 2007a). NR2A diffusion coefficients in both synaptic and extrasynaptic compartments are slower than those of NR2B (Groc et al., 2006), and a greater proportion of NR2A is immobile in these two compartments. Conversely, the dwell time of NR2B in synapses is 3-fold shorter at DIV15 than at DIV8, and the dwell time of NR2A was significantly longer than NR2B at DIV15. Electrophysiological data also reveal increased mobility of NR2B-containing receptors and exchange at synapses. In 3-week-old hippocampal slices, mobile NR2B-containing NMDA receptors are responsible for the recovery of sEPSCs following MK-801 block (Zhao et al., 2008). Surprisingly, after the NR2B-mediated recovery, an LTP stimulation protocol leads to induction of LTD at these synapses, indicating that NMDA receptor subunit switching can lead to a novel type of metaplasticity (Zhao et al., 2008). Taken together, these observations demonstrate that subunit-specific differences in NMDA receptor synaptic targeting correlate with distinct diffusional and synaptic properties.
In addition to PSD scaffold proteins, the extracellular matrix also influences subunit-specific synaptic targeting of NMDA receptors. For example, developmental acceleration in synaptic NR1/2B receptor diffusion correlates with increased expression of reelin (Groc et al., 2007a), an extracellular matrix protein involved in developmental maturation and synaptic plasticity, which carries out these functions through binding to β1 integrin (Rodriguez et al., 2000) and lipoprotein receptors (Dityatev and Schachner, 2006; Qiu et al., 2006). SPT of NR2B-containing receptors revealed that antibody-mediated inhibition of reelin decreases the mobility of native NR2B receptors and increases synaptic dwell time, whereas addition of recombinant reelin to young hippocampal neurons accelerates synaptic maturation by reducing NR2B-mediated synaptic currents and receptor dwell time within synapses (Groc et al., 2007a).
Providing further evidence for mobile NMDA receptors during synapse maturation is the finding that NMDA receptor subunits exchange at neonatal synapses but not more mature synapses (Bellone and Nicoll, 2007). Although LTP-inducing stimuli do not affect the exchange kinetics of EYFP-NR1 in cultured hippocampal neurons (Sharma et al., 2006), LTP induction at neonatal CA1 synapses can lead to subunit switching of synaptic NR2A receptors for NR2B receptors within seconds. This effect can endure for at least 1 hr and can be reversed by depotentiating stimuli (Bellone and Nicoll, 2007). Although the precise molecular mechanisms are not yet clear, this reversible switch between NR2B- and NR2A-containing receptors supports tight regulation of NMDA receptor trafficking.
One possible mechanism that could lead to preferential down-regulation of NR2B- versus NR2A-containing receptors is their differential binding to the clathrin adaptor AP-2. The C terminus of NR2B contains a tyrosine-based AP-2 clathrin internalization sequence (Lavezzari et al., 2003) adjacent to its PSD-95-binding site, suggesting that NR2B receptor interactions with PSD proteins prevent NMDA receptor downregulation by clathrin-mediated endocytosis. Indeed, NR2B interactions with PSD-95, SAP97, and PSD-93 inhibit NR2B endocytosis (Roche et al., 2001; Lavezzari et al., 2003). In addition, Fyn kinase-mediated phosphorylation of the NR2B AP-2-binding motif prevents endocytosis and thus may be a mechanism to increase synaptic targeting of NR2B (Prybylowski et al., 2005). The NR2A subunit also contains a tyrosine-based sorting motif, but this sequence is not required for endocytosis. Instead, a dileucine-based AP-2 internalization motif is utilized (Prybylowski et al., 2005). Following endocytosis, NMDA receptors can be sorted for either recycling or degradation (Lavezzari et al., 2004; Scott et al., 2004). The membrane-proximal sorting signals of both NR1 and NR2 drive NMDA receptors into lysosomes for degradation, whereas the NR2 distal sorting motifs direct NMDA receptors to a recycling pathway (Scott et al., 2004). Currently, it is not known how these sorting motifs are differentially utilized, although the NR2 distal motif is sufficient to drive recycling even in the presence of both the NR1 and NR2 proximal degradation motifs (Scott et al., 2004).
An additional factor controlling NMDA receptor channel properties, surface expression, and synaptic exchange during development is the NR3A subunit. Unlike NR2 subunits, the nonconventional NMDA receptor subunit NR3A has a strict window of expression during development, which peaks around postnatal day 8 and begins to decline at postnatal day 12 into adulthood in most brain regions in the rat (Wong et al., 2002). When associated with NR1/NR2 NMDA receptors, NR3A attenuates NMDA responses by reducing single-channel conductance (Das et al., 1998; Sasaki et al., 2002). NR3A knockout mice have enhanced NMDA receptor responses, and expression of NR3A in oocytes reduces the unitary conductance of NMDA receptors (Das et al., 1998). In addition, NR3A-containing channels have decreased Ca2+ permeability and are less sensitive to Mg2+ (Sasaki et al., 2002). The decreased sensitivity of NR3A-containing receptors to Mg2+ reduces the voltage dependence of these NMDA receptors, potentially altering the Hebbian learning rules for plasticity during synaptogenesis. Interestingly, the postnatal decline in NR3A expression corresponds to sensitive periods of plasticity in several brain regions. Endocytosis and downregulation of NR3A occurs in an activity-dependent manner and is mediated by direct interaction of the NR3A intracellular domain with the dynamin-associated F-BAR protein syndapin-1/PACSIN-1, whose upregulation also coincides with the developmental loss of NR3A (Perez-Otano et al., 2006). Thus, activity-dependent endocytosis of NR3A by syndapin-1/PACSIN-1 may contribute to experience-driven developmental changes in synaptic NMDA receptors.
Spine-Localized Endocytic Cycling Regulates Surface Glutamate Receptor Distribution
Glutamate receptors are transported through diverse intracellular compartments (Kennedy and Ehlers, 2006); this vesicular transport to and from the plasma membrane tunes the abundance of receptors in both synaptic and extrasynaptic regions (Lau and Zukin, 2007; Shepherd and Huganir, 2007). Initial evidence for dynamic glutamate receptor trafficking demonstrated that AMPA receptors undergo continual endocytosis and exocytosis, even in the absence of synaptic activity (Carroll et al., 1999b; Luscher et al., 1999; Shi et al., 1999; Beattie et al., 2000; Ehlers, 2000; Lin et al., 2000; Man et al., 2000) (Figures 4 and 5). Endocytosis of glutamate receptors occurs through a dynamin-dependent pathway (Carroll et al., 1999a; Luscher et al., 1999), and both dendritic spines and shafts contain the full assembly of clathrin-coat proteins and adaptors that sort, trap, and remove glutamate receptors from the cell surface (Blanpied et al., 2002; Racz et al., 2004; Lu et al., 2007). Both NMDA and AMPA receptor subunits contain sorting sequences that allow them to bind directly to the cargo-sorting adaptor AP-2 (Roche et al., 2001; Lee et al., 2002; Lavezzari et al., 2003; Prybylowski et al., 2005; Kastning et al., 2007). Endocytosis of glutamate receptors is dependent on dynamin function, based on evidence that an inhibitory peptide perturbing dynamin function blocks internalization (Luscher et al., 1999). In addition, snapshots of endocytosis captured by immunogold EM demonstrate internalization of GluR2/3 subunits within spine clathrin-coated pits (Petralia et al., 2003). Although most dendritic spines of hippocampal neurons contain endocytic machinery (Blanpied et al., 2002; Racz et al., 2004; Lu et al., 2007), it currently remains unknown whether endocytosis of glutamate receptors occurs preferentially in spines or dendritic shafts and whether the location of receptor endocytosis influences recycling or degradation.
Figure 4. Spine-Localized Endocytic Zones Maintain Synaptic Glutamate Receptors.
(A) Electron micrograph of a dendritic spine from CA1 hippocampus of adult brain. The arrowhead points to an invaginating clathrin-coated pit located adjacent to the electron-dense PSD. Scale bar, 200 nm. Adapted from Racz et al. (2004); reprinted with permission from Nature Publishing Group, copyright 2004.
(B) Examples of endocytic zones in spines marked by clathrin-DsRed (red), which are distinct from PSD-95-EGFP (green). Scale bar, 1 μm. Adapted from Blanpied et al. (2002).
(C) (Left) Endocytic zone positive (EZ+) spines maintain a local supply of extrasynaptic AMPA receptors. The spine EZ acts as a diffusional trap to recapture AMPA receptors that have escaped the synapse. Endocytic cycling through spines maintains a pool of local extrasynaptic AMPA receptors leading to increased filling of AMPA receptor slots in synapses. (Right) In the absence of an endocytic zone, receptors that escape the synapse are not recaptured, leading to a decrease in the extrasynaptic receptor abundance in spines and subsequently loss of AMPA receptors from the synapse. Model based on Lu et al. (2007).
Figure 5. Integrating Models for Receptor Trafficking and Diffusion during Synaptic Plasticity.
Induction of LTP by Ca2+ influx through NMDA receptors leads to activation (lightning bolt) of PKA and CaMKII, which in turn promotes the mobilization of recycling endosomes (RE) into spines, exocytosis from recycling endosomes, and appearance of AMPA receptors at the spine membrane. The number of available slots in the PSD increases through unknown mechanisms, which can be filled by increased levels of extrasynaptic AMPA receptors. Receptor diffusion inside synapses decreases due to stronger scaffold interactions and/or receptor confinement. The EZ may also contribute to LTP by maintaining local recycling of AMPA receptors and preventing their escape from the spine membrane. On the other hand, induction of LTD leads to activation of protein phosphatases (lightning bolt), including PP2B and PP1, triggering clathrin-, dynamin-, and Rab5-dependent endocytosis of AMPA receptors, likely at the spine EZ. Receptor downregulation occurs by trafficking through early (EE) and late endosomes (LE). Loss of synaptic slot positions through unknown mechanisms reduces AMPA receptor capacity and increases the diffusion of synaptic AMPA receptors. EZ, endocytic zone; P-GluR1, phosphorylated GluR1.
Dendritic spines appear to contain all of the core endocytic machinery (clathrin, AP-2, dynamin-2, epsin, Hip1) at a location that has been designated the endocytic zone (EZ) (Blanpied et al., 2002; Racz et al., 2004; Yao et al., 2006; Lu et al., 2007), which consists of stable clathrin coats located lateral to the PSD (Figures 4A–4C). This positioning may maintain tight spatial control over glutamate receptor levels in response to synaptic activity, providing a mechanism for input-specific plasticity. Unlike the dynamic clathrin-coated pits observed in immature neurons and other cell types (Blanpied et al., 2002; Ehrlich et al., 2004; Newpher et al., 2005), the spine EZ resembles the stable behavior of nonterminal clathrin-coated pits (Merrifield et al., 2005), which can persist for tens of minutes (Blanpied et al., 2002). Given this long-term stability, the EZ could also act as a secondary diffusion trap on the spine membrane to help prevent lateral escape of receptors from the spine. This trapping may also prevent diffusion of cargo into neighboring synapses, which, in addition to receptor-scaffold interactions, would provide a synergistic mechanism for tuning postsynaptic receptor levels on a spine-by-spine basis (Figure 4C).
How does the endocytic machinery target to spines, and what is its functional role in synaptic transmission? A recent study has shown that dynamin-3 couples EZs to PSDs (Figures 2 and 4C) (Lu et al., 2007). Whereas the brain-specific dynamin-1 localizes to presynaptic terminals and the ubiquitous dynamin-2, which mediates endocytosis in all cell types, is thought to be both pre- and postsynaptic, dynamin-3 is enriched at the postsynaptic specialization and is the only isoform known to bind to the postsynaptic adaptor homer (Brakeman et al., 1997; Tu et al., 1998; Xiao et al., 1998; Gray et al., 2003, 2005). Consistent with a direct scaffolding function, disrupting dynamin-3 expression or its interaction with homer uncouples the EZ from the PSD, leading to a marked decrease in PSDs with an adjacent EZ (Lu et al., 2007). The functional consequences of EZ loss from the PSD are counterintuitive. Rather than increasing synaptic AMPA receptor levels, as would be expected for blocking receptor endocytosis, spatial uncoupling of the EZ depletes synaptic AMPA receptors and decreases the amplitude and frequency of mEPSCs (Lu et al., 2007). These results suggest a model where the EZ, by allowing for spine-localized endocytic cycling, serves as a trap to recapture and maintain the extrasynaptic pool of AMPA receptors, thereby limiting the escape of AMPA receptors from spines (Lu et al., 2007) (Figure 4C). Consistent with this notion, spine-localized recycling endosomes are important for maintaining a mobilizable pool of synaptic AMPA receptors (Park et al., 2004).
There are many unanswered questions regarding the functions of the spine EZ. What types of postsynaptic cargo are trafficked by the EZ? Does the EZ contribute to spatial differences in AMPA receptor abundance across dendritic segments? Interestingly, in nonneuronal cells, different types of cargo influence clathrin-coated pit dynamics and rates of endocytosis. For example, clathrin-coated pits containing G protein-coupled receptors have very long lifetimes, which are thought to occur because of cargo-cytoskeleton interactions (Puthenveedu and von Zastrow, 2006). A similar cargo-scaffold interaction could occur at the spine EZ, perhaps with perisynaptic mGluRs that also bind directly to the PSD scaffold homer (Brakeman et al., 1997; Tu et al., 1998; Xiao et al., 1998). In addition to cytoskeletal interactions, the most obvious contributing factor to coated-pit formation is the lipid composition of the plasma membrane. AP-2, AP180/CALM, epsins, amphiphysin, endophilins, and dynamin all interact with phosphatidylinositol-4-5 bisphosphate, PI(4,5)P2 (Traub, 2003; Legendre-Guillemin et al., 2004; Wenk and De Camilli, 2004; Itoh and De Camilli, 2006). Does the spine membrane somehow maintain a region of high PI(4,5)P2, which ultimately recruits the EZ, and if so, how does this lipid microdo-main become established adjacent to the PSD?
Cholesterol/sphingolipid-rich lipid rafts exert important control over endocytosis and surface targeting of glutamate receptors (Hering et al., 2003; Hou et al., 2008b). AMPA receptors associate with mobile lipid rafts on the cell surface, and this association is controlled by NMDA receptor activation (Hou et al., 2008b). Interestingly, lipid raft depletion results in elevated AMPA receptor endocytosis and decreased AMPA receptor exocytosis (Hering et al., 2003; Hou et al., 2008b), indicating that the positioning of lipid rafts could lead to tight spatial control of receptor insertion and removal, an effect that could be especially important within dendritic spines to alter the abundance of synaptic AMPA receptors. It remains unknown how surface diffusion of glutamate receptors is affected by interactions with lipid rafts and what role lipid rafts play in the lateral exchange of synaptic glutamate receptors.
A major remaining question is the role of the EZ in synaptic plasticity. Clathrin-mediated endocytosis is required for downregulation of AMPA receptors and expression of LTD (Carroll et al., 1999b; Beattie et al., 2000; Man et al., 2000). In fact, perturbing endocytosis by dynamin inhibition leads to increased AMPA receptor currents, while blocking exocytosis leads to decreased AMPA receptor-mediated currents (Luscher et al., 2000). This led to a simple idea that exocytosis increases AMPA receptors for LTP, and endocytosis downregulates receptors for LTD. However, these experiments have relied on global inhibition of exo/endocytosis throughout the cell; it is not clear whether the location of receptor endocytosis leads to different outcomes for the expression of plasticity. Given the finding that a loss of spine EZs decreases synaptic AMPA receptor levels (Lu et al., 2007), an obvious question is whether LTP or LTD can occur at synapses that lack an adjacent EZ (Figure 5).
Once internalized, AMPA receptors and NMDA receptors can be sorted in endosomes for either recycling or degradation (Kennedy and Ehlers, 2006; Lau and Zukin, 2007; Shepherd and Huganir, 2007) (Figure 5). Differential AMPA receptor sorting is dependent upon the type of receptor activated. Stimulation of AMPA receptors alone leads to their downregulation, whereas activation of NMDA receptors initially leads to AMPA receptor endocytosis (10 min) and later recycling back to the cell surface (30 min) (Ehlers, 2000). It is not clear how this sorting occurs, although the phosphorylation state of the receptor may be an important factor. For example, dephosphorylation of serine 845 of GluR1 is associated with LTD and may be the sorting signal that leads to lysosomal degradation (Lee et al., 1998; Ehlers, 2000), while phosphorylation of GluR1 at serine 845 accompanies reinsertion into the spine membrane via trafficking from recycling endosomes (Ehlers, 2000; Lee et al., 2000; Esteban et al., 2003). Following induction of LTD, Ca2+-dependent activation of Rab5 leads to the formation of AMPA receptor-containing endocytic vesicles, likely derived from the EZ, which are then delivered to the early endosome (Brown et al., 2005) (Figure 5). GluR1 dephosphorylation also occurs following Rab5 activation, which could provide the sorting signal needed for GluR1 delivery to lysosomes. Therefore, expression of LTD requires activation of a Rab5-dependent sorting pathway and AMPA receptor dephosphorylation. It remains unknown whether AMPA receptors destined for recycling or degradation are internalized through the spine EZ.
The EZ is typically visualized as a single discrete patch of clathrin adjacent to the PSD (Blanpied et al., 2002; Lu et al., 2007). An interesting possibility is that the trafficking or lateral diffusion of receptors in spines is inherently directed, polarized, or channeled due to the asymmetric positioning of the EZ relative to the PSD. If the EZ acts as a polarized center for receptor endocytosis (Blanpied et al., 2002; Lu et al., 2007) and spine-localized recycling endosomes direct the exocytosis of cargo at distinct spine membrane domains (Park et al., 2006), this could establish a gradient for receptor movement in spines (Figure 5). Such a polarized cargo delivery system in spines could produce specific entry and exit sites within the PSD. Elucidation of the exact sites of receptor exocytosis, which is still debated, will be an important step in the evaluation of this model. Indeed, whereas cargo from recycling endosomes can be directly exocytosed in spines (Park et al., 2006), AMPA receptors can either accumulate in spines (Kopec et al., 2006) or be exocytosed in the dendritic shaft (Yudowski et al., 2007).
Glutamate Receptor Exocytosis and Synaptic Incorporation
It remains unknown whether there are dedicated sites of glutamate receptor exocytosis or whether these events occur randomly. The spatial positioning of exocytic sites could be important to control the rate at which receptors enter into synapses, as receptors inserted more closely to the PSD would encounter fewer obstacles preventing diffusion into the PSD. Not only would receptor insertion near the PSD be important for the timing of plasticity induction, but spine-localized insertion events could target receptors to individual spines for input-specific plasticity. Conversely, random insertion of receptors into all sites throughout the cell could contribute to global changes in the available pool of extrasynaptic receptors. Several different approaches have been used to map the locations of AMPA receptor and recycling endosome cargo insertion. One method employed thrombin cleavage of exogenously expressed HA-tagged AMPA receptors (Passafaro et al., 2001), allowing temporal and spatial mapping of newly inserted receptors (Rosenberg et al., 2001), although not in real time. Using the thrombin cleavage assay, GluR2 insertion and synaptic accumulation occurred more quickly than GluR1 insertion (Passafaro et al., 2001). Consistent with electrophysiological studies (Shi et al., 2001), swapping the C-terminal tails of GluR1 and GluR2 conferred the corresponding trafficking properties to the other subunit, indicating that C-terminal domains dictate insertion kinetics and synaptic targeting, presumably by directing the sorting of receptors to specific intracellular compartments or by interactions with scaffolding proteins.
Pharmacological approaches have also been used to track the location of receptor insertion. Using a photoreactive irreversible antagonist of AMPA receptors to rapidly and focally block surface AMPA receptors, the time course of receptor insertion was measured (Adesnik et al., 2005). Fast exchange of intracellular receptors with surface receptors occurred within the soma, but very little synaptic exchange was detected. SEP-tagging of glutamate receptors has also been used to visualize insertion of AMPA receptors into the spine membrane and dendrites, demonstrating differences in the insertion rates of distinct glutamate receptor subunits (Kopec et al., 2006; Yudowski et al., 2007). Under basal conditions SEP-tagged GluR2, NR2B, and NR2A have higher synaptic enrichment than GluR1. Following induction of chemical LTP, both SEP-tagged GluR1 and GluR2 subunits accumulate at the spine surface, whereas NMDA receptor subunit levels do not change (Kopec et al., 2006). However, these experiments did not directly visualize exocytic events, and thus the exact sites of exocytosis could not be determined.
Rapid time-lapse imaging of SEP-GluR1 exocytosis demonstrated that local insertion of GluR1 AMPA receptors in the dendritic shaft is important to target receptors to the spine membrane (Yudowski et al., 2007). SEP-GluR1 exocytosis occurs under basal conditions in the somata and dendrites, and potentiating stimuli increase the frequency of these individual exocytic events. Some exocytic events involved membrane insertion of GluR1 and rapid lateral dispersion, which subsequently led to a short-lived increase of surface GluR1 within 2–5 μm of the insertion site (Yudowski et al., 2007). Other persistent events were characterized by slow lateral dispersion of GluR1 following exocytosis, which led to a less obvious increase in local surface receptor concentration (Yudowski et al., 2007). Importantly, the transient insertion events in dendritic shafts were able to supply nearby spines by short-range lateral diffusion. Therefore, even in the absence of direct spine insertion, receptors can still enter into nearby synapses from dendritic shafts. Direct GluR1 insertion events were not detected in spines (Yudowski et al., 2007), although similar stimuli evoke robust spine exocytosis from recycling endosomes (Park et al., 2006). The finding that newly inserted GluR2 accumulates at synapses faster than GluR1 (Passafaro et al., 2001) suggests that AMPA receptors with different subunit compositions may be inserted in different locations. An important caveat here is that it is not clear whether heterologously expressed AMPA receptor subunits, which are typically homomers and in the case of GluR1 signal differently due to high Ca2+-permeability, undergo intracellular trafficking that differs from endogenous heteromeric receptors. Indeed, the high rate of synthesis of recombinant receptors expressed from foreign expression plasmids would favor cargo loading in the biosynthetic secretory pathway far in excess of that for endogenous receptors, whose rate of synthesis is much lower. Nonetheless, local exocytosis of AMPA receptors in dendrites or spines coupled with short-range diffusion to the postsynaptic membrane provides a general basis for short-range targeting of AMPA receptor to synapses.
Perhaps the strongest evidence for spine-localized exocytosis is the exocytic trafficking from dendritic recycling endosomes (Park et al., 2004, 2006) (Figure 5). The translocation of recycling endosomes into spines correlates with spine enlargement and spine-localized exocytosis (Park et al., 2006), and blocking recycling endosome transport reduces insertion of synaptic GluR1 for LTP (Park et al., 2004). Furthermore, time-lapse imaging of transferrin receptor (TfR)-SEP, which populates recycling endosomes at steady state, shows a rapid insertion of recycling endosome cargo into the spine membrane (Park et al., 2006). Vesicles derived from recycling endosomes may be targeted to the postsynaptic membrane by the exocyst (Gerges et al., 2006). In addition to mobilized recycling endosomes, endosomal compartments are also found in the dendritic shaft and extend between several spines (Cooney et al., 2002), suggesting that a single endosome could deliver cargo to multiple spines. The mechanisms responsible for sorting endosomal cargo traffic between neighboring spines remain to be determined.
Chemically induced LTP leads to increased mobilization of recycling endosomes into spines and exocytosis of endosomal cargo during spine growth (Park et al., 2004, 2006). These findings provide an attractive explanation for how spines increase size and AMPA receptor content concomitantly during the expression of LTP (Matsuzaki et al., 2004). However, given the differences in the rate of insertion and targeting of GluR1 and GluR2 into synapses (Passafaro et al., 2001), it is possible that AMPA receptor subunits are sorted through different endosomes or undergo sorting within endosomes. Studies on exogenous homomeric AMPA receptors have found large differences in the endosomal sorting of GluR1 and GluR2 (Lee et al., 2004), although these differences were not seen with endogenous receptors (Ehlers, 2000). Notably, endocytic cycling of AMPA receptors is intact in neurons from mutant mice lacking both GluR2 and GluR3 (Biou et al., 2008), suggesting that these two subunits are not absolutely required for endosomal trafficking.
Do recycling endosomes contain both GluR1 and GluR2 AMPA receptor subunits to the same degree, or do different populations of endosomes carry different receptor subunits? Recent evidence indicates that a single spine may contain multiple endosomal pathways with distinct trafficking functions. One population of endosomes is Rab11 dependent and transports AMPA receptors into synapses (Brown et al., 2007), consistent with the observation that Rab11-dependent endosomes deliver GluR1 AMPA receptors for expression of LTP (Park et al., 2004). An additional class of endosomes controlled by Rab4 mediates constitutive recycling within spines (Brown et al., 2007). These findings are consistent with the morphological heterogeneity of recycling endosomes, tubules, and vesicles observed in dendrites and spines (Cooney et al., 2002; Park et al., 2006). Taken together, these data suggest that dendritic spines are highly complex autonomous units, which contain many of the trafficking compartments needed to maintain spine morphology, receptor targeting, and synapse function. Despite these advances and the potential involvement of distinct classes of motor proteins (Setou et al., 2002; Wu et al., 2002; Hoogenraad et al., 2005; Osterweil et al., 2005; Lise et al., 2006; Correia et al., 2008), there remains a fundamental lack of information on the molecular machinery that transports endosomal compartments and their cargo in spines and the mechanisms by which such machinery is activated or modified during synaptic plasticity.
Integrating Models for Glutamate Receptor Trafficking and Diffusion during Synaptic Plasticity
Many different trafficking events contribute to synaptic glutamate receptor abundance. How do these pathways converge in a cooperative manner for synaptic plasticity? As mentioned above, Ca2+ fluxes control the dynamics of endocytosis, exocytosis, and lateral diffusion. In presynaptic terminals, Ca2+ influx controls the assembly dynamics of clathrin-coated pits and endocytic adaptors via regulation of phosphorylation and dephosphorylation (Nichols et al., 1994; Bauerfeind et al., 1997; Marks and McMahon, 1998; Slepnev et al., 1998; Lai et al., 1999; Cousin and Robinson, 2001; Tan et al., 2003; Anggono et al., 2006). Ca2+-dependent activation of calcineurin leads to the dephosphorylation of multiple endocytic proteins that drive synaptic vesicle recycling. Most prominently, dynamin-1 undergoes calcineurin-dependent dephosphorylation at serines 774 and 778 during synaptic vesicle endocytosis (Tan et al., 2003), and this dephosphorylation increases its association with the F-BAR protein syndapin-1 (Anggono et al., 2006). Rephosphorylation of dynamin-1 at these same sites by Cdk5 sustains the cycle of phosphorylation and dephosphorylation and is required for synaptic vesicle endocytosis (Tan et al., 2003; Tomizawa et al., 2003). It is tempting to speculate that a similar series of events could occur during endocytosis in dendritic spines. Calcineurin activity is required for expression of LTD and endocytosis of AMPA receptors (Beattie et al., 2000; Ehlers, 2000). It is possible that dephosphorylation of postsynaptic cargo and adaptors that regulate EZ dynamics is controlled by synaptic activity and Ca2+ levels. Although dynamin-1 is almost exclusively presynaptic, the related dynamin-3 contains conserved phosphorylation sites (Graham et al., 2007) and is concentrated in dendritic spines where it is required for spatial coupling of the EZ to the PSD (Lu et al., 2007). Moreover, syndapin-1/PACSIN-1, which associates with dynamin-1 in a phosphorylation-regulated manner in presynaptic terminals (Anggono et al., 2006), is also found in postsynaptic compartments (Perez-Otano et al., 2006). Postsynaptic exocytosis is also enhanced by influx of Ca2+ driven by potentiating stimuli (Maletic-Savatic et al., 1998; Park et al., 2006). Although the SNARE-dependent exocytosis of synaptic vesicles in presynaptic terminals evoked by Ca2+ requires synaptotagmin (Yoshihara and Littleton, 2002; Rizo et al., 2006), the exact targets of Ca2+ regulation for spine exocytosis have yet to be determined.
As Ca2+ entry into spines can potentially drive both endocytosis and exocytosis, how are the relative rates of each balanced to express long-term potentiation and depression? Presumably, LTD effectors such as calcineurin are activated by smaller but more sustained calcium transients than the targets of AMPA receptor exocytosis activated during LTP. Moreover, it is likely that, much like AMPA receptors (Lee et al., 2000), the core machinery for endocytosis and exocytosis is itself a reversible target for kinase and phosphatase signaling during LTP and LTD. Indeed, LTD-inducing stimuli activate the endocytic Rab family GTPase Rab5 (Brown et al., 2005), whereas LTP-inducing stimuli augment generalized endosomal recycling (Park et al., 2004) (Figure 5). Thus, although both types of trafficking machinery are regulated by Ca2+, the magnitude and kinetics of stimulation will ultimately determine which types of trafficking events are activated, and this activation will likely be reflected by a change in the rates of ongoing endocytosis or exocytosis or by the precise spatial location of membrane trafficking events. In addition, because exocytosis and endocytosis are not thought to occur immediately within the PSD, scaffold proteins within the PSD must also release or accept receptors in response to synaptic activity, whose exchange rate at the PSD could easily be influenced by tuning the available pool of extrasynaptic receptors through simple mass action.
In the case of LTD-inducing stimuli, endocytosis of extrasynaptic receptors would lessen the extrasynaptic pool and decrease the number of free receptors that could enter synapses, eventually leading to depletion of synaptic receptors. In addition, increased mobility of synaptic receptors driven by reversible chemical interactions would lead to decreased synaptic AMPA receptors and reduced dwell times at synapses (Figure 5). In the case of LTP induction, increased AMPA receptor membrane insertion along with decreased mobility of synaptic receptors could lead to receptor accumulation and increased dwell times at synapses (Figure 5). Further, activity-dependent regulation of the number of available scaffold binding sites (or “slots”) or their affinity for AMPA receptors may be the key gatekeepers of synaptic receptor content, whose effects would also be amplified through molecular crowding (Lisman and Raghavachari, 2006).
Similar to experimental data (Luscher et al., 1999), mathematical modeling suggests that blocking endocytosis will double the PSD AMPA receptor number within 1 hr, while blocking exocytosis halves synaptic AMPA receptors levels in less than 10 min (Earnshaw and Bressloff, 2006). The modeling also found that, during expression of plasticity, GluR1 insertion alone could not account for the increase of synaptic receptors that occurs during LTP. Additional mechanisms, such as increasing the number of PSD scaffold positions and synaptic affinity, would also be required. Modeling of LTD expression led to a similar conclusion, where decreases in synaptic receptor levels were only achieved in the long term if the number of synaptic slot positions in the PSD were also decreased (Earnshaw and Bressloff, 2006). One possibility is that receptor-scaffold complexes may be added coordinately, which would allow for large-scale changes in receptor abundance (Lisman and Raghavachari, 2006). Thus, both exocytosis of AMPA receptors and addition of receptor-binding proteins may contribute to the increase of GluR1-containing AMPA receptors that occurs during LTP. Such analysis also emphasizes the importance of receptor density (rather than absolute number) and nanopositioning as potential determinants of synaptic strength. Given the very low affinity of AMPA receptors for glutamate, the precise positioning or packing of AMPA receptors relative to presynaptic release sites may impact synaptic strength.
Conclusions and Remaining Questions
Advances in imaging technology now permit direct analysis of glutamate receptor movements in the plasma membrane. Besides supporting earlier findings demonstrating receptor exchange at synapses, these techniques have also allowed a glimpse of receptor behavior inside synapses. The relatively few types of receptors characterized by SPT have provided an expanded understanding of glutamate receptor behavior. In future studies, it will be important to analyze the mobility of the remaining glutamate receptors. In addition, other postsynaptic membrane proteins, such as adhesion molecules and ion channels that control plasticity, have yet to be analyzed in detail. Multiprobe imaging experiments or high-density single-molecule imaging techniques would allow simultaneous tracking of individual receptors in a whole population (Manley et al., 2008), providing a better understanding of how individual receptor movements reflect the behavior of the population. In addition, developing smaller probes to label receptors would facilitate tracking of single receptors as they undergo diffusion, endocytosis, and exocytosis (Lasne et al., 2006). Combined tracking of receptor lateral mobility and intracellular membrane trafficking will give a greater understanding of the dynamic movements of glutamate receptors and allow for visualization of receptor cycling in individual spines. Moreover, smaller probes have the advantage of increased synaptic access, which will allow for a more accurate and thorough analysis of synaptic receptor interactions.
At the interface of lateral diffusion and intracellular trafficking is spine endocytosis and exocytosis. We have only scratched the surface of possible functions of the spine endocytic zone (EZ). Are specific receptors excluded from EZ internalization? How does plasticity induction change the function and activity of the EZ? Does the spine EZ trap glutamate receptors? Does the size or position of the EZ affect trapping? Does the EZ provide a reserve pool of receptors under conditions where receptor-binding sites in the PSD, or slots, have become saturated? In addition, how often do such receptor slots change in the PSD and will it be possible to label free slots? What are the signaling pathways that regulate scaffold-receptor binding properties? Finally, is there a nanoarchitecture within the PSD that concentrates or rarefies receptors within synaptic subdomains, or perhaps generates receptor entry and exit points?
Even less is clear regarding the exocytosis of glutamate receptors. Where does such exocytosis occur? How is it regulated? What is the relevant trafficking machinery, and how does it interface with plasticity signaling? What is the spatial distribution of exocytic sources and endocytic sinks for diffusible pools of extrasynaptic glutamate receptors at individual synapses or across dendritic segments? Which intracellular compartments transport various types of AMPA and NMDA receptors?
Filling in these large gaps will move us closer to a more complete cell-biological and biophysical understanding of postsynaptic plasticity. Given the ever-expanding appreciation of glutamate receptor trafficking in the development and plasticity of diverse neural circuits, the pathological plasticity that occurs during addiction (Nestler, 2002; Lau and Zukin, 2007), neurobehavioral disorders including autism and mental retardations (Pardo and Eberhart, 2007), chronic pain syndromes (Bleakman et al., 2006), the diminished synaptic function during aging and early Alzheimer’s disease (Almeida et al., 2005; Chang et al., 2006; Hsieh et al., 2006; Lau and Zukin, 2007; Shankar et al., 2007), the neural damage accompanying stroke and brain injury (Hardingham et al., 2002), and the altered states of mood and perception in depression and schizophrenia (Tsai and Coyle, 2002; Lau and Zukin, 2007), uncovering the molecular mechanisms underlying glutamate receptor mobility promises to unleash new insight into how the brain develops computes, stores, and suffers.
Acknowledgments
We thank Benjamin Arenkial, Daniel Choquet, Ian Davison, Cyril Hanus, Juliet Hernandez, Matthew Kennedy, Angela Mabb, Sridhar Raghavachari, Zhiping Wang, Richard Weinberg, and Ryohei Yasuda for critical review of the manuscript. Work in the lab of M.D.E. is supported by grants from the NIH. M.D.E. is an Investigator of the Howard Hughes Medical Institute. T.M.N. is supported by an NRSA postdoctoral fellowship from the NIH.
References
- Adesnik H, Nicoll RA, England PM. Photoinactivation of native AMPA receptors reveals their real-time trafficking. Neuron. 2005;48:977–985. doi: 10.1016/j.neuron.2005.11.030. [DOI] [PubMed] [Google Scholar]
- Akaaboune M, Culican SM, Turney SG, Lichtman JW. Rapid and reversible effects of activity on acetylcholine receptor density at the neuromuscular junction in vivo. Science. 1999;286:503–507. doi: 10.1126/science.286.5439.503. [DOI] [PubMed] [Google Scholar]
- Akaaboune M, Grady RM, Turney S, Sanes JR, Lichtman JW. Neurotransmitter receptor dynamics studied in vivo by reversible photounbinding of fluorescent ligands. Neuron. 2002;34:865–876. doi: 10.1016/s0896-6273(02)00739-0. [DOI] [PubMed] [Google Scholar]
- Allison DW, Gelfand VI, Spector I, Craig AM. Role of actin in anchoring postsynaptic receptors in cultured hippocampal neurons: differential attachment of NMDA versus AMPA receptors. J Neurosci. 1998;18:2423–2436. doi: 10.1523/JNEUROSCI.18-07-02423.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison DW, Chervin AS, Gelfand VI, Craig AM. Postsynaptic scaffolds of excitatory and inhibitory synapses in hippocampal neurons: maintenance of core components independent of actin filaments and microtubules. J Neurosci. 2000;20:4545–4554. doi: 10.1523/JNEUROSCI.20-12-04545.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida CG, Tampellini D, Takahashi RH, Greengard P, Lin MT, Snyder EM, Gouras GK. Beta-amyloid accumulation in APP mutant neurons reduces PSD-95 and GluR1 in synapses. Neurobiol Dis. 2005;20:187–198. doi: 10.1016/j.nbd.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Anderson MJ, Cohen MW. Nerve-induced and spontaneous redistribution of acetylcholine receptors on cultured muscle cells. J Physiol. 1977;268:757–773. doi: 10.1113/jphysiol.1977.sp011880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrasfalvy BK, Magee JC. Distance-dependent increase in AMPA receptor number in the dendrites of adult hippocampal CA1 pyramidal neurons. J Neurosci. 2001;21:9151–9159. doi: 10.1523/JNEUROSCI.21-23-09151.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anggono V, Smillie KJ, Graham ME, Valova VA, Cousin MA, Robinson PJ. Syndapin I is the phosphorylation-regulated dynamin I partner in synaptic vesicle endocytosis. Nat Neurosci. 2006;9:752–760. doi: 10.1038/nn1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki C, Venkatesan C, Go CG, Mong JA, Dawson TM. Cellular and subcellular localization of NMDA-R1 subunit immunoreactivity in the visual cortex of adult and neonatal rats. J Neurosci. 1994;14:5202–5222. doi: 10.1523/JNEUROSCI.14-09-05202.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashby MC, De La Rue SA, Ralph GS, Uney J, Collingridge GL, Henley JM. Removal of AMPA receptors (AMPARs) from synapses is preceded by transient endocytosis of extrasynaptic AMPARs. J Neurosci. 2004;24:5172–5176. doi: 10.1523/JNEUROSCI.1042-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashby MC, Maier SR, Nishimune A, Henley JM. Lateral diffusion drives constitutive exchange of AMPA receptors at dendritic spines and is regulated by spine morphology. J Neurosci. 2006;26:7046–7055. doi: 10.1523/JNEUROSCI.1235-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod D, Ravdin PM, Podleski TR. Control of acetylcholine receptor mobility and distribution in cultured muscle membranes. A fluorescence study. Biochim Biophys Acta. 1978;511:23–38. doi: 10.1016/0005-2736(78)90062-7. [DOI] [PubMed] [Google Scholar]
- Barria A, Malinow R. Subunit-specific NMDA receptor trafficking to synapses. Neuron. 2002;35:345–353. doi: 10.1016/s0896-6273(02)00776-6. [DOI] [PubMed] [Google Scholar]
- Bats C, Groc L, Choquet D. The interaction between Stargazin and PSD-95 regulates AMPA receptor surface trafficking. Neuron. 2007;53:719–734. doi: 10.1016/j.neuron.2007.01.030. [DOI] [PubMed] [Google Scholar]
- Baude A, Nusser Z, Roberts JD, Mulvihill E, McIlhinney RA, Somogyi P. The metabotropic glutamate receptor (mGluR1 alpha) is concentrated at perisynaptic membrane of neuronal subpopulations as detected by immunogold reaction. Neuron. 1993;11:771–787. doi: 10.1016/0896-6273(93)90086-7. [DOI] [PubMed] [Google Scholar]
- Baude A, Molnar E, Latawiec D, McIlhinney RA, Somogyi P. Synaptic and nonsynaptic localization of the GluR1 subunit of the AMPA-type excitatory amino acid receptor in the rat cerebellum. J Neurosci. 1994;14:2830–2843. doi: 10.1523/JNEUROSCI.14-05-02830.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baude A, Nusser Z, Molnar E, McIlhinney RA, Somogyi P. High-resolution immunogold localization of AMPA type glutamate receptor subunits at synaptic and non-synaptic sites in rat hippocampus. Neuroscience. 1995;69:1031–1055. doi: 10.1016/0306-4522(95)00350-r. [DOI] [PubMed] [Google Scholar]
- Bauerfeind R, Takei K, De Camilli P. Amphiphysin I is associated with coated endocytic intermediates and undergoes stimulation-dependent dephosphorylation in nerve terminals. J Biol Chem. 1997;272:30984–30992. doi: 10.1074/jbc.272.49.30984. [DOI] [PubMed] [Google Scholar]
- Beattie EC, Carroll RC, Yu X, Morishita W, Yasuda H, von Zastrow M, Malenka RC. Regulation of AMPA receptor endocytosis by a signaling mechanism shared with LTD. Nat Neurosci. 2000;3:1291–1300. doi: 10.1038/81823. [DOI] [PubMed] [Google Scholar]
- Beique JC, Lin DT, Kang MG, Aizawa H, Takamiya K, Huganir RL. Synapse-specific regulation of AMPA receptor function by PSD-95. Proc Natl Acad Sci USA. 2006;103:19535–19540. doi: 10.1073/pnas.0608492103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellone C, Nicoll RA. Rapid bidirectional switching of synaptic NMDA receptors. Neuron. 2007;55:779–785. doi: 10.1016/j.neuron.2007.07.035. [DOI] [PubMed] [Google Scholar]
- Berg DK, Hall ZW. Loss of alpha-bungarotoxin from junctional and extrajunctional acetylcholine receptors in rat diaphragm muscle in vivo and in organ culture. J Physiol. 1975;252:771–789. doi: 10.1113/jphysiol.1975.sp011169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biou V, Bhattacharyya S, Malenka RC. Endocytosis and recycling of AMPA receptors lacking GluR2/3. Proc Natl Acad Sci USA. 2008;105:1038–1043. doi: 10.1073/pnas.0711412105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackstone CD, Moss SJ, Martin LJ, Levey AI, Price DL, Huganir RL. Biochemical characterization and localization of a non-N-methyl-D-aspartate glutamate receptor in rat brain. J Neurochem. 1992;58:1118–1126. doi: 10.1111/j.1471-4159.1992.tb09370.x. [DOI] [PubMed] [Google Scholar]
- Blanpied TA, Scott DB, Ehlers MD. Dynamics and regulation of clathrin coats at specialized endocytic zones of dendrites and spines. Neuron. 2002;36:435–449. doi: 10.1016/s0896-6273(02)00979-0. [DOI] [PubMed] [Google Scholar]
- Bleakman D, Alt A, Nisenbaum ES. Glutamate receptors and pain. Semin Cell Dev Biol. 2006;17:592–604. doi: 10.1016/j.semcdb.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Bloodgood BL, Sabatini BL. Neuronal activity regulates diffusion across the neck of dendritic spines. Science. 2005;310:866–869. doi: 10.1126/science.1114816. [DOI] [PubMed] [Google Scholar]
- Bogdanov Y, Michels G, Armstrong-Gold C, Haydon PG, Lindstrom J, Pangalos M, Moss SJ. Synaptic GABAA receptors are directly recruited from their extrasynaptic counterparts. EMBO J. 2006;25:4381–4389. doi: 10.1038/sj.emboj.7601309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgdorff AJ, Choquet D. Regulation of AMPA receptor lateral movements. Nature. 2002;417:649–653. doi: 10.1038/nature00780. [DOI] [PubMed] [Google Scholar]
- Bourne JN, Harris KM. Balancing structure and function at hippocampal dendritic spines. Annu Rev Neurosci. 2008 doi: 10.1146/annurev.neuro.31.060407.125646. in press. Published online February 19, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brakeman PR, Lanahan AA, O’Brien R, Roche K, Barnes CA, Huganir RL, Worley PF. Homer: a protein that selectively binds metabotropic glutamate receptors. Nature. 1997;386:284–288. doi: 10.1038/386284a0. [DOI] [PubMed] [Google Scholar]
- Bresler T, Shapira M, Boeckers T, Dresbach T, Futter M, Garner CC, Rosenblum K, Gundelfinger ED, Ziv NE. Postsynaptic density assembly is fundamentally different from presynaptic active zone assembly. J Neurosci. 2004;24:1507–1520. doi: 10.1523/JNEUROSCI.3819-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressloff PC, Earnshaw BA. Diffusion-trapping model of receptor trafficking in dendrites. Phys Rev E Stat Nonlin Soft Matter Phys. 2007;75:041915. doi: 10.1103/PhysRevE.75.041915. [DOI] [PubMed] [Google Scholar]
- Brown TC, Tran IC, Backos DS, Esteban JA. NMDA receptor-dependent activation of the small GTPase Rab5 drives the removal of synaptic AMPA receptors during hippocampal LTD. Neuron. 2005;45:81–94. doi: 10.1016/j.neuron.2004.12.023. [DOI] [PubMed] [Google Scholar]
- Brown TC, Correia SS, Petrok CN, Esteban JA. Functional compartmentalization of endosomal trafficking for the synaptic delivery of AMPA receptors during long-term potentiation. J Neurosci. 2007;27:13311–13315. doi: 10.1523/JNEUROSCI.4258-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin RK, Grab DJ, Cohen RS, Siekevitz P. Isolation and characterization of postsynaptic densities from various brain regions: enrichment of different types of postsynaptic densities. J Cell Biol. 1980;86:831–845. doi: 10.1083/jcb.86.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmignoto G, Vicini S. Activity-dependent decrease in NMDA receptor responses during development of the visual cortex. Science. 1992;258:1007–1011. doi: 10.1126/science.1279803. [DOI] [PubMed] [Google Scholar]
- Carroll RC, Beattie EC, Xia H, Luscher C, Altschuler Y, Nicoll RA, Malenka RC, von Zastrow M. Dynamin-dependent endocytosis of ionotropic glutamate receptors. Proc Natl Acad Sci USA. 1999a;96:14112–14117. doi: 10.1073/pnas.96.24.14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll RC, Lissin DV, von Zastrow M, Nicoll RA, Malenka RC. Rapid redistribution of glutamate receptors contributes to long-term depression in hippocampal cultures. Nat Neurosci. 1999b;2:454–460. doi: 10.1038/8123. [DOI] [PubMed] [Google Scholar]
- Chang EH, Savage MJ, Flood DG, Thomas JM, Levy RB, Mahadomrongkul V, Shirao T, Aoki C, Huerta PT. AMPA receptor down-scaling at the onset of Alzheimer’s disease pathology in double knockin mice. Proc Natl Acad Sci USA. 2006;103:3410–3415. doi: 10.1073/pnas.0507313103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charrier C, Ehrensperger MV, Dahan M, Levi S, Triller A. Cytoskeleton regulation of glycine receptor number at synapses and diffusion in the plasma membrane. J Neurosci. 2006;26:8502–8511. doi: 10.1523/JNEUROSCI.1758-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Chetkovich DM, Petralia RS, Sweeney NT, Kawasaki Y, Wenthold RJ, Bredt DS, Nicoll RA. Stargazin regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature. 2000;408:936–943. doi: 10.1038/35050030. [DOI] [PubMed] [Google Scholar]
- Chen L, El-Husseini A, Tomita S, Bredt DS, Nicoll RA. Stargazin differentially controls the trafficking of alpha-amino-3-hydroxyl-5-methyl-4-isoxazolepropionate and kainate receptors. Mol Pharmacol. 2003;64:703–706. doi: 10.1124/mol.64.3.703. [DOI] [PubMed] [Google Scholar]
- Chen X, Vinade L, Leapman RD, Petersen JD, Nakagawa T, Phillips TM, Sheng M, Reese TS. Mass of the postsynaptic density and enumeration of three key molecules. Proc Natl Acad Sci USA. 2005;102:11551–11556. doi: 10.1073/pnas.0505359102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Lagerholm BC, Yang B, Jacobson K. Methods to measure the lateral diffusion of membrane lipids and proteins. Methods. 2006;39:147–153. doi: 10.1016/j.ymeth.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Chen X, Winters C, Azzam R, Li X, Galbraith JA, Leapman RD, Reese TS. Organization of the core structure of the postsynaptic density. Proc Natl Acad Sci USA. 2008;105:4453–4458. doi: 10.1073/pnas.0800897105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng D, Hoogenraad CC, Rush J, Ramm E, Schlager MA, Duong DM, Xu P, Wijayawardana SR, Hanfelt J, Nakagawa T, et al. Relative and absolute quantification of postsynaptic density proteome isolated from rat forebrain and cerebellum. Mol Cell Proteomics. 2006;5:1158–1170. doi: 10.1074/mcp.D500009-MCP200. [DOI] [PubMed] [Google Scholar]
- Chetkovich DM, Chen L, Stocker TJ, Nicoll RA, Bredt DS. Phosphorylation of the postsynaptic density-95 (PSD-95)/discs large/zona occludens-1 binding site of stargazin regulates binding to PSD-95 and synaptic targeting of AMPA receptors. J Neurosci. 2002;22:5791–5796. doi: 10.1523/JNEUROSCI.22-14-05791.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choquet D, Triller A. The role of receptor diffusion in the organization of the postsynaptic membrane. Nat Rev Neurosci. 2003;4:251–265. doi: 10.1038/nrn1077. [DOI] [PubMed] [Google Scholar]
- Chung HJ, Xia J, Scannevin RH, Zhang X, Huganir RL. Phosphorylation of the AMPA receptor subunit GluR2 differentially regulates its interaction with PDZ domain-containing proteins. J Neurosci. 2000;20:7258–7267. doi: 10.1523/JNEUROSCI.20-19-07258.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HJ, Steinberg JP, Huganir RL, Linden DJ. Requirement of AMPA receptor GluR2 phosphorylation for cerebellar long-term depression. Science. 2003;300:1751–1755. doi: 10.1126/science.1082915. [DOI] [PubMed] [Google Scholar]
- Cooney JR, Hurlburt JL, Selig DK, Harris KM, Fiala JC. Endosomal compartments serve multiple hippocampal dendritic spines from a widespread rather than a local store of recycling membrane. J Neurosci. 2002;22:2215–2224. doi: 10.1523/JNEUROSCI.22-06-02215.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia SS, Bassani S, Brown TC, Lise MF, Backos DS, El-Husseini A, Passafaro M, Esteban JA. Motor protein-dependent transport of AMPA receptors into spines during long-term potentiation. Nat Neurosci. 2008;11:457–466. doi: 10.1038/nn2063. [DOI] [PubMed] [Google Scholar]
- Cousin MA, Robinson PJ. The dephosphins: dephosphorylation by calcineurin triggers synaptic vesicle endocytosis. Trends Neurosci. 2001;24:659–665. doi: 10.1016/s0166-2236(00)01930-5. [DOI] [PubMed] [Google Scholar]
- Cuadra AE, Kuo SH, Kawasaki Y, Bredt DS, Chetkovich DM. AMPA receptor synaptic targeting regulated by stargazin interactions with the Golgi-resident PDZ protein nPIST. J Neurosci. 2004;24:7491–7502. doi: 10.1523/JNEUROSCI.1255-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahan M, Levi S, Luccardini C, Rostaing P, Riveau B, Triller A. Diffusion dynamics of glycine receptors revealed by single-quantum dot tracking. Science. 2003;302:442–445. doi: 10.1126/science.1088525. [DOI] [PubMed] [Google Scholar]
- Dakoji S, Tomita S, Karimzadegan S, Nicoll RA, Bredt DS. Interaction of transmembrane AMPA receptor regulatory proteins with multiple membrane associated guanylate kinases. Neuropharmacology. 2003;45:849–856. doi: 10.1016/s0028-3908(03)00267-3. [DOI] [PubMed] [Google Scholar]
- Das S, Sasaki YF, Rothe T, Premkumar LS, Takasu M, Crandall JE, Dikkes P, Conner DA, Rayudu PV, Cheung W, et al. Increased NMDA current and spine density in mice lacking the NMDA receptor subunit NR3A. Nature. 1998;393:377–381. doi: 10.1038/30748. [DOI] [PubMed] [Google Scholar]
- Daw MI, Chittajallu R, Bortolotto ZA, Dev KK, Duprat F, Henley JM, Collingridge GL, Isaac JT. PDZ proteins interacting with C-terminal GluR2/3 are involved in a PKC-dependent regulation of AMPA receptors at hippocampal synapses. Neuron. 2000;28:873–886. doi: 10.1016/s0896-6273(00)00160-4. [DOI] [PubMed] [Google Scholar]
- Derkach VA, Oh MC, Guire ES, Soderling TR. Regulatory mechanisms of AMPA receptors in synaptic plasticity. Nat Rev Neurosci. 2003;8:101–113. doi: 10.1038/nrn2055. [DOI] [PubMed] [Google Scholar]
- Dityatev A, Schachner M. The extracellular matrix and synapses. Cell Tissue Res. 2006;326:647–654. doi: 10.1007/s00441-006-0217-1. [DOI] [PubMed] [Google Scholar]
- Dong H, O’Brien RJ, Fung ET, Lanahan AA, Worley PF, Huganir RL. GRIP: a synaptic PDZ domain-containing protein that interacts with AMPA receptors. Nature. 1997;386:279–284. doi: 10.1038/386279a0. [DOI] [PubMed] [Google Scholar]
- Earnshaw BA, Bressloff PC. Biophysical model of AMPA receptor trafficking and its regulation during long-term potentiation/long-term depression. J Neurosci. 2006;26:12362–12373. doi: 10.1523/JNEUROSCI.3601-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers MD. Reinsertion or degradation of AMPA receptors determined by activity-dependent endocytic sorting. Neuron. 2000;28:511–525. doi: 10.1016/s0896-6273(00)00129-x. [DOI] [PubMed] [Google Scholar]
- Ehlers MD. Activity level controls postsynaptic composition and signaling via the ubiquitin-proteasome system. Nat Neurosci. 2003;6:231–242. doi: 10.1038/nn1013. [DOI] [PubMed] [Google Scholar]
- Ehlers MD, Heine M, Groc L, Lee MC, Choquet D. Diffusional trapping of GluR1 AMPA receptors by input-specific synaptic activity. Neuron. 2007;54:447–460. doi: 10.1016/j.neuron.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich I, Malinow R. Postsynaptic density 95 controls AMPA receptor incorporation during long-term potentiation and experience-driven synaptic plasticity. J Neurosci. 2004;24:916–927. doi: 10.1523/JNEUROSCI.4733-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich I, Klein M, Rumpel S, Malinow R. PSD-95 is required for activity-driven synapse stabilization. Proc Natl Acad Sci USA. 2007;104:4176–4181. doi: 10.1073/pnas.0609307104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich M, Boll W, Van Oijen A, Hariharan R, Chandran K, Nibert ML, Kirchhausen T. Endocytosis by random initiation and stabilization of clathrin-coated pits. Cell. 2004;118:591–605. doi: 10.1016/j.cell.2004.08.017. [DOI] [PubMed] [Google Scholar]
- El-Husseini AE, Schnell E, Chetkovich DM, Nicoll RA, Bredt DS. PSD-95 involvement in maturation of excitatory synapses. Science. 2000;290:1364–1368. [PubMed] [Google Scholar]
- Esteban JA, Shi SH, Wilson C, Nuriya M, Huganir RL, Malinow R. PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nat Neurosci. 2003;6:136–143. doi: 10.1038/nn997. [DOI] [PubMed] [Google Scholar]
- Fertuck HC, Salpeter MM. Quantitation of junctional and extra-junctional acetylcholine receptors by electron microscope autoradiography after 125I-alpha-bungarotoxin binding at mouse neuromuscular junctions. J Cell Biol. 1976;69:144–158. doi: 10.1083/jcb.69.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer M, Kaech S, Knutti D, Matus A. Rapid actin-based plasticity in dendritic spines. Neuron. 1998;20:847–854. doi: 10.1016/s0896-6273(00)80467-5. [DOI] [PubMed] [Google Scholar]
- Franks KM, Stevens CF, Sejnowski TJ. Independent sources of quantal variability at single glutamatergic synapses. J Neurosci. 2003;23:3186–3195. doi: 10.1523/JNEUROSCI.23-08-03186.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman HV, Bresler T, Garner CC, Ziv NE. Assembly of new individual excitatory synapses: time course and temporal order of synaptic molecule recruitment. Neuron. 2000;27:57–69. doi: 10.1016/s0896-6273(00)00009-x. [DOI] [PubMed] [Google Scholar]
- Gaertner TR, Kolodziej SJ, Wang D, Kobayashi R, Koomen JM, Stoops JK, Waxham MN. Comparative analyses of the three-dimensional structures and enzymatic properties of alpha, beta, gamma and delta isoforms of Ca2+-calmodulin-dependent protein kinase II. J Biol Chem. 2004;279:12484–12494. doi: 10.1074/jbc.M313597200. [DOI] [PubMed] [Google Scholar]
- Gardner SM, Takamiya K, Xia J, Suh JG, Johnson R, Yu S, Huganir RL. Calcium-permeable AMPA receptor plasticity is mediated by subunit-specific interactions with PICK1 and NSF. Neuron. 2005;45:903–915. doi: 10.1016/j.neuron.2005.02.026. [DOI] [PubMed] [Google Scholar]
- Gerges NZ, Backos DS, Rupasinghe CN, Spaller MR, Esteban JA. Dual role of the exocyst in AMPA receptor targeting and insertion into the postsynaptic membrane. EMBO J. 2006;25:1623–1634. doi: 10.1038/sj.emboj.7601065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham ME, Anggono V, Bache N, Larsen MR, Craft GE, Robinson PJ. The in vivo phosphorylation sites of rat brain dynamin I. J Biol Chem. 2007;282:14695–14707. doi: 10.1074/jbc.M609713200. [DOI] [PubMed] [Google Scholar]
- Gray NW, Fourgeaud L, Huang B, Chen J, Cao H, Oswald BJ, Hemar A, McNiven MA. Dynamin 3 is a component of the postsynapse, where it interacts with mGluR5 and Homer. Curr Biol. 2003;13:510–515. doi: 10.1016/s0960-9822(03)00136-2. [DOI] [PubMed] [Google Scholar]
- Gray NW, Kruchten AE, Chen J, McNiven MA. A dynamin-3 spliced variant modulates the actin/cortactin-dependent morphogenesis of dendritic spines. J Cell Sci. 2005;118:1279–1290. doi: 10.1242/jcs.01711. [DOI] [PubMed] [Google Scholar]
- Gray NW, Weimer RM, Bureau I, Svoboda K. Rapid redistribution of synaptic PSD-95 in the neocortex in vivo. PLoS Biol. 2006;4:e370. doi: 10.1371/journal.pbio.0040370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greger IH, Ziff EB, Penn AC. Molecular determinants of AMPA receptor subunit assembly. Trends Neurosci. 2007;30:407–416. doi: 10.1016/j.tins.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Groc L, Heine M, Cognet L, Brickley K, Stephenson FA, Lounis B, Choquet D. Differential activity-dependent regulation of the lateral mobilities of AMPA and NMDA receptors. Nat Neurosci. 2004;7:695–696. doi: 10.1038/nn1270. [DOI] [PubMed] [Google Scholar]
- Groc L, Heine M, Cousins SL, Stephenson FA, Lounis B, Cognet L, Choquet D. NMDA receptor surface mobility depends on NR2A–2B subunits. Proc Natl Acad Sci USA. 2006;103:18769–18774. doi: 10.1073/pnas.0605238103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groc L, Choquet D, Stephenson FA, Verrier D, Manzoni OJ, Chavis P. NMDA receptor surface trafficking and synaptic subunit composition are developmentally regulated by the extracellular matrix protein Reelin. J Neurosci. 2007a;27:10165–10175. doi: 10.1523/JNEUROSCI.1772-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groc L, Lafourcade M, Heine M, Renner M, Racine V, Sibarita JB, Lounis B, Choquet D, Cognet L. Surface trafficking of neurotransmitter receptor: comparison between single-molecule/quantum dot strategies. J Neurosci. 2007b;27:12433–12437. doi: 10.1523/JNEUROSCI.3349-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulley RL, Reese TS. Cytoskeletal organization at the postsynaptic complex. J Cell Biol. 1981;91:298–302. doi: 10.1083/jcb.91.1.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley JG, Henley JM. PICK1 is a calcium-sensor for NMDA-induced AMPA receptor trafficking. EMBO J. 2005;24:3266–3278. doi: 10.1038/sj.emboj.7600801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham GE, Bading H. Coupling of extrasynaptic NMDA receptors to a CREB shut-off pathway is developmentally regulated. Biochim Biophys Acta. 2002;1600:148–153. doi: 10.1016/s1570-9639(02)00455-7. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci. 2002;5:405–414. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- Harms KJ, Tovar KR, Craig AM. Synapse-specific regulation of AMPA receptor subunit composition by activity. J Neurosci. 2005;25:6379–6388. doi: 10.1523/JNEUROSCI.0302-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KM, Stevens JK. Dendritic spines of CA 1 pyramidal cells in the rat hippocampus: serial electron microscopy with reference to their biophysical characteristics. J Neurosci. 1989;9:2982–2997. doi: 10.1523/JNEUROSCI.09-08-02982.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KM, Jensen FE, Tsao B. Three-dimensional structure of dendritic spines and synapses in rat hippocampus (CA1) at postnatal day 15 and adult ages: implications for the maturation of synaptic physiology and long-term potentiation. J Neurosci. 1992;12:2685–2705. doi: 10.1523/JNEUROSCI.12-07-02685.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AZ, Pettit DL. Extrasynaptic And Synaptic NMDARs Form Stable And Uniform Pools In Hippocampal Slices. J Neurophysiol. 2007;99:524–533. [Google Scholar]
- Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science. 2000;287:2262–2267. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- He Y, Janssen WG, Vissavajjhala P, Morrison JH. Synaptic distribution of GluR2 in hippocampal GABAergic interneurons and pyramidal cells: a double-label immunogold analysis. Exp Neurol. 1998;150:1–13. doi: 10.1006/exnr.1997.6720. [DOI] [PubMed] [Google Scholar]
- He Y, Hof PR, Janssen WG, Vissavajjhala P, Morrison JH. AMPA GluR2 subunit is differentially distributed on GABAergic neurons and pyramidal cells in the macaque monkey visual cortex. Brain Res. 2001;921:60–67. doi: 10.1016/s0006-8993(01)03083-9. [DOI] [PubMed] [Google Scholar]
- Heine M, Groc L, Frischknecht R, Beique JC, Lounis B, Rumbaugh G, Huganir RL, Cognet L, Choquet D. Surface mobility of postsynaptic AMPARs tunes synaptic transmission. Science. 2008;320:201–205. doi: 10.1126/science.1152089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hering H, Lin CC, Sheng M. Lipid rafts in the maintenance of synapses, dendritic spines, and surface AMPA receptor stability. J Neurosci. 2003;23:3262–3271. doi: 10.1523/JNEUROSCI.23-08-03262.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirbec H, Francis JC, Lauri SE, Braithwaite SP, Coussen F, Mulle C, Dev KK, Coutinho V, Meyer G, Isaac JT, et al. Rapid and differential regulation of AMPA and kainate receptors at hippocampal mossy fibre synapses by PICK1 and GRIP. Neuron. 2003;37:625–638. doi: 10.1016/s0896-6273(02)01191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holcman D, Triller A. Modeling synaptic dynamics driven by receptor lateral diffusion. Biophys J. 2006;91:2405–2415. doi: 10.1529/biophysj.106.081935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogenraad CC, Milstein AD, Ethell IM, Henkemeyer M, Sheng M. GRIP1 controls dendrite morphogenesis by regulating EphB receptor trafficking. Nat Neurosci. 2005;8:906–915. doi: 10.1038/nn1487. [DOI] [PubMed] [Google Scholar]
- Hou Q, Zhang D, Jarzylo L, Huganir RL, Man HY. Homeostatic regulation of AMPA receptor expression at single hippocampal synapses. Proc Natl Acad Sci USA. 2008a;105:775–780. doi: 10.1073/pnas.0706447105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Q, Huang Y, Amato S, Snyder SH, Huganir RL, Man HY. Regulation of AMPA receptor localization in lipid rafts. Mol Cell Neurosci. 2008b doi: 10.1016/j.mcn.2008.02.010. in press. Published online March 5, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howarth M, Chinnapen DJ, Gerrow K, Dorrestein PC, Grandy MR, Kelleher NL, El-Husseini A, Ting AY. A monovalent streptavidin with a single femtomolar biotin binding site. Nat Methods. 2006;3:267–273. doi: 10.1038/NMETHXXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh H, Boehm J, Sato C, Iwatsubo T, Tomita T, Sisodia S, Malinow R. AMPAR removal underlies Abeta-induced synaptic depression and dendritic spine loss. Neuron. 2006;52:831–843. doi: 10.1016/j.neuron.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac JT. Postsynaptic silent synapses: evidence and mechanisms. Neuropharmacology. 2003;45:450–460. doi: 10.1016/s0028-3908(03)00229-6. [DOI] [PubMed] [Google Scholar]
- Isaac JT, Ashby M, McBain CJ. The role of the GluR2 subunit in AMPA receptor function and synaptic plasticity. Neuron. 2007;54:859–871. doi: 10.1016/j.neuron.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Itoh T, De Camilli P. BAR, F-BAR (EFC) and ENTH/ANTH domains in the regulation of membrane-cytosol interfaces and membrane curvature. Biochim Biophys Acta. 2006;1761:897–912. doi: 10.1016/j.bbalip.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Jin W, Ge WP, Xu J, Cao M, Peng L, Yung W, Liao D, Duan S, Zhang M, Xia J. Lipid binding regulates synaptic targeting of PICK1, AMPA receptor trafficking, and synaptic plasticity. J Neurosci. 2006;26:2380–2390. doi: 10.1523/JNEUROSCI.3503-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas P, Sakmann B. Glutamate receptor channels in isolated patches from CA1 and CA3 pyramidal cells of rat hippocampal slices. J Physiol. 1992;455:143–171. doi: 10.1113/jphysiol.1992.sp019294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastning K, Kukhtina V, Kittler JT, Chen G, Pechstein A, Enders S, Lee SH, Sheng M, Yan Z, Haucke V. Molecular determinants for the interaction between AMPA receptors and the clathrin adaptor complex AP-2. Proc Natl Acad Sci USA. 2007;104:2991–2996. doi: 10.1073/pnas.0611170104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy MJ, Ehlers MD. Organelles and trafficking machinery for postsynaptic plasticity. Annu Rev Neurosci. 2006;29:325–362. doi: 10.1146/annurev.neuro.29.051605.112808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharazia VN, Phend KD, Rustioni A, Weinberg RJ. EM co-localization of AMPA and NMDA receptor subunits at synapses in rat cerebral cortex. Neurosci Lett. 1996a;210:37–40. doi: 10.1016/0304-3940(96)12658-6. [DOI] [PubMed] [Google Scholar]
- Kharazia VN, Wenthold RJ, Weinberg RJ. GluR1-immunopositive interneurons in rat neocortex. J Comp Neurol. 1996b;368:399–412. doi: 10.1002/(SICI)1096-9861(19960506)368:3<399::AID-CNE6>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Kim CH, Chung HJ, Lee HK, Huganir RL. Interaction of the AMPA receptor subunit GluR2/3 with PDZ domains regulates hippocampal long-term depression. Proc Natl Acad Sci USA. 2001;98:11725–11730. doi: 10.1073/pnas.211132798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Futai K, Jo J, Hayashi Y, Cho K, Sheng M. Synaptic accumulation of PSD-95 and synaptic function regulated by phosphorylation of serine-295 of PSD-95. Neuron. 2007;56:488–502. doi: 10.1016/j.neuron.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Kneussel M, Loebrich S. Trafficking and synaptic anchoring of ionotropic inhibitory neurotransmitter receptors. Biol Cell. 2007;99:297–309. doi: 10.1042/BC20060120. [DOI] [PubMed] [Google Scholar]
- Kohr G. NMDA receptor function: subunit composition versus spatial distribution. Cell Tissue Res. 2006;326:439–446. doi: 10.1007/s00441-006-0273-6. [DOI] [PubMed] [Google Scholar]
- Kopec CD, Li B, Wei W, Boehm J, Malinow R. Glutamate receptor exocytosis and spine enlargement during chemically induced long-term potentiation. J Neurosci. 2006;26:2000–2009. doi: 10.1523/JNEUROSCI.3918-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornau HC, Schenker LT, Kennedy MB, Seeburg PH. Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science. 1995;269:1737–1740. doi: 10.1126/science.7569905. [DOI] [PubMed] [Google Scholar]
- Kuriu T, Inoue A, Bito H, Sobue K, Okabe S. Differential control of postsynaptic density scaffolds via actin-dependent and -independent mechanisms. J Neurosci. 2006;26:7693–7706. doi: 10.1523/JNEUROSCI.0522-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusumi A, Sako Y, Yamamoto M. Confined lateral diffusion of membrane receptors as studied by single particle tracking (nanovid microscopy). Effects of calcium-induced differentiation in cultured epithelial cells. Biophys J. 1993;65:2021–2040. doi: 10.1016/S0006-3495(93)81253-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusumi A, Nakada C, Ritchie K, Murase K, Suzuki K, Murakoshi H, Kasai RS, Kondo J, Fujiwara T. Paradigm shift of the plasma membrane concept from the two-dimensional continuum fluid to the partitioned fluid: high-speed single-molecule tracking of membrane molecules. Annu Rev Biophys Biomol Struct. 2005;34:351–378. doi: 10.1146/annurev.biophys.34.040204.144637. [DOI] [PubMed] [Google Scholar]
- Lai MM, Hong JJ, Ruggiero AM, Burnett PE, Slepnev VI, De Camilli P, Snyder SH. The calcineurin-dynamin 1 complex as a calcium sensor for synaptic vesicle endocytosis. J Biol Chem. 1999;274:25963–25966. doi: 10.1074/jbc.274.37.25963. [DOI] [PubMed] [Google Scholar]
- Landis DM, Reese TS. Cytoplasmic organization in cerebellar dendritic spines. J Cell Biol. 1983;97:1169–1178. doi: 10.1083/jcb.97.4.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasne D, Blab GA, Berciaud S, Heine M, Groc L, Choquet D, Cognet L, Lounis B. Single nanoparticle photothermal tracking (SNaPT) of 5-nm gold beads in live cells. Biophys J. 2006;91:4598–4604. doi: 10.1529/biophysj.106.089771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau CG, Zukin RS. NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat Rev Neurosci. 2007;8:413–426. doi: 10.1038/nrn2153. [DOI] [PubMed] [Google Scholar]
- Lavezzari G, McCallum J, Lee R, Roche KW. Differential binding of the AP-2 adaptor complex and PSD-95 to the C-terminus of the NMDA receptor subunit NR2B regulates surface expression. Neuropharmacology. 2003;45:729–737. doi: 10.1016/s0028-3908(03)00308-3. [DOI] [PubMed] [Google Scholar]
- Lavezzari G, McCallum J, Dewey CM, Roche KW. Subunit-specific regulation of NMDA receptor endocytosis. J Neurosci. 2004;24:6383–6391. doi: 10.1523/JNEUROSCI.1890-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HK, Kameyama K, Huganir RL, Bear MF. NMDA induces long-term synaptic depression and dephosphorylation of the GluR1 subunit of AMPA receptors in hippocampus. Neuron. 1998;21:1151–1162. doi: 10.1016/s0896-6273(00)80632-7. [DOI] [PubMed] [Google Scholar]
- Lee HK, Barbarosie M, Kameyama K, Bear MF, Huganir RL. Regulation of distinct AMPA receptor phosphorylation sites during bidirectional synaptic plasticity. Nature. 2000;405:955–959. doi: 10.1038/35016089. [DOI] [PubMed] [Google Scholar]
- Lee SH, Liu L, Wang YT, Sheng M. Clathrin adaptor AP2 and NSF interact with overlapping sites of GluR2 and play distinct roles in AMPA receptor trafficking and hippocampal LTD. Neuron. 2002;36:661–674. doi: 10.1016/s0896-6273(02)01024-3. [DOI] [PubMed] [Google Scholar]
- Lee SH, Simonetta A, Sheng M. Subunit rules governing the sorting of internalized AMPA receptors in hippocampal neurons. Neuron. 2004;43:221–236. doi: 10.1016/j.neuron.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Legendre-Guillemin V, Wasiak S, Hussain NK, Angers A, McPherson PS. ENTH/ANTH proteins and clathrin-mediated membrane budding. J Cell Sci. 2004;117:9–18. doi: 10.1242/jcs.00928. [DOI] [PubMed] [Google Scholar]
- Li JH, Wang YH, Wolfe BB, Krueger KE, Corsi L, Stocca G, Vicini S. Developmental changes in localization of NMDA receptor subunits in primary cultures of cortical neurons. Eur J Neurosci. 1998;10:1704–1715. doi: 10.1046/j.1460-9568.1998.00169.x. [DOI] [PubMed] [Google Scholar]
- Lin JW, Ju W, Foster K, Lee SH, Ahmadian G, Wyszynski M, Wang YT, Sheng M. Distinct molecular mechanisms and divergent endocytotic pathways of AMPA receptor internalization. Nat Neurosci. 2000;3:1282–1290. doi: 10.1038/81814. [DOI] [PubMed] [Google Scholar]
- Lin DT, Huganir RL. PICK1 and phosphorylation of the glutamate receptor 2 (GluR2) AMPA receptor subunit regulates GluR2 recycling after NMDA receptor-induced internalization. J Neurosci. 2007;27:13903–13908. doi: 10.1523/JNEUROSCI.1750-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lise MF, Wong TP, Trinh A, Hines RM, Liu L, Kang R, Hines DJ, Lu J, Goldenring JR, Wang YT, El-Husseini A. Involvement of myosin Vb in glutamate receptor trafficking. J Biol Chem. 2006;281:3669–3678. doi: 10.1074/jbc.M511725200. [DOI] [PubMed] [Google Scholar]
- Lisman J, Raghavachari S. A unified model of the presynaptic and postsynaptic changes during LTP at CA1 synapses. Sci STKE. 2006;2006:re11. doi: 10.1126/stke.3562006re11. [DOI] [PubMed] [Google Scholar]
- Liu SQ, Cull-Candy SG. Synaptic activity at calcium-permeable AMPA receptors induces a switch in receptor subtype. Nature. 2000;405:454–458. doi: 10.1038/35013064. [DOI] [PubMed] [Google Scholar]
- Liu SJ, Cull-Candy SG. Subunit interaction with PICK and GRIP controls Ca2+ permeability of AMPARs at cerebellar synapses. Nat Neurosci. 2005;8:768–775. doi: 10.1038/nn1468. [DOI] [PubMed] [Google Scholar]
- Lu W, Ziff EB. PICK1 interacts with ABP/GRIP to regulate AMPA receptor trafficking. Neuron. 2005;47:407–421. doi: 10.1016/j.neuron.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Lu W, Man H, Ju W, Trimble WS, MacDonald JF, Wang YT. Activation of synaptic NMDA receptors induces membrane insertion of new AMPA receptors and LTP in cultured hippocampal neurons. Neuron. 2001;29:243–254. doi: 10.1016/s0896-6273(01)00194-5. [DOI] [PubMed] [Google Scholar]
- Lu J, Helton TD, Blanpied TA, Racz B, Newpher TM, Weinberg RJ, Ehlers MD. Postsynaptic positioning of endocytic zones and AMPA receptor cycling by physical coupling of dynamin-3 to homer. Neuron. 2007;55:874–889. doi: 10.1016/j.neuron.2007.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujan R, Roberts JD, Shigemoto R, Ohishi H, Somogyi P. Differential plasma membrane distribution of metabotropic glutamate receptors mGluR1 alpha, mGluR2 and mGluR5, relative to neurotransmitter release sites. J Chem Neuroanat. 1997;13:219–241. doi: 10.1016/s0891-0618(97)00051-3. [DOI] [PubMed] [Google Scholar]
- Luscher C, Xia H, Beattie EC, Carroll RC, von Zastrow M, Malenka RC, Nicoll RA. Role of AMPA receptor cycling in synaptic transmission and plasticity. Neuron. 1999;24:649–658. doi: 10.1016/s0896-6273(00)81119-8. [DOI] [PubMed] [Google Scholar]
- Luscher C, Nicoll RA, Malenka RC, Muller D. Synaptic plasticity and dynamic modulation of the postsynaptic membrane. Nat Neurosci. 2000;3:545–550. doi: 10.1038/75714. [DOI] [PubMed] [Google Scholar]
- Magee JC, Cook EP. Somatic EPSP amplitude is independent of synapse location in hippocampal pyramidal neurons. Nat Neurosci. 2000;3:895–903. doi: 10.1038/78800. [DOI] [PubMed] [Google Scholar]
- Maletic-Savatic M, Koothan T, Malinow R. Calcium-evoked dendritic exocytosis in cultured hippocampal neurons. Part II: mediation by calcium/calmodulin-dependent protein kinase II. J Neurosci. 1998;18:6814–6821. doi: 10.1523/JNEUROSCI.18-17-06814.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man HY, Lin JW, Ju WH, Ahmadian G, Liu L, Becker LE, Sheng M, Wang YT. Regulation of AMPA receptor-mediated synaptic transmission by clathrin-dependent receptor internalization. Neuron. 2000;25:649–662. doi: 10.1016/s0896-6273(00)81067-3. [DOI] [PubMed] [Google Scholar]
- Manley S, Gillette JM, Patterson GH, Shroff H, Hess HF, Betzig E, Lippincott-Schwartz J. High-density mapping of single-molecule trajectories with photoactivated localization microscopy. Nat Methods. 2008;5:155–157. doi: 10.1038/nmeth.1176. [DOI] [PubMed] [Google Scholar]
- Marks B, McMahon HT. Calcium triggers calcineurin-dependent synaptic vesicle recycling in mammalian nerve terminals. Curr Biol. 1998;8:740–749. doi: 10.1016/s0960-9822(98)70297-0. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Blackstone CD, Levey AI, Huganir RL, Price DL. AMPA glutamate receptor subunits are differentially distributed in rat brain. Neuroscience. 1993a;53:327–358. doi: 10.1016/0306-4522(93)90199-p. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Blackstone CD, Levey AI, Huganir RL, Price DL. Cellular localizations of AMPA glutamate receptors within the basal forebrain magnocellular complex of rat and monkey. J Neurosci. 1993b;13:2249–2263. doi: 10.1523/JNEUROSCI.13-05-02249.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey PV, Johnson BE, Moult PR, Auberson YP, Brown MW, Molnar E, Collingridge GL, Bashir ZI. Differential roles of NR2A and NR2B-containing NMDA receptors in cortical long-term potentiation and long-term depression. J Neurosci. 2004;24:7821–7828. doi: 10.1523/JNEUROSCI.1697-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masugi-Tokita M, Tarusawa E, Watanabe M, Molnar E, Fujimoto K, Shigemoto R. Number and density of AMPA receptors in individual synapses in the rat cerebellum as revealed by SDS-digested freeze-fracture replica labeling. J Neurosci. 2007;27:2135–2144. doi: 10.1523/JNEUROSCI.2861-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda S, Miura E, Matsuda K, Kakegawa W, Kohda K, Watanabe M, Yuzaki M. Accumulation of AMPA receptors in autophagosomes in neuronal axons lacking adaptor protein AP-4. Neuron. 2008;57:730–745. doi: 10.1016/j.neuron.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Matsuzaki M, Ellis-Davies GC, Nemoto T, Miyashita Y, Iino M, Kasai H. Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nat Neurosci. 2001;4:1086–1092. doi: 10.1038/nn736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature. 2004;429:761–766. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister AK, Stevens CF. Nonsaturation of AMPA and NMDA receptors at hippocampal synapses. Proc Natl Acad Sci USA. 2000;97:6173–6178. doi: 10.1073/pnas.100126497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier J, Vannier C, Serge A, Triller A, Choquet D. Fast and reversible trapping of surface glycine receptors by gephyrin. Nat Neurosci. 2001;4:253–260. doi: 10.1038/85099. [DOI] [PubMed] [Google Scholar]
- Merrifield CJ, Perrais D, Zenisek D. Coupling between clathrin-coated-pit invagination, cortactin recruitment, and membrane scission observed in live cells. Cell. 2005;121:593–606. doi: 10.1016/j.cell.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Mi R, Tang X, Sutter R, Xu D, Worley P, O’Brien RJ. Differing mechanisms for glutamate receptor aggregation on dendritic spines and shafts in cultured hippocampal neurons. J Neurosci. 2002;22:7606–7616. doi: 10.1523/JNEUROSCI.22-17-07606.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalet X, Pinaud FF, Bentolila LA, Tsay JM, Doose S, Li JJ, Sundaresan G, Wu AM, Gambhir SS, Weiss S. Quantum dots for live cells, in vivo imaging, and diagnostics. Science. 2005;307:538–544. doi: 10.1126/science.1104274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michels G, Moss SJ. GABAA receptors: properties and trafficking. Crit Rev Biochem Mol Biol. 2007;42:3–14. doi: 10.1080/10409230601146219. [DOI] [PubMed] [Google Scholar]
- Mohrmann R, Kohr G, Hatt H, Sprengel R, Gottmann K. Deletion of the C-terminal domain of the NR2B subunit alters channel properties and synaptic targeting of N-methyl-D-aspartate receptors in nascent neocortical synapses. J Neurosci Res. 2002;68:265–275. doi: 10.1002/jnr.10219. [DOI] [PubMed] [Google Scholar]
- Molnar E, Baude A, Richmond SA, Patel PB, Somogyi P, McIlhinney RA. Biochemical and immunocytochemical characterization of antipeptide antibodies to a cloned GluR1 glutamate receptor subunit: cellular and subcellular distribution in the rat forebrain. Neuroscience. 1993;53:307–326. doi: 10.1016/0306-4522(93)90198-o. [DOI] [PubMed] [Google Scholar]
- Molnar E, McIlhinney RA, Baude A, Nusser Z, Somogyi P. Membrane topology of the GluR1 glutamate receptor subunit: epitope mapping by site-directed antipeptide antibodies. J Neurochem. 1994;63:683–693. doi: 10.1046/j.1471-4159.1994.63020683.x. [DOI] [PubMed] [Google Scholar]
- Mori H, Manabe T, Watanabe M, Satoh Y, Suzuki N, Toki S, Nakamura K, Yagi T, Kushiya E, Takahashi T, et al. Role of the carboxy-terminal region of the GluR epsilon2 subunit in synaptic localization of the NMDA receptor channel. Neuron. 1998;21:571–580. doi: 10.1016/s0896-6273(00)80567-x. [DOI] [PubMed] [Google Scholar]
- Morishita W, Lu W, Smith GB, Nicoll RA, Bear MF, Malenka RC. Activation of NR2B-containing NMDA receptors is not required for NMDA receptor-dependent long-term depression. Neuropharmacology. 2007;52:71–76. doi: 10.1016/j.neuropharm.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Common molecular and cellular substrates of addiction and memory. Neurobiol Learn Mem. 2002;78:637–647. doi: 10.1006/nlme.2002.4084. [DOI] [PubMed] [Google Scholar]
- Newpher TM, Smith RP, Lemmon V, Lemmon SK. In vivo dynamics of clathrin and its adaptor-dependent recruitment to the actin-based endocytic machinery in yeast. Dev Cell. 2005;9:87–98. doi: 10.1016/j.devcel.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Nichols RA, Suplick GR, Brown JM. Calcineurin-mediated protein dephosphorylation in brain nerve terminals regulates the release of glutamate. J Biol Chem. 1994;269:23817–23823. [PubMed] [Google Scholar]
- Niethammer M, Kim E, Sheng M. Interaction between the C terminus of NMDA receptor subunits and multiple members of the PSD-95 family of membrane-associated guanylate kinases. J Neurosci. 1996;16:2157–2163. doi: 10.1523/JNEUROSCI.16-07-02157.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen A, England PM. A subtype-selective, use-dependent inhibitor of native AMPA receptors. J Am Chem Soc. 2007;129:4902–4903. doi: 10.1021/ja0705801. [DOI] [PubMed] [Google Scholar]
- Nimchinsky EA, Yasuda R, Oertner TG, Svoboda K. The number of glutamate receptors opened by synaptic stimulation in single hippocampal spines. J Neurosci. 2004;24:2054–2064. doi: 10.1523/JNEUROSCI.5066-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuriya M, Huganir RL. Regulation of AMPA receptor trafficking by N-cadherin. J Neurochem. 2006;97:652–661. doi: 10.1111/j.1471-4159.2006.03740.x. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Lujan R, Laube G, Roberts JD, Molnar E, Somogyi P. Cell type and pathway dependence of synaptic AMPA receptor number and variability in the hippocampus. Neuron. 1998;21:545–559. doi: 10.1016/s0896-6273(00)80565-6. [DOI] [PubMed] [Google Scholar]
- O’Brien RJ, Xu D, Petralia RS, Steward O, Huganir RL, Worley P. Synaptic clustering of AMPA receptors by the extracellular immediate-early gene product Narp. Neuron. 1999;23:309–323. doi: 10.1016/s0896-6273(00)80782-5. [DOI] [PubMed] [Google Scholar]
- O’Brien R, Xu D, Mi R, Tang X, Hopf C, Worley P. Synaptically targeted narp plays an essential role in the aggregation of AMPA receptors at excitatory synapses in cultured spinal neurons. J Neurosci. 2002;22:4487–4498. doi: 10.1523/JNEUROSCI.22-11-04487.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh MC, Derkach VA, Guire ES, Soderling TR. Extrasynaptic membrane trafficking regulated by GluR1 serine 845 phosphorylation primes AMPA receptors for long-term potentiation. J Biol Chem. 2006;281:752–758. doi: 10.1074/jbc.M509677200. [DOI] [PubMed] [Google Scholar]
- Okabe S. Molecular anatomy of the postsynaptic density. Mol Cell Neurosci. 2007;34:503–518. doi: 10.1016/j.mcn.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Nagai T, Miyawaki A, Hayashi Y. Rapid and persistent modulation of actin dynamics regulates postsynaptic reorganization underlying bidirectional plasticity. Nat Neurosci. 2004;7:1104–1112. doi: 10.1038/nn1311. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Narayanan R, Lee SH, Murata K, Hayashi Y. The role of CaMKII as an F-actin-bundling protein crucial for maintenance of dendritic spine structure. Proc Natl Acad Sci USA. 2007;104:6418–6423. doi: 10.1073/pnas.0701656104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osten P, Khatri L, Perez JL, Kohr G, Giese G, Daly C, Schulz TW, Wensky A, Lee LM, Ziff EB. Mutagenesis reveals a role for ABP/GRIP binding to GluR2 in synaptic surface accumulation of the AMPA receptor. Neuron. 2000;27:313–325. doi: 10.1016/s0896-6273(00)00039-8. [DOI] [PubMed] [Google Scholar]
- Osterweil E, Wells DG, Mooseker MS. A role for myosin VI in postsynaptic structure and glutamate receptor endocytosis. J Cell Biol. 2005;168:329–338. doi: 10.1083/jcb.200410091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panicker S, Brown K, Nicoll RA. Synaptic AMPA receptor subunit trafficking is independent of the C terminus in the GluR2-lacking mouse. Proc Natl Acad Sci USA. 2008;105:1032–1037. doi: 10.1073/pnas.0711313105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo CA, Eberhart CG. The neurobiology of autism. Brain Pathol. 2007;17:434–447. doi: 10.1111/j.1750-3639.2007.00102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M, Penick EC, Edwards JG, Kauer JA, Ehlers MD. Recycling endosomes supply AMPA receptors for LTP. Science. 2004;305:1972–1975. doi: 10.1126/science.1102026. [DOI] [PubMed] [Google Scholar]
- Park M, Salgado JM, Ostroff L, Helton TD, Robinson CG, Harris KM, Ehlers MD. Plasticity-induced growth of dendritic spines by exocytic trafficking from recycling endosomes. Neuron. 2006;52:817–830. doi: 10.1016/j.neuron.2006.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passafaro M, Piech V, Sheng M. Subunit-specific temporal and spatial patterns of AMPA receptor exocytosis in hippocampal neurons. Nat Neurosci. 2001;4:917–926. doi: 10.1038/nn0901-917. [DOI] [PubMed] [Google Scholar]
- Peng J, Kim MJ, Cheng D, Duong DM, Gygi SP, Sheng M. Semiquantitative proteomic analysis of rat forebrain postsynaptic density fractions by mass spectrometry. J Biol Chem. 2004;279:21003–21011. doi: 10.1074/jbc.M400103200. [DOI] [PubMed] [Google Scholar]
- Perez-Otano I, Ehlers MD. Learning from NMDA receptor trafficking: clues to the development and maturation of glutamatergic synapses. Neurosignals. 2004;13:175–189. doi: 10.1159/000077524. [DOI] [PubMed] [Google Scholar]
- Perez-Otano I, Lujan R, Tavalin SJ, Plomann M, Modregger J, Liu XB, Jones EG, Heinemann SF, Lo DC, Ehlers MD. Endocytosis and synaptic removal of NR3A-containing NMDA receptors by PACSIN1/syndapin1. Nat Neurosci. 2006;9:611–621. doi: 10.1038/nn1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestronk A, Drachman DB, Griffin JW. Effects of aging on nerve sprouting and regeneration. Exp Neurol. 1980;70:65–82. doi: 10.1016/0014-4886(80)90006-0. [DOI] [PubMed] [Google Scholar]
- Peter BJ, Kent HM, Mills IG, Vallis Y, Butler PJ, Evans PR, McMahon HT. BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science. 2004;303:495–499. doi: 10.1126/science.1092586. [DOI] [PubMed] [Google Scholar]
- Petersen JD, Chen X, Vinade L, Dosemeci A, Lisman JE, Reese TS. Distribution of postsynaptic density (PSD)-95 and Ca2+/calmodulin-dependent protein kinase II at the PSD. J Neurosci. 2003;23:11270–11278. doi: 10.1523/JNEUROSCI.23-35-11270.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petralia RS, Wenthold RJ. Light and electron immunocytochemical localization of AMPA-selective glutamate receptors in the rat brain. J Comp Neurol. 1992;318:329–354. doi: 10.1002/cne.903180309. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Wenthold RJ. Immunocytochemistry of NMDA receptors. Methods Mol Biol. 1999;128:73–92. doi: 10.1385/1-59259-683-5:73. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Wang YX, Wenthold RJ. Internalization at glutamatergic synapses during development. Eur J Neurosci. 2003;18:3207–3217. doi: 10.1111/j.1460-9568.2003.03074.x. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Sans N, Wang YX, Wenthold RJ. Ontogeny of postsynaptic density proteins at glutamatergic synapses. Mol Cell Neurosci. 2005;29:436–452. doi: 10.1016/j.mcn.2005.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettit DL, Augustine GJ. Distribution of functional glutamate and GABA receptors on hippocampal pyramidal cells and interneurons. J Neurophysiol. 2000;84:28–38. doi: 10.1152/jn.2000.84.1.28. [DOI] [PubMed] [Google Scholar]
- Pettit DL, Wang SS, Gee KR, Augustine GJ. Chemical two-photon uncaging: a novel approach to mapping glutamate receptors. Neuron. 1997;19:465–471. doi: 10.1016/s0896-6273(00)80361-x. [DOI] [PubMed] [Google Scholar]
- Prybylowski K, Chang K, Sans N, Kan L, Vicini S, Wenthold RJ. The synaptic localization of NR2B-containing NMDA receptors is controlled by interactions with PDZ proteins and AP-2. Neuron. 2005;47:845–857. doi: 10.1016/j.neuron.2005.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthenveedu MA, von Zastrow M. Cargo regulates clathrin-coated pit dynamics. Cell. 2006;127:113–124. doi: 10.1016/j.cell.2006.08.035. [DOI] [PubMed] [Google Scholar]
- Qian H, Sheetz MP, Elson EL. Single particle tracking. Analysis of diffusion and flow in two-dimensional systems. Biophys J. 1991;60:910–921. doi: 10.1016/S0006-3495(91)82125-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu S, Zhao LF, Korwek KM, Weeber EJ. Differential reelin-induced enhancement of NMDA and AMPA receptor activity in the adult hippocampus. J Neurosci. 2006;26:12943–12955. doi: 10.1523/JNEUROSCI.2561-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racca C, Stephenson FA, Streit P, Roberts JD, Somogyi P. NMDA receptor content of synapses in stratum radiatum of the hippocampal CA1 area. J Neurosci. 2000;20:2512–2522. doi: 10.1523/JNEUROSCI.20-07-02512.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racz B, Blanpied TA, Ehlers MD, Weinberg RJ. Lateral organization of endocytic machinery in dendritic spines. Nat Neurosci. 2004;7:917–918. doi: 10.1038/nn1303. [DOI] [PubMed] [Google Scholar]
- Raghavachari S, Lisman JE. Properties of quantal transmission at CA1 synapses. J Neurophysiol. 2004;92:2456–2467. doi: 10.1152/jn.00258.2004. [DOI] [PubMed] [Google Scholar]
- Reits EA, Neefjes JJ. From fixed to FRAP: measuring protein mobility and activity in living cells. Nat Cell Biol. 2001;3:E145–E147. doi: 10.1038/35078615. [DOI] [PubMed] [Google Scholar]
- Richards DA, De Paola V, Caroni P, Gahwiler BH, McKinney RA. AMPA-receptor activation regulates the diffusion of a membrane marker in parallel with dendritic spine motility in the mouse hippocampus. J Physiol. 2004;558:503–512. doi: 10.1113/jphysiol.2004.062091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizo J, Chen X, Arac D. Unraveling the mechanisms of synaptotagmin and SNARE function in neurotransmitter release. Trends Cell Biol. 2006;16:339–350. doi: 10.1016/j.tcb.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Rocca DL, Martin S, Jenkins EL, Hanley JG. Inhibition of Arp2/3-mediated actin polymerization by PICK1 regulates neuronal morphology and AMPA receptor endocytosis. Nat Cell Biol. 2008;10:259–271. doi: 10.1038/ncb1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche KW, Standley S, McCallum J, Dune Ly C, Ehlers MD, Wenthold RJ. Molecular determinants of NMDA receptor internalization. Nat Neurosci. 2001;4:794–802. doi: 10.1038/90498. [DOI] [PubMed] [Google Scholar]
- Rodriguez MA, Pesold C, Liu WS, Kriho V, Guidotti A, Pappas GD, Costa E. Colocalization of integrin receptors and reelin in dendritic spine postsynaptic densities of adult nonhuman primate cortex. Proc Natl Acad Sci USA. 2000;97:3550–3555. doi: 10.1073/pnas.050589797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SW, Hughes TE, Hollmann M, Gasic GP, Deneris ES, Heinemann S. The characterization and localization of the glutamate receptor subunit GluR1 in the rat brain. J Neurosci. 1991;11:2713–2724. doi: 10.1523/JNEUROSCI.11-09-02713.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M, Meier J, Triller A, Vannier C. Dynamics of glycine receptor insertion in the neuronal plasma membrane. J Neurosci. 2001;21:5036–5044. doi: 10.1523/JNEUROSCI.21-14-05036.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostaing P, Real E, Siksou L, Lechaire JP, Boudier T, Boeckers TM, Gertler F, Gundelfinger ED, Triller A, Marty S. Analysis of synaptic ultrastructure without fixative using high-pressure freezing and tomography. Eur J Neurosci. 2006;24:3463–3474. doi: 10.1111/j.1460-9568.2006.05234.x. [DOI] [PubMed] [Google Scholar]
- Rumbaugh G, Vicini S. Distinct synaptic and extrasynaptic NMDA receptors in developing cerebellar granule neurons. J Neurosci. 1999;19:10603–10610. doi: 10.1523/JNEUROSCI.19-24-10603.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saglietti L, Dequidt C, Kamieniarz K, Rousset MC, Valnegri P, Thoumine O, Beretta F, Fagni L, Choquet D, Sala C, et al. Extracellular interactions between GluR2 and N-cadherin in spine regulation. Neuron. 2007;54:461–477. doi: 10.1016/j.neuron.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Sala C, Rudolph-Correia S, Sheng M. Developmentally regulated NMDA receptor-dependent dephosphorylation of cAMP response element-binding protein (CREB) in hippocampal neurons. J Neurosci. 2000;20:3529–3536. doi: 10.1523/JNEUROSCI.20-10-03529.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala C, Piech V, Wilson NR, Passafaro M, Liu G, Sheng M. Regulation of dendritic spine morphology and synaptic function by Shank and Homer. Neuron. 2001;31:115–130. doi: 10.1016/s0896-6273(01)00339-7. [DOI] [PubMed] [Google Scholar]
- Sans N, Petralia RS, Wang YX, Blahos J, 2nd, Hell JW, Wenthold RJ. A developmental change in NMDA receptor-associated proteins at hippocampal synapses. J Neurosci. 2000a;20:1260–1271. doi: 10.1523/JNEUROSCI.20-03-01260.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sans NA, Montcouquiol ME, Raymond J. Postnatal developmental changes in AMPA and NMDA receptors in the rat vestibular nuclei. Brain Res Dev Brain Res. 2000b;123:41–52. doi: 10.1016/s0165-3806(00)00082-1. [DOI] [PubMed] [Google Scholar]
- Santamaria F, Wils S, De Schutter E, Augustine GJ. Anomalous diffusion in Purkinje cell dendrites caused by spines. Neuron. 2006;52:635–648. doi: 10.1016/j.neuron.2006.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki YF, Rothe T, Premkumar LS, Das S, Cui J, Talantova MV, Wong HK, Gong X, Chan SF, Zhang D, et al. Characterization and comparison of the NR3A subunit of the NMDA receptor in recombinant systems and primary cortical neurons. J Neurophysiol. 2002;87:2052–2063. doi: 10.1152/jn.00531.2001. [DOI] [PubMed] [Google Scholar]
- Sattler R, Xiong Z, Lu WY, MacDonald JF, Tymianski M. Distinct roles of synaptic and extrasynaptic NMDA receptors in excitotoxicity. J Neurosci. 2000;20:22–33. doi: 10.1523/JNEUROSCI.20-01-00022.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton MJ. Lateral diffusion in an archipelago. Single-particle diffusion. Biophys J. 1993;64:1766–1780. doi: 10.1016/S0006-3495(93)81548-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell E, Sizemore M, Karimzadegan S, Chen L, Bredt DS, Nicoll RA. Direct interactions between PSD-95 and stargazin control synaptic AMPA receptor number. Proc Natl Acad Sci USA. 2002;99:13902–13907. doi: 10.1073/pnas.172511199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott DB, Michailidis I, Mu Y, Logothetis D, Ehlers MD. Endocytosis and degradative sorting of NMDA receptors by conserved membrane-proximal signals. J Neurosci. 2004;24:7096–7109. doi: 10.1523/JNEUROSCI.0780-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidenman KJ, Steinberg JP, Huganir R, Malinow R. Glutamate receptor subunit 2 Serine 880 phosphorylation modulates synaptic transmission and mediates plasticity in CA1 pyramidal cells. J Neurosci. 2003;23:9220–9228. doi: 10.1523/JNEUROSCI.23-27-09220.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitanidou T, Triller A, Korn H. Distribution of glycine receptors on the membrane of a central neuron: an immunoelectron microscopy study. J Neurosci. 1988;8:4319–4333. doi: 10.1523/JNEUROSCI.08-11-04319.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serge A, Fourgeaud L, Hemar A, Choquet D. Receptor activation and homer differentially control the lateral mobility of metabotropic glutamate receptor 5 in the neuronal membrane. J Neurosci. 2002;22:3910–3920. doi: 10.1523/JNEUROSCI.22-10-03910.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setou M, Seog DH, Tanaka Y, Kanai Y, Takei Y, Kawagishi M, Hirokawa N. Glutamate-receptor-interacting protein GRIP1 directly steers kinesin to dendrites. Nature. 2002;417:83–87. doi: 10.1038/nature743. [DOI] [PubMed] [Google Scholar]
- Shankar GM, Bloodgood BL, Townsend M, Walsh DM, Selkoe DJ, Sabatini BL. Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J Neurosci. 2007;27:2866–2875. doi: 10.1523/JNEUROSCI.4970-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma K, Fong DK, Craig AM. Postsynaptic protein mobility in dendritic spines: long-term regulation by synaptic NMDA receptor activation. Mol Cell Neurosci. 2006;31:702–712. doi: 10.1016/j.mcn.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Shen K, Meyer T. Dynamic control of CaMKII translocation and localization in hippocampal neurons by NMDA receptor stimulation. Science. 1999;284:162–166. doi: 10.1126/science.284.5411.162. [DOI] [PubMed] [Google Scholar]
- Sheng M, Hoogenraad CC. The postsynaptic architecture of excitatory synapses: a more quantitative view. Annu Rev Biochem. 2007;76:823–847. doi: 10.1146/annurev.biochem.76.060805.160029. [DOI] [PubMed] [Google Scholar]
- Sheng M, Cummings J, Roldan LA, Jan YN, Jan LY. Changing subunit composition of heteromeric NMDA receptors during development of rat cortex. Nature. 1994;368:144–147. doi: 10.1038/368144a0. [DOI] [PubMed] [Google Scholar]
- Shepherd JD, Huganir RL. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu Rev Cell Dev Biol. 2007;23:613–643. doi: 10.1146/annurev.cellbio.23.090506.123516. [DOI] [PubMed] [Google Scholar]
- Shi SH, Hayashi Y, Petralia RS, Zaman SH, Wenthold RJ, Svoboda K, Malinow R. Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science. 1999;284:1811–1816. doi: 10.1126/science.284.5421.1811. [DOI] [PubMed] [Google Scholar]
- Shi S, Hayashi Y, Esteban JA, Malinow R. Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell. 2001;105:331–343. doi: 10.1016/s0092-8674(01)00321-x. [DOI] [PubMed] [Google Scholar]
- Shouval HZ, Kalantzis G. Stochastic properties of synaptic transmission affect the shape of spike time-dependent plasticity curves. J Neurophysiol. 2005;93:1069–1073. doi: 10.1152/jn.00504.2004. [DOI] [PubMed] [Google Scholar]
- Sia GM, Beique JC, Rumbaugh G, Cho R, Worley PF, Huganir RL. Interaction of the N-terminal domain of the AMPA receptor GluR4 subunit with the neuronal pentraxin NP1 mediates GluR4 synaptic recruitment. Neuron. 2007;55:87–102. doi: 10.1016/j.neuron.2007.06.020. [DOI] [PubMed] [Google Scholar]
- Siegel SJ, Brose N, Janssen WG, Gasic GP, Jahn R, Heinemann SF, Morrison JH. Regional, cellular, and ultrastructural distribution of N-methyl-D-aspartate receptor subunit 1 in monkey hippocampus. Proc Natl Acad Sci USA. 1994;91:564–568. doi: 10.1073/pnas.91.2.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JB, Restituito S, Lu W, Lee-Edwards L, Khatri L, Ziff EB. Synaptic anchorage of AMPA receptors by cadherins through neural plakophilin-related arm protein AMPA receptor-binding protein complexes. J Neurosci. 2007;27:8505–8516. doi: 10.1523/JNEUROSCI.1395-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slepnev VI, Ochoa GC, Butler MH, Grabs D, De Camilli P. Role of phosphorylation in regulation of the assembly of endocytic coat complexes. Science. 1998;281:821–824. doi: 10.1126/science.281.5378.821. [DOI] [PubMed] [Google Scholar]
- Smith MA, Ellis-Davies GC, Magee JC. Mechanism of the distance-dependent scaling of Schaffer collateral synapses in rat CA1 pyramidal neurons. J Physiol. 2003;548:245–258. doi: 10.1113/jphysiol.2002.036376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spacek J, Harris KM. Three-dimensional organization of smooth endoplasmic reticulum in hippocampal CA1 dendrites and dendritic spines of the immature and mature rat. J Neurosci. 1997;17:190–203. doi: 10.1523/JNEUROSCI.17-01-00190.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruston N, Jonas P, Sakmann B. Dendritic glutamate receptor channels in rat hippocampal CA3 and CA1 pyramidal neurons. J Physiol. 1995;482:325–352. doi: 10.1113/jphysiol.1995.sp020521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S, Osten P, Vilim FS, Khatri L, Inman G, States B, Daly C, DeSouza S, Abagyan R, Valtschanoff JG, et al. Novel anchorage of GluR2/3 to the postsynaptic density by the AMPA receptor-binding protein ABP. Neuron. 1998;21:581–591. doi: 10.1016/s0896-6273(00)80568-1. [DOI] [PubMed] [Google Scholar]
- Star EN, Kwiatkowski DJ, Murthy VN. Rapid turnover of actin in dendritic spines and its regulation by activity. Nat Neurosci. 2002;5:239–246. doi: 10.1038/nn811. [DOI] [PubMed] [Google Scholar]
- Steigerwald F, Schulz TW, Schenker LT, Kennedy MB, Seeburg PH, Kohr G. C-Terminal truncation of NR2A subunits impairs synaptic but not extrasynaptic localization of NMDA receptors. J Neurosci. 2000;20:4573–4581. doi: 10.1523/JNEUROSCI.20-12-04573.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein V, House DR, Bredt DS, Nicoll RA. Postsynaptic density-95 mimics and occludes hippocampal long-term potentiation and enhances long-term depression. J Neurosci. 2003;23:5503–5506. doi: 10.1523/JNEUROSCI.23-13-05503.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg JP, Takamiya K, Shen Y, Xia J, Rubio ME, Yu S, Jin W, Thomas GM, Linden DJ, Huganir RL. Targeted in vivo mutations of the AMPA receptor subunit GluR2 and its interacting protein PICK1 eliminate cerebellar long-term depression. Neuron. 2006;49:845–860. doi: 10.1016/j.neuron.2006.02.025. [DOI] [PubMed] [Google Scholar]
- Sugiyama Y, Kawabata I, Sobue K, Okabe S. Determination of absolute protein numbers in single synapses by a GFP-based calibration technique. Nat Methods. 2005;2:677–684. doi: 10.1038/nmeth783. [DOI] [PubMed] [Google Scholar]
- Sun L, June Liu S. Activation of extrasynaptic NMDA receptors induces a PKC-dependent switch in AMPA receptor subtypes in mouse cerebellar stellate cells. J Physiol. 2007;583:537–553. doi: 10.1113/jphysiol.2007.136788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takumi Y, Matsubara A, Rinvik E, Ottersen OP. The arrangement of glutamate receptors in excitatory synapses. Ann N Y Acad Sci. 1999a;868:474–482. doi: 10.1111/j.1749-6632.1999.tb11316.x. [DOI] [PubMed] [Google Scholar]
- Takumi Y, Ramirez-Leon V, Laake P, Rinvik E, Ottersen OP. Different modes of expression of AMPA and NMDA receptors in hippocampal synapses. Nat Neurosci. 1999b;2:618–624. doi: 10.1038/10172. [DOI] [PubMed] [Google Scholar]
- Tan TC, Valova VA, Malladi CS, Graham ME, Berven LA, Jupp OJ, Hansra G, McClure SJ, Sarcevic B, Boadle RA, et al. Cdk5 is essential for synaptic vesicle endocytosis. Nat Cell Biol. 2003;5:701–710. doi: 10.1038/ncb1020. [DOI] [PubMed] [Google Scholar]
- Tanaka J, Matsuzaki M, Tarusawa E, Momiyama A, Molnar E, Kasai H, Shigemoto R. Number and density of AMPA receptors in single synapses in immature cerebellum. J Neurosci. 2005;25:799–807. doi: 10.1523/JNEUROSCI.4256-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardin C, Cognet L, Bats C, Lounis B, Choquet D. Direct imaging of lateral movements of AMPA receptors inside synapses. EMBO J. 2003;22:4656–4665. doi: 10.1093/emboj/cdg463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terashima A, Pelkey KA, Rah JC, Suh YH, Roche KW, Collingridge GL, McBain CJ, Isaac JT. An essential role for PICK1 in NMDA receptor-dependent bidirectional synaptic plasticity. Neuron. 2008;57:872–882. doi: 10.1016/j.neuron.2008.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas CG, Miller AJ, Westbrook GL. Synaptic and extrasynaptic NMDA receptor NR2 subunits in cultured hippocampal neurons. J Neurophysiol. 2006;95:1727–1734. doi: 10.1152/jn.00771.2005. [DOI] [PubMed] [Google Scholar]
- Tomita S, Fukata M, Nicoll RA, Bredt DS. Dynamic interaction of stargazin-like TARPs with cycling AMPA receptors at synapses. Science. 2004;303:1508–1511. doi: 10.1126/science.1090262. [DOI] [PubMed] [Google Scholar]
- Tomita S, Stein V, Stocker TJ, Nicoll RA, Bredt DS. Bidirectional synaptic plasticity regulated by phosphorylation of stargazin-like TARPs. Neuron. 2005;45:269–277. doi: 10.1016/j.neuron.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Tomizawa K, Sunada S, Lu YF, Oda Y, Kinuta M, Ohshima T, Saito T, Wei FY, Matsushita M, Li ST, et al. Cophosphorylation of amphiphysin I and dynamin I by Cdk5 regulates clathrin-mediated endocytosis of synaptic vesicles. J Cell Biol. 2003;163:813–824. doi: 10.1083/jcb.200308110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar KR, Westbrook GL. The incorporation of NMDA receptors with a distinct subunit composition at nascent hippocampal synapses in vitro. J Neurosci. 1999;19:4180–4188. doi: 10.1523/JNEUROSCI.19-10-04180.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar KR, Westbrook GL. Mobile NMDA receptors at hippocampal synapses. Neuron. 2002;34:255–264. doi: 10.1016/s0896-6273(02)00658-x. [DOI] [PubMed] [Google Scholar]
- Traub LM. Sorting it out: AP-2 and alternate clathrin adaptors in endocytic cargo selection. J Cell Biol. 2003;163:203–208. doi: 10.1083/jcb.200309175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triller A, Choquet D. Surface trafficking of receptors between synaptic and extrasynaptic membranes: and yet they do move! Trends Neurosci. 2005;28:133–139. doi: 10.1016/j.tins.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Triller A, Cluzeaud F, Pfeiffer F, Betz H, Korn H. Distribution of glycine receptors at central synapses: an immunoelectron microscopy study. J Cell Biol. 1985;101:683–688. doi: 10.1083/jcb.101.2.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai G, Coyle JT. Glutamatergic mechanisms in schizophrenia. Annu Rev Pharmacol Toxicol. 2002;42:165–179. doi: 10.1146/annurev.pharmtox.42.082701.160735. [DOI] [PubMed] [Google Scholar]
- Tsuriel S, Geva R, Zamorano P, Dresbach T, Boeckers T, Gundelfinger ED, Garner CC, Ziv NE. Local sharing as a predominant determinant of synaptic matrix molecular dynamics. PLoS Biol. 2006;4:e271. doi: 10.1371/journal.pbio.0040271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu JC, Xiao B, Yuan JP, Lanahan AA, Leoffert K, Li M, Linden DJ, Worley PF. Homer binds a novel proline-rich motif and links group 1 metabotropic glutamate receptors with IP3 receptors. Neuron. 1998;21:717–726. doi: 10.1016/s0896-6273(00)80589-9. [DOI] [PubMed] [Google Scholar]
- Tu JC, Xiao B, Naisbitt S, Yuan JP, Petralia RS, Brakeman P, Doan A, Aakalu VK, Lanahan AA, Sheng M, Worley PF. Coupling of mGluR/Homer and PSD-95 complexes by the Shank family of postsynaptic density proteins. Neuron. 1999;23:583–592. doi: 10.1016/s0896-6273(00)80810-7. [DOI] [PubMed] [Google Scholar]
- Uchida N, Honjo Y, Johnson KR, Wheelock MJ, Takeichi M. The catenin/cadherin adhesion system is localized in synaptic junctions bordering transmitter release zones. J Cell Biol. 1996;135:767–779. doi: 10.1083/jcb.135.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valtschanoff JG, Weinberg RJ. Laminar organization of the NMDA receptor complex within the postsynaptic density. J Neurosci. 2001;21:1211–1217. doi: 10.1523/JNEUROSCI.21-04-01211.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberghe W, Nicoll RA, Bredt DS. Interaction with the unfolded protein response reveals a role for stargazin in biosynthetic AMPA receptor transport. J Neurosci. 2005;25:1095–1102. doi: 10.1523/JNEUROSCI.3568-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YH, Bosy TZ, Yasuda RP, Grayson DR, Vicini S, Pizzorusso T, Wolfe BB. Characterization of NMDA receptor subunit-specific antibodies: distribution of NR2A and NR2B receptor subunits in rat brain and ontogenic profile in the cerebellum. J Neurochem. 1995;65:176–183. doi: 10.1046/j.1471-4159.1995.65010176.x. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Inoue Y, Sakimura K, Mishina M. Developmental changes in distribution of NMDA receptor channel subunit mRNAs. Neuroreport. 1992;3:1138–1140. doi: 10.1097/00001756-199212000-00027. [DOI] [PubMed] [Google Scholar]
- Wenk MR, De Camilli P. Protein-lipid interactions and phosphoinositide metabolism in membrane traffic: insights from vesicle recycling in nerve terminals. Proc Natl Acad Sci USA. 2004;101:8262–8269. doi: 10.1073/pnas.0401874101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K, Russell SL, Shen YM, Molinoff PB. Developmental switch in the expression of NMDA receptors occurs in vivo and in vitro. Neuron. 1993;10:267–278. doi: 10.1016/0896-6273(93)90317-k. [DOI] [PubMed] [Google Scholar]
- Wong HK, Liu XB, Matos MF, Chan SF, Perez-Otano I, Boysen M, Cui J, Nakanishi N, Trimmer JS, Jones EG, et al. Temporal and regional expression of NMDA receptor subunit NR3A in the mammalian brain. J Comp Neurol. 2002;450:303–317. doi: 10.1002/cne.10314. [DOI] [PubMed] [Google Scholar]
- Wu H, Nash JE, Zamorano P, Garner CC. Interaction of SAP97 with minus-end-directed actin motor myosin VI. Implications for AMPA receptor trafficking. J Biol Chem. 2002;277:30928–30934. doi: 10.1074/jbc.M203735200. [DOI] [PubMed] [Google Scholar]
- Xia J, Zhang X, Staudinger J, Huganir RL. Clustering of AMPA receptors by the synaptic PDZ domain-containing protein PICK1. Neuron. 1999;22:179–187. doi: 10.1016/s0896-6273(00)80689-3. [DOI] [PubMed] [Google Scholar]
- Xia J, Chung HJ, Wihler C, Huganir RL, Linden DJ. Cerebellar long-term depression requires PKC-regulated interactions between GluR2/3 and PDZ domain-containing proteins. Neuron. 2000;28:499–510. doi: 10.1016/s0896-6273(00)00128-8. [DOI] [PubMed] [Google Scholar]
- Xiao B, Tu JC, Petralia RS, Yuan JP, Doan A, Breder CD, Ruggiero A, Lanahan AA, Wenthold RJ, Worley PF. Homer regulates the association of group 1 metabotropic glutamate receptors with multivalent complexes of homer-related, synaptic proteins. Neuron. 1998;21:707–716. doi: 10.1016/s0896-6273(00)80588-7. [DOI] [PubMed] [Google Scholar]
- Xu D, Hopf C, Reddy R, Cho RW, Guo L, Lanahan A, Petralia RS, Wenthold RJ, O’Brien RJ, Worley P. Narp and NP1 form heterocomplexes that function in developmental and activity-dependent synaptic plasticity. Neuron. 2003;39:513–528. doi: 10.1016/s0896-6273(03)00463-x. [DOI] [PubMed] [Google Scholar]
- Yao PJ, Bushlin I, Petralia RS. Partially overlapping distribution of epsin1 and HIP1 at the synapse: analysis by immunoelectron microscopy. J Comp Neurol. 2006;494:368–379. doi: 10.1002/cne.20810. [DOI] [PubMed] [Google Scholar]
- Yi Z, Petralia RS, Fu Z, Swanwick CC, Wang YX, Prybylowski K, Sans N, Vicini S, Wenthold RJ. The role of the PDZ protein GIPC in regulating NMDA receptor trafficking. J Neurosci. 2007;27:11663–11675. doi: 10.1523/JNEUROSCI.3252-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara M, Littleton JT. Synaptotagmin I functions as a calcium sensor to synchronize neurotransmitter release. Neuron. 2002;36:897–908. doi: 10.1016/s0896-6273(02)01065-6. [DOI] [PubMed] [Google Scholar]
- Young SH, Poo MM. Rapid lateral diffusion of extrajunctional acetylcholine receptors in the developing muscle membrane of Xenopus tadpole. J Neurosci. 1983;3:225–231. doi: 10.1523/JNEUROSCI.03-01-00225.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudowski GA, Puthenveedu MA, von Zastrow M. Distinct modes of regulated receptor insertion to the somatodendritic plasma membrane. Nat Neurosci. 2006;9:622–627. doi: 10.1038/nn1679. [DOI] [PubMed] [Google Scholar]
- Yudowski GA, Puthenveedu MA, Leonoudakis D, Panicker S, Thorn KS, Beattie EC, von Zastrow M. Real-time imaging of discrete exocytic events mediating surface delivery of AMPA receptors. J Neurosci. 2007;27:11112–11121. doi: 10.1523/JNEUROSCI.2465-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Cao YQ, Tsien RW. Quantum dots provide an optical signal specific to full collapse fusion of synaptic vesicles. Proc Natl Acad Sci USA. 2007;104:17843–17848. doi: 10.1073/pnas.0706906104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Peng Y, Xu Z, Chen RQ, Gu QH, Chen Z, Lu W. Synaptic metaplasticity through NMDA receptor lateral diffusion. J Neurosci. 2008;28:3060–3070. doi: 10.1523/JNEUROSCI.5450-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zhu JJ, Esteban JA, Hayashi Y, Malinow R. Postnatal synaptic potentiation: delivery of GluR4-containing AMPA receptors by spontaneous activity. Nat Neurosci. 2000;3:1098–1106. doi: 10.1038/80614. [DOI] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]