Abstract
The endogenous adrenocortical response to sepsis is critical for host survival. The in vivo interactions among the endogenous glucocorticoid response, the induction of cytokines, and host survival during endotoxemia were explored in this study by use of the glucocorticoid receptor antagonist RU 486. Male Lewis rats underwent sterile insertion of a right jugular venous catheter. After a 72-h recovery period, animals received a 50% lethal dose of Escherichia coli endotoxin (2.5 mg/kg) via the catheter after pretreatment for 30 min prior to lipopolysaccharide (LPS) treatment with (i) vehicle alone intravenously (i.v.) (-corticosterone [-Cort]/-RU 486/+LPS) (n = 10), (ii) the antiglucocorticoid RU 486 (10 mg/kg) i.v. (-Cort/+RU 486/+LPS) (n = 11), or (iii) RU 486 (10 mg/kg) i.v. in animals that had undergone subcutaneous implantation of a corticosterone pellet at the time of catheter insertion (+Cort/+RU 486/+LPS) (n = 10). Except in animals receiving corticosterone pretreatment, baseline plasma corticosterone levels were low in all groups. Plasma corticosterone levels increased significantly (P less than 0.001) above the baseline following LPS administration. Animals in the -Cort/+RU 486/+LPS-treated group exhibited significantly increased mortality (P less than 0.001), with only 9% of the animals surviving at 72 h, as well as significantly increased plasma interleukin-6 levels, compared with animals receiving the vehicle alone (-Cort/-RU 486/+LPS), which showed 50% mortality. Pretreatment with corticosterone and RU 486 (+Cort/+RU 486/+LPS) significantly (P less than 0.001) reversed the mortality observed with RU 486 pretreatment alone (-Cort/+RU 486/+LPS), with 70% of the animals surviving at 72 h, and significantly attenuated the peak plasma tumor necrosis factor and interleukin-6 responses to LPS, compared with those in the animals treated with vehicle alone. These data demonstrate that the blockade of glucocorticoid binding by RU 486 increases LPS-induced mortality. The reversal of this effect by the induction of hypercorticosteronemia prior to RU 486 and LPS exposure (+Cort/+RU 486/+LPS) improves survival and is further associated with significant attenuation of cytokine production. Therefore, these data suggest that the protective effect of the endogenous glucocorticoid response to acute endotoxemia may result from the down-regulation of a potentially lethal cytokine response.
Full text
PDF

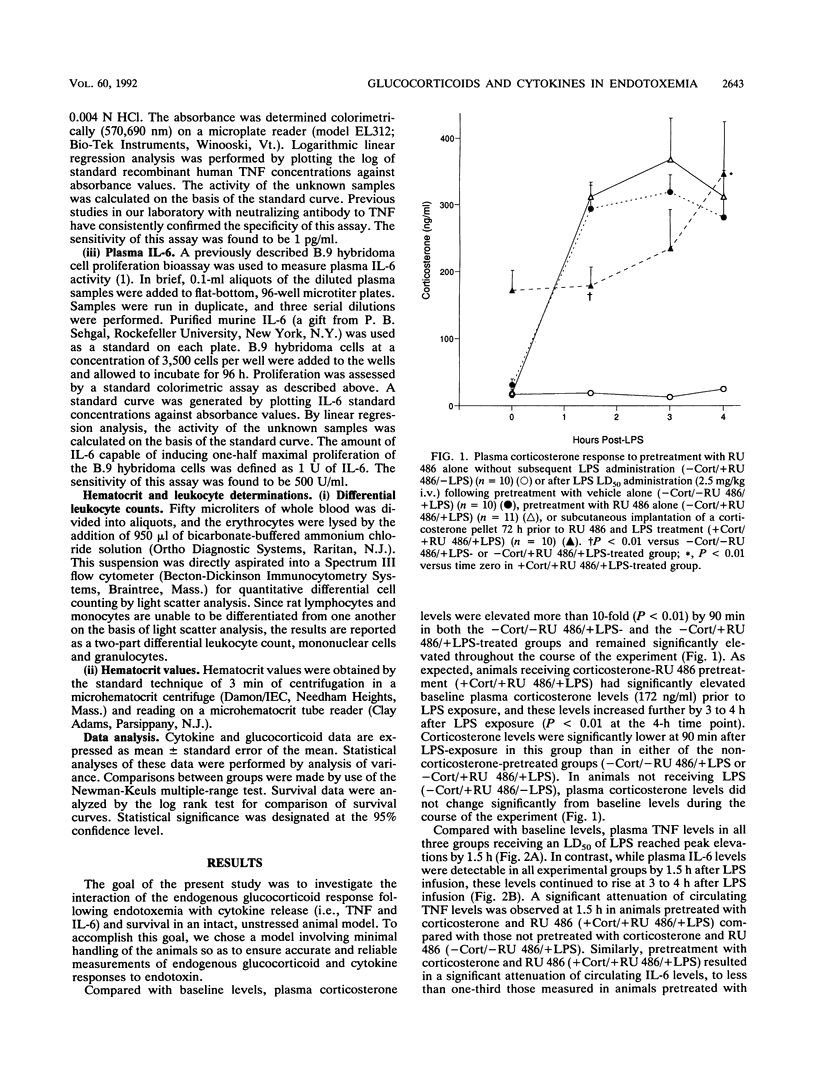
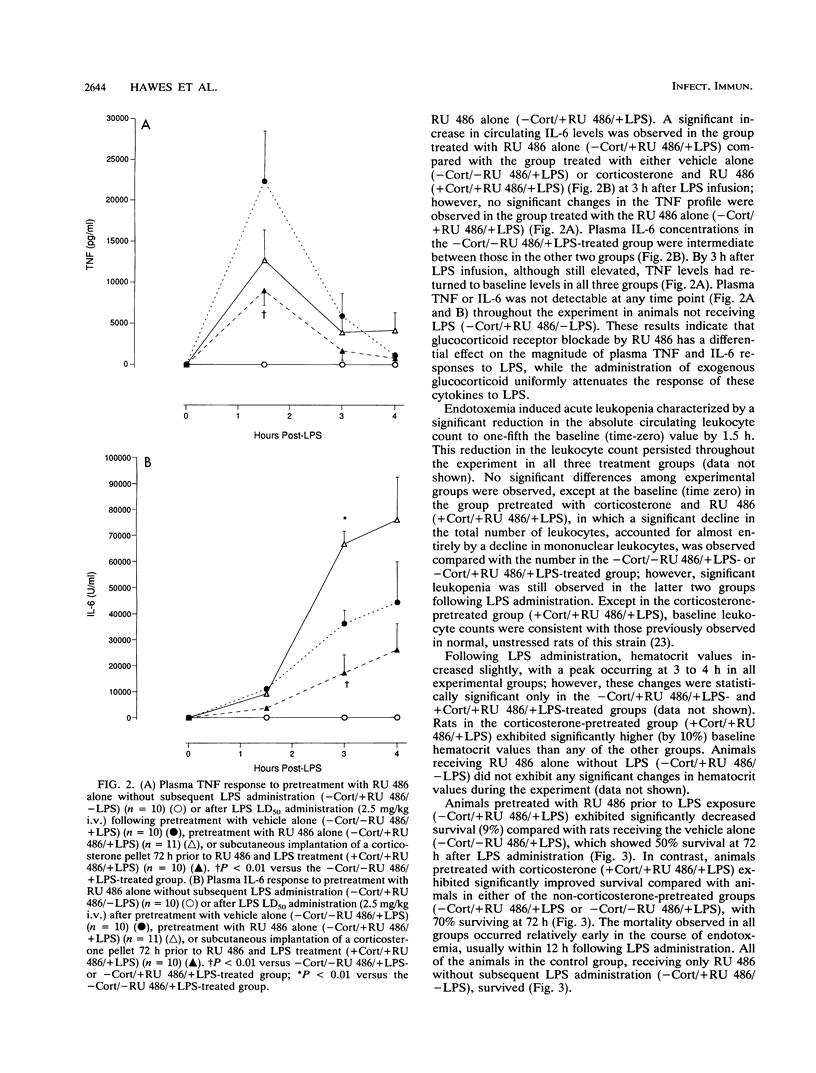
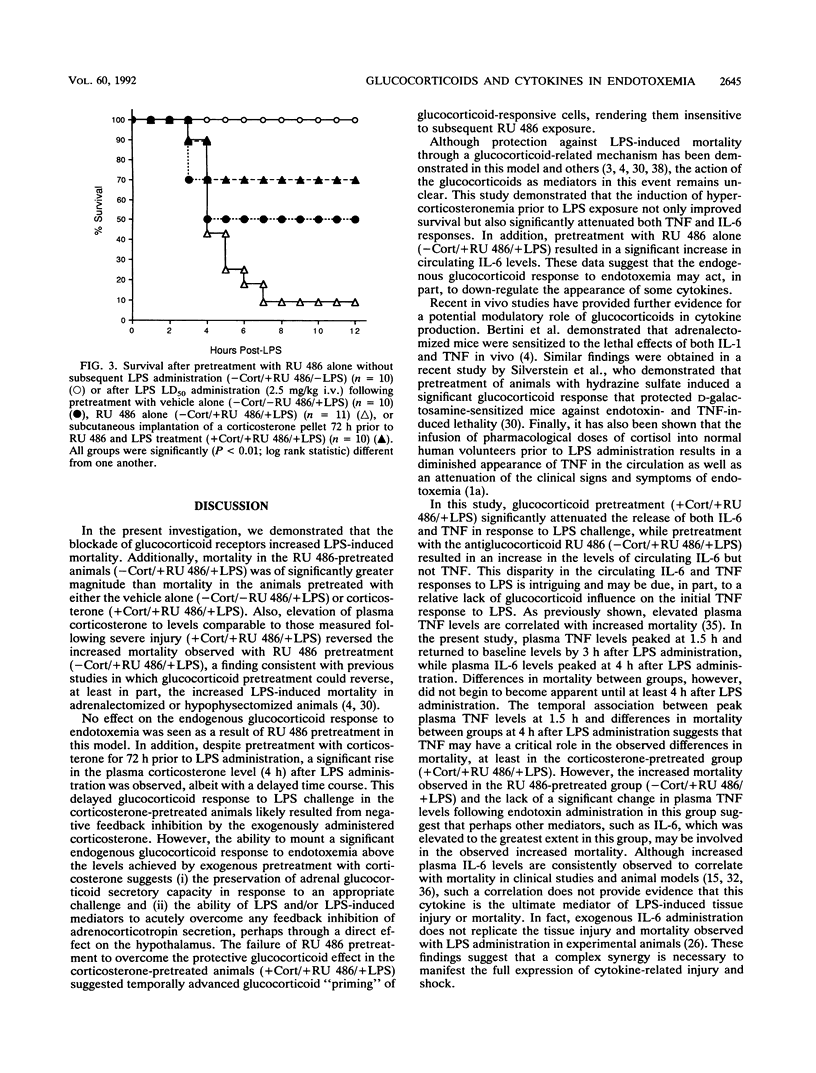


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aarden L. A., De Groot E. R., Schaap O. L., Lansdorp P. M. Production of hybridoma growth factor by human monocytes. Eur J Immunol. 1987 Oct;17(10):1411–1416. doi: 10.1002/eji.1830171004. [DOI] [PubMed] [Google Scholar]
- BERRY L. J., SMYTHE D. S. EFFECTS OF BACTERIAL ENDOTOXINS ON METABOLISM. VII. ENZYME INDUCTION AND CORTISONE PROTECTION. J Exp Med. 1964 Nov 1;120:721–732. doi: 10.1084/jem.120.5.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann H., Prowse K. R., Marinković S., Won K. A., Jahreis G. P. Stimulation of hepatic acute phase response by cytokines and glucocorticoids. Ann N Y Acad Sci. 1989;557:280-95, discussion 295-6. doi: 10.1111/j.1749-6632.1989.tb24021.x. [DOI] [PubMed] [Google Scholar]
- Bertini R., Bianchi M., Ghezzi P. Adrenalectomy sensitizes mice to the lethal effects of interleukin 1 and tumor necrosis factor. J Exp Med. 1988 May 1;167(5):1708–1712. doi: 10.1084/jem.167.5.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler B., Krochin N., Milsark I. W., Luedke C., Cerami A. Control of cachectin (tumor necrosis factor) synthesis: mechanisms of endotoxin resistance. Science. 1986 May 23;232(4753):977–980. doi: 10.1126/science.3754653. [DOI] [PubMed] [Google Scholar]
- Beutler B., Milsark I. W., Cerami A. C. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985 Aug 30;229(4716):869–871. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- Claman H. N. Corticosteroids and lymphoid cells. N Engl J Med. 1972 Aug 24;287(8):388–397. doi: 10.1056/NEJM197208242870806. [DOI] [PubMed] [Google Scholar]
- Eskandari M. K., Nguyen D. T., Kunkel S. L., Remick D. G. WEHI 164 subclone 13 assay for TNF: sensitivity, specificity, and reliability. Immunol Invest. 1990 Feb;19(1):69–79. doi: 10.3109/08820139009042026. [DOI] [PubMed] [Google Scholar]
- Fauci A. S., Dale D. C. The effect of in vivo hydrocortisone on subpopulations of human lymphocytes. J Clin Invest. 1974 Jan;53(1):240–246. doi: 10.1172/JCI107544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer E., Marano M. A., Barber A. E., Hudson A., Lee K., Rock C. S., Hawes A. S., Thompson R. C., Hayes T. J., Anderson T. D. Comparison between effects of interleukin-1 alpha administration and sublethal endotoxemia in primates. Am J Physiol. 1991 Aug;261(2 Pt 2):R442–R452. doi: 10.1152/ajpregu.1991.261.2.R442. [DOI] [PubMed] [Google Scholar]
- Floch A., Bousseau A., Hetier E., Floc'h F., Bost P. E., Cavero I. RP 55778, a PAF receptor antagonist, prevents and reverses LPS-induced hemoconcentration and TNF release. J Lipid Mediat. 1989 Nov-Dec;1(6):349–360. [PubMed] [Google Scholar]
- Fong Y. M., Marano M. A., Moldawer L. L., Wei H., Calvano S. E., Kenney J. S., Allison A. C., Cerami A., Shires G. T., Lowry S. F. The acute splanchnic and peripheral tissue metabolic response to endotoxin in humans. J Clin Invest. 1990 Jun;85(6):1896–1904. doi: 10.1172/JCI114651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong Y., Moldawer L. L., Marano M., Wei H., Tatter S. B., Clarick R. H., Santhanam U., Sherris D., May L. T., Sehgal P. B. Endotoxemia elicits increased circulating beta 2-IFN/IL-6 in man. J Immunol. 1989 Apr 1;142(7):2321–2324. [PubMed] [Google Scholar]
- Gagne D., Pons M., Philibert D. RU 38486: a potent antiglucocorticoid in vitro and in vivo. J Steroid Biochem. 1985 Sep;23(3):247–251. doi: 10.1016/0022-4731(85)90401-7. [DOI] [PubMed] [Google Scholar]
- Hack C. E., De Groot E. R., Felt-Bersma R. J., Nuijens J. H., Strack Van Schijndel R. J., Eerenberg-Belmer A. J., Thijs L. G., Aarden L. A. Increased plasma levels of interleukin-6 in sepsis. Blood. 1989 Oct;74(5):1704–1710. [PubMed] [Google Scholar]
- Jones W. G., 2nd, Barber A. E., Kapur S., Hawes A. J., Fahey T. J., 3rd, Minei J. P., Shires G. T., 3rd, Calvano S. E., Shires G. T. Pathophysiologic glucocorticoid levels and survival of translocating bacteria. Arch Surg. 1991 Jan;126(1):50–55. doi: 10.1001/archsurg.1991.01410250056009. [DOI] [PubMed] [Google Scholar]
- Kass E. H. High-dose corticosteroids for septic shock. N Engl J Med. 1984 Nov 1;311(18):1178–1179. doi: 10.1056/NEJM198411013111809. [DOI] [PubMed] [Google Scholar]
- Keith L. D., Winslow J. R., Reynolds R. W. A general procedure for estimation of corticosteroid response in individual rats. Steroids. 1978 Apr;31(4):523–531. doi: 10.1016/0039-128x(78)90034-x. [DOI] [PubMed] [Google Scholar]
- Kettelhut I. C., Fiers W., Goldberg A. L. The toxic effects of tumor necrosis factor in vivo and their prevention by cyclooxygenase inhibitors. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4273–4277. doi: 10.1073/pnas.84.12.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lähteenmäki P., Heikinheimo O., Croxatto H., Spitz I., Shoupe D., Birgerson L., Luukkainen T. Pharmacokinetics and metabolism of RU 486. J Steroid Biochem. 1987;27(4-6):859–863. doi: 10.1016/0022-4731(87)90160-9. [DOI] [PubMed] [Google Scholar]
- Mathison J. C., Wolfson E., Ulevitch R. J. Participation of tumor necrosis factor in the mediation of gram negative bacterial lipopolysaccharide-induced injury in rabbits. J Clin Invest. 1988 Jun;81(6):1925–1937. doi: 10.1172/JCI113540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michie H. R., Manogue K. R., Spriggs D. R., Revhaug A., O'Dwyer S., Dinarello C. A., Cerami A., Wolff S. M., Wilmore D. W. Detection of circulating tumor necrosis factor after endotoxin administration. N Engl J Med. 1988 Jun 9;318(23):1481–1486. doi: 10.1056/NEJM198806093182301. [DOI] [PubMed] [Google Scholar]
- Morrison D. C., Ryan J. L. Endotoxins and disease mechanisms. Annu Rev Med. 1987;38:417–432. doi: 10.1146/annurev.me.38.020187.002221. [DOI] [PubMed] [Google Scholar]
- Ohlsson K., Björk P., Bergenfeldt M., Hageman R., Thompson R. C. Interleukin-1 receptor antagonist reduces mortality from endotoxin shock. Nature. 1990 Dec 6;348(6301):550–552. doi: 10.1038/348550a0. [DOI] [PubMed] [Google Scholar]
- Preiser J. C., Schmartz D., Van der Linden P., Content J., Vanden Bussche P., Buurman W., Sebald W., Dupont E., Pinsky M. R., Vincent J. L. Interleukin-6 administration has no acute hemodynamic or hematologic effect in the dog. Cytokine. 1991 Jan;3(1):1–4. doi: 10.1016/1043-4666(91)90002-u. [DOI] [PubMed] [Google Scholar]
- Richardson R. P., Rhyne C. D., Fong Y., Hesse D. G., Tracey K. J., Marano M. A., Lowry S. F., Antonacci A. C., Calvano S. E. Peripheral blood leukocyte kinetics following in vivo lipopolysaccharide (LPS) administration to normal human subjects. Influence of elicited hormones and cytokines. Ann Surg. 1989 Aug;210(2):239–245. doi: 10.1097/00000658-198908000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal S. J. Mifepristone (RU 486) N Engl J Med. 1990 Mar 8;322(10):691–693. doi: 10.1056/NEJM199003083221009. [DOI] [PubMed] [Google Scholar]
- Silverstein R., Christoffersen C. A., Morrison D. C. Modulation of endotoxin lethality in mice by hydrazine sulfate. Infect Immun. 1989 Jul;57(7):2072–2078. doi: 10.1128/iai.57.7.2072-2078.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein R., Turley B. R., Christoffersen C. A., Johnson D. C., Morrison D. C. Hydrazine sulfate protects D-galactosamine-sensitized mice against endotoxin and tumor necrosis factor/cachectin lethality: evidence of a role for the pituitary. J Exp Med. 1991 Feb 1;173(2):357–365. doi: 10.1084/jem.173.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestre L., Dubois C., Renault M., Rezvani Y., Baulieu E. E., Ulmann A. Voluntary interruption of pregnancy with mifepristone (RU 486) and a prostaglandin analogue. A large-scale French experience. N Engl J Med. 1990 Mar 8;322(10):645–648. doi: 10.1056/NEJM199003083221001. [DOI] [PubMed] [Google Scholar]
- Starnes H. F., Jr, Pearce M. K., Tewari A., Yim J. H., Zou J. C., Abrams J. S. Anti-IL-6 monoclonal antibodies protect against lethal Escherichia coli infection and lethal tumor necrosis factor-alpha challenge in mice. J Immunol. 1990 Dec 15;145(12):4185–4191. [PubMed] [Google Scholar]
- Tracey K. J., Fong Y., Hesse D. G., Manogue K. R., Lee A. T., Kuo G. C., Lowry S. F., Cerami A. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987 Dec 17;330(6149):662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- Tracey K. J., Lowry S. F., Fahey T. J., 3rd, Albert J. D., Fong Y., Hesse D., Beutler B., Manogue K. R., Calvano S., Wei H. Cachectin/tumor necrosis factor induces lethal shock and stress hormone responses in the dog. Surg Gynecol Obstet. 1987 May;164(5):415–422. [PubMed] [Google Scholar]
- Waage A., Brandtzaeg P., Halstensen A., Kierulf P., Espevik T. The complex pattern of cytokines in serum from patients with meningococcal septic shock. Association between interleukin 6, interleukin 1, and fatal outcome. J Exp Med. 1989 Jan 1;169(1):333–338. doi: 10.1084/jem.169.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren R. S., Starnes H. F., Jr, Alcock N., Calvano S., Brennan M. F. Hormonal and metabolic response to recombinant human tumor necrosis factor in rat: in vitro and in vivo. Am J Physiol. 1988 Aug;255(2 Pt 1):E206–E212. doi: 10.1152/ajpendo.1988.255.2.E206. [DOI] [PubMed] [Google Scholar]


