Abstract
Rationale
Substance abuse is more prevalent among patients with schizophrenia than in the general population. The considerable overlap in neurobiological disruptions thought to underlie each condition suggests that addictive behavior may represent a primary symptom of schizophrenia.
Objective
This study investigated drug-seeking in a neurodevelopmental animal model of schizophrenia, the neonatal ventral hippocampal lesion (NVHL) model.
Materials and methods
At postnatal day 7, rats received an excitotoxic ventral hippocampus lesion or a sham procedure and were trained as adults to self-administer methamphetamine (0.1 mg/kg/infusion) or respond for natural reinforcement (water or food).
Results
NVHL rats were faster than shams to acquire the operant response for either drug self-administration or water reinforcement, suggesting that simple instrumental learning may be enhanced in these animals. NVHL and sham rats displayed no differences in fixed-ratio (FR) responding for either methamphetamine or food, and both groups of animals were equally sensitive to methamphetamine dose changes (0.05, 0.1, or 0.2 mg/kg/infusion). However, under a progressive-ratio (PR) schedule, NVHL animals reached significantly higher break points (NVHL 18 infusions; sham 12 infusions) for methamphetamine but not food reinforcement, suggesting enhanced motivation to acquire drug and/or elevated incentive value of the drug that did not generalize to another form of reinforcement.
Conclusions
These data indicate that developmental disruption of the hippocampus elevates rats’ vulnerability to drug-seeking behavior under PR conditions. Furthermore, drug self-administration in the NVHL animal emulates addictive behavior in schizophrenia, making this model useful for investigating the mechanisms of dual diagnosis, including the neurobiological and behavioral similarities between addiction and schizophrenia.
Keywords: Schizophrenia, Self-administration, Rat, Hippocampus, Addiction, Dual diagnosis, Comorbidity
Introduction
Patients with schizophrenia often abuse psychoactive substances, including nicotine, alcohol, cannabis, and psychostimulants (Regier et al. 1990; Selzer and Lieberman 1993; Krystal et al. 1999). Increased substance abuse among patients may represent attempts at self-medication (Khantzian 1997; Krystal et al. 1999). However, this hypothesis is not generally supported by empirical data; for example, patients’ symptoms do not predict the substance that is abused (Blanchard et al. 2000). Alternatively, both schizophrenia and substance abuse could arise from overlapping pathophysiological mechanisms conferring vulnerability to both disorders (Khantzian 1997; Chambers et al. 2001). In support of such a model, parallel dysfunctions in corticolimbic dopamine and glutamate systems are implicated in schizophrenia and addiction.
Hypofrontality has been characterized as an abnormal activation of the prefrontal cortex (PFC) in response to stimulation (e.g., increased working memory load) in schizophrenia patients (Perlstein et al. 2001; Egan et al. 2001). Abnormal prefrontal neuronal firing is also observed in multiple animal models of schizophrenia (O’Donnell et al. 2002; Jackson et al. 2004; Goto and Grace 2006; Moore et al. 2006), and PFC-dependent cognitive performance is disrupted in these animal models (Chambers et al. 1996; Moghaddam et al. 1997; Lipska and Weinberger 2000; Saul et al. 2006; Moore et al. 2006). In human drug users, PFC dysfunction is hypothesized to underlie compulsive drug-seeking behavior and impaired decision-making (Volkow et al. 2003). In animals, drug self-administration depends upon the activation of the PFC (McGregor and Roberts 1995; Park et al. 2002), and repeated psychostimulant treatment alters both the basic neurophysiological properties of PFC neurons (Trantham et al. 2002; Peterson et al. 2006) and the synaptic interactions between the PFC and nucleus accumbens (Brady et al. 2005).
In schizophrenic patients, there are also abnormalities in cellular organization and volume in the hippocampus (e.g., Szeszko et al. 2003), although these findings are somewhat inconsistent (Weinberger 1999). Drug-craving activates medial temporal lobe structures in addicted individuals (Grant et al. 1996; Kilts et al. 2001; but see Childress et al. 1999), and the hippocampus is implicated in drug-seeking behavior in animal models (Caine et al. 2001; Sun and Rebec 2003).
Finally, mesolimbic dopamine overactivity has long been associated with schizophrenia (Laruelle et al. 1996), and drugs of abuse powerfully increase dopamine transmission in the nucleus accumbens (Koob 1992). Long-term abuse of psychoactive drugs may result in repeated supraphysiological increases in accumbens dopamine transmission (Paulson and Robinson 1995), leading to sensitization of incentive salience assigned to drugs and drug-associated cues (Robinson and Berridge 2000; Volkow et al. 2003).
Elucidation of the shared neurobiological mechanisms that may underlie schizophrenia and addictive behavior requires the development of animal models that concurrently address components of both clinical conditions. The neonatal ventral hippocampal lesion (NVHL) model reproduces several behavioral abnormalities observed in schizophrenia, including hypersensitivity to stimulants, hyperactivity, reduced social interactions, and impaired working memory (Lipska and Weinberger 2000). A recent investigation demonstrated enhanced reinstatement of cocaine-seeking behavior in NVHL rats (Chambers and Self 2002). In this study, we further investigated drug-seeking behavior in the NVHL model by assessing methamphetamine self-administration under both fixed-ratio (FR) and progressive-ratio (PR) schedules of reinforcement.
Materials and methods
Subjects
Timed pregnant Sprague–Dawley females were obtained at embryonic days 15–18 from Taconic Farms (Germantown, NY, USA) and were individually housed with free access to food and water in a temperature- and humidity-controlled environment with a 12-h:12-h light/dark cycle (lights on at 7:00 A.M.). Neonatal pups were left undisturbed until postnatal day (PD) 6 or 7, when they were weighed and sex was determined. Healthy male pups received surgery on PD6–8, and behavioral testing began after PD56 (see below). These experiments were conducted in accordance with the US Public Health Service Guide for the Care and Use of Laboratory Animals, and all procedures were approved by the Institutional Animal Care and Use Committees at Albany Medical College or St. Mary’s College of Maryland.
Neonatal ventral hippocampal lesion
Between PD6 and PD8, male pups (15–20 g) received either an excitotoxic lesion of the ventral hippocampus (NVHL; n=44) or a sham procedure (n=35). Pups were anesthetized via hypothermia and secured to a modified platform placed in a stereotaxic apparatus (David Kopf, Tujunga, CA, USA), and the scalp was incised. NVHL animals received bilateral infusions (0.3 μl per side; 0.15 μl/min) of ibotenic acid (10 μg/μl in artificial CSF [aCSF]) into the ventral hippocampus, at coordinates of 3 mm posterior to bregma, 3.5 mm lateral to bregma, and 5 mm from the surface of the skull. Sham animals received bilateral infusions of aCSF into the ventral hippocampus at the same coordinates. The infusion needle was left in place for 3 min after each infusion to allow for diffusion away from the needle tip. The wound was closed with wound clips, and pups were placed on a warming pad until their respiration and activity levels had returned to normal. Pups were then returned to their mothers where they remained undisturbed except for husbandry, until the wound clips were removed at approximately PD23. At approximately PD26, animals were weaned and housed in pairs of like lesion status. Upon reaching adulthood (PD56), animals were single-housed and were handled for at least 2 days before beginning behavioral training.
Behavioral testing
Three separate groups of animals were utilized in this study. The first group (n=39; 20 NVHL, 19 sham) was trained to lever press for water reinforcement before the acquisition of methamphetamine self-administration. The second group (n=19; 10 NVHL, 9 sham) received no pretraining before acquiring methamphetamine self-administration. The third group (n=21; 14 NVHL, 7 sham) was trained to lever press for food reinforcement only and was never trained on methamphetamine self-administration. Groups 1 and 2 were trained and tested at Albany Medical College; group 3 was trained and tested at St. Mary’s College of Maryland.
Water pretraining
After handling, animals in group 1 were maintained on a 23-h water deprivation schedule with free access to food in the home cage. Acquisition of the lever-pressing behavior took place in 16-h overnight sessions in operant conditioning chambers located inside of sound-attenuating chambers (Coulbourn Instruments, Allentown, PA, USA). A lever press on either the left or right lever resulted in delivery of water (10 μl) under a fixed-ratio-1 (FR1) schedule of reinforcement. For training on all types of reinforcement, two levers were used so that endogenous side preferences would not impede acquisition of responding (e.g., Glick and Hinds 1985). Each water delivery was followed by a 10-s time-out period marked by illumination of the houselight. Overnight sessions continued until animals met the criterion of 300 presses in a single session. Animals were then further trained in daily 1-h sessions with the same parameters until they met the criterion of four consecutive days of at least 700 presses. Upon reaching criterion, animals were given free access to water for the rest of the experiment.
Catheter implantation
Animals were surgically implanted under sterile conditions with a polyethylene/silastic catheter (total volume approximately 0.03 ml), constructed according to the design of Weeks (1972), in the external jugular vein. Animals were anesthetized with ketamine (90 mg/kg, i.p.) and xylazine (6 mg/kg, i.p.), and an incision was made on the ventral surface of the neck. The external jugular vein was dissected and isolated, and the silastic portion of the catheter (4.0–4.5 cm length) was inserted caudally into the vein. The catheter was loosely sutured to the vein and to the underlying muscle tissue, and the wound was sutured closed. The remainder of the catheter (PE20 tubing) was threaded subcutaneously to exit through an incision on the dorsal midscapular region where it was sutured to the muscle and the distal end occluded with a stainless steel stylet. The dorsal wound was closed with sutures or wound clips and treated with topical antibiotic. On the second night after the day of surgery, catheterized animals began overnight acquisition of methamphetamine self-administration.
Self-administration
Animals were trained to lever press for infusions of methamphetamine (0.1 mg/kg/infusion) under an FR1 schedule in the same operant conditioning chambers used for water pretraining. Before each daily session, approximately 0.05 ml of sterile saline (0.9%) was infused into each animal’s catheter. The catheter was then connected via a stainless steel commutator to a length of Tygon tubing (I.D. 0.51 cm) connected to a liquid swivel (Instech Laboratories, Plymouth Meeting, PA, USA) inside the chamber. One noncontingent drug infusion was administered at the beginning of each daily session. A response on either lever resulted in the delivery of 50 μl of methamphetamine (in sterile saline) in 1 s, delivered by activation of a syringe pump (55-2222; Harvard Apparatus, Holliston, MA, USA) located outside of the chamber and connected to the liquid swivel via Tygon tubing. The houselight was illuminated during the duration of each infusion. After each infusion, there was a 20-s time-out period during which the levers were not active. At the conclusion of each daily session, each animal’s catheter was filled with 0.05 ml of a mixture of penicillin, heparin, and streptokinase in sterile saline. Catheters were tested for patency once a week by infusing 0.2–0.4 ml of thiopental (25 mg/ml). Animals that did not respond with complete and immediate loss of muscle tone were either recatheterized on the opposite side or removed from the experiment.
Acquisition of self-administration first took place in 16-h overnight sessions until animals met the criterion of 100 lever presses in a single session. Animals were then trained in daily 3-h sessions until they made 20 responses in a single session. Upon reaching this second criterion, animals were trained under the FR1 schedule for 10 daily 1-h sessions. A subset of animals from group 1 (n=9) was then given 20 additional sessions to establish a dose–response relationship for self-administration of methamphetamine. These animals were trained on either a lower dose (0.05 mg/kg/infusion) or a higher dose (0.2 mg/kg/infusion) for 10 days, followed by 10 days at the opposite dose. Finally, 16 animals from group 1 and 12 animals from group 2 were trained under a PR schedule where the number of required lever presses (ratio) was exponentially increased with each successive infusion (0.1 mg/kg/infusion). The PR schedule used in this study was: 1, 3, 6, 9, 12, 17, 24, 32, 42, 56, 73, 95, 124, 161, 208, 268, 346, 445, 573, 737, 948, 1,218, 1,566, etc. (Lorrain et al. 2000). The highest ratio completed before 60 min elapsed without completion of a ratio was defined as the break point. The maximum session length for PR sessions was 5 h.
Sucrose training
After handling at age PD56, animals in group 3 were gradually food-restricted (over a period of 3 days) to approximately 90% of their free weight with free access to water in the home cage. Animals were trained 6 days/week to respond on either the left or right lever for sucrose pellet reinforcers (45 mg, Noyes) under an FR1 schedule. Acquisition took place in 16-h overnight sessions until animals met the criterion of 300 lever presses in a single session. After acquisition, animals were trained under the FR1 schedule for 10 daily 30-min sessions. Animals were then trained under a PR schedule with the same schedule of successive reinforcement as described above, but with the break point defined as the highest ratio completed before 5 min elapsed without completion of a ratio and the maximum session length set at 60 min. Although these parameters differ from those used for the PR schedule of methamphetamine self-administration, they are in accordance with the literature on food-based PR testing (e.g., Mobini et al. 2000; Solinas and Goldberg 2005). In addition, as it has been suggested that different parameters be used for reinforcers that support different response patterns (Richardson and Roberts 1996), we chose to use a shorter break point criterion for food reinforcement, for which animals are likely to respond (and satiate) more quickly than for drug self-administration. Finally, a subset of sucrose-trained animals was tested for possible perseveration behaviors. Animals were given three additional daily sessions under the FR1 schedule, and each animal’s preferred lever was defined as the lever receiving greater than 50% of responses on the third day. Beginning the next day, animals were then reinforced (under an FR1 schedule) for responses only on this preferred lever until stable responding was established (95% of free response levels for three consecutive days). Animals were then switched to reinforcement (FR1) only on the nonpreferred lever until 95% of free response levels were observed for three consecutive days. Both levers were available throughout all of these perseveration sessions; responding on the inactive lever had no programmed consequences.
Lesion verification
After the completion of behavioral testing, animals were transcardially perfused with cold saline (0.9%), followed by 4% paraformaldehyde in 0.1 M phosphate buffer. Brains were removed and postfixed in 4% paraformaldehyde for at least 24 h before being transferred to 30% sucrose in 0.1 M phosphate buffer for cryoprotection. Sections (40 μm) through the dorsal and ventral hippocampus were taken using a freezing microtome, mounted on glass slides, and Nissl stained. The hippocampus was examined microscopically for evidence of bilateral damage, which typically included cell loss, thinning, enlarged ventricles, gliosis, and/or cellular disorganization. Based on previous observations of a lack of correlation between lesion size and behavioral impairments (e.g., O’Donnell et al. 2002), any observable evidence of bilateral hippocampal damage was considered acceptable. Animals with significant damage to surrounding areas were removed from the study.
Statistical analysis
Acquisition of lever pressing for water, food, and methamphetamine was analyzed using independent t tests to compare NVHL and sham animals. Postacquisition responding for water, methamphetamine, or food reinforcement was analyzed with mixed ANOVAs using factors of lesion group and session, and additional factors of dose as appropriate. The Huynh–Feldt correction for sphericity was applied to all repeated-measures analyses; uncorrected df and corrected F and p values are reported in the text. Significant findings were followed by post hoc t tests. When multiple t tests were required, the Bonferroni correction was used to adjust the alpha level. With this exception, an alpha level of 0.05 was applied to all analyses.
Results
Lesions
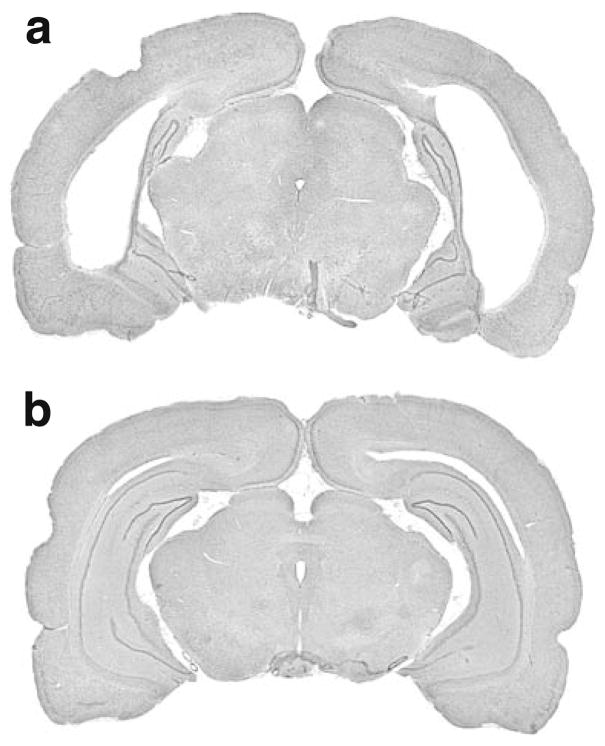
Nissl-stained hippocampal sections from NVHL animals exhibited varying degrees of cell loss, cavitation, enlarged ventricles, and cellular disorganization. A representative photomicrograph from an NVHL brain with significant cell loss is shown in Fig. 1a. Sham-treated animals showed no evidence of damage to either the hippocampus or adjacent areas (Fig. 1b). No animals from group 1 were excluded based on histological findings. From group 2, one NVHL rat was excluded due to lack of bilateral damage and one sham rat died before completing the study. From group 3, five NVHL rats were removed due to insufficient bilateral damage. Thus, the final sample sizes for each group were as follows: group 1, n=39 (20 NVHL, 19 sham); group 2, n= 17 (9 NVHL, 8 sham); group 3, n=16 (9 NVHL, 7 sham).
Fig. 1.
Representative Nissl-stained brains from NVHL (a) and sham-treated (b) animals. a Extensive cavitation and ventricular enlargement in a brain from an NVHL rat. b No damage is evident in the sham-treated brain. NVHL neonatal ventral hippocampal lesion
Acquisition of lever-pressing behavior
Water pretraining
NVHL animals in group 1 initially acquired the lever-press response for water reinforcement in fewer days (overnight sessions) than sham animals (Table 1; t37 =2.21, p=0.04). On the night on which criterion (≥300 lever presses) was reached, NVHL and sham animals made a similar number of lever presses (Table 1; p=0.41). After daily 1-h training sessions, NVHL and sham-treated animals met the next phase of water reinforcement criterion in a similar number of days (p= 0.092; NVHL, 5.6±1.3 days; sham, 4.8±1.0 days). All animals increased their responding over the last 4 days at criterion performance (F3, 69=16.53, p <0.001; session 1, 725±241 presses; session 4, 1,010±304 presses) with no differences between NVHL and sham-treated rats either overall or across days (both ps>0.46).
Table 1.
Acquisition of lever-pressing responses
| Group 1 (water/METH)
|
Group 2 (METH only)
|
Group 3 (sucrose only)
|
||||
|---|---|---|---|---|---|---|
| NVHL | Sham | NVHL | Sham | NVHL | Sham | |
| Water or sucrose | ||||||
| Days to criterion | 1.5±0.5* | 3.2±3.2 | n/a | n/a | 1.0±0 | 1.0±0 |
| Number of presses | 1415±866 | 1626±694 | n/a | n/a | 476±26 | 470±26 |
| Methamphetamine | ||||||
| Days to criterion | 1.3±0.8 | 1.7±1.3 | 1.0±0** | 3.5±1.2 | n/a | n/a |
| Number of presses | 215±86* | 158±64 | 180±58 | 133±48 | n/a | n/a |
| Latency (min) to 100 presses (criterion) | 563±207* | 721±154 | 561±196** | 869±93 | n/a | n/a |
| n per groupa | 20 | 19 | 8 | 8 | 9 | 7 |
All values are mean±standard deviation (SD).
METH: methamphetamine, NVHL: neonatal ventral hippocampal lesion
One NVHL rat from group 2 was recatheterized during self-administration acquisition; data from this rat was not included in the analysis of acquisition.
p<0.05,
p<0.01 vs. sham; independent t tests
Methamphetamine self-administration
NVHL and sham animals in group 1 (those that received water pretraining before methamphetamine) did not differ in the number of overnight sessions needed to acquire methamphetamine self-administration (Table 1; p=0.56). However, on the night on which criterion (≥100 presses) was reached, NVHL animals did make a higher number of lever presses (t37=2.34, p =0.025) and took less time to complete the first 100 lever presses (t36=2.66, p =0.012) than sham animals.
In contrast, NVHL animals in group 2 (those that did not receive water pretraining) acquired methamphetamine self-administration in significantly fewer days than sham animals (Table 1; t14=5.92, p=0.001). These animals did not differ in the total number of lever-press responses on the final criterion night (p=0.104), but NVHL animals were faster to reach the criterion requirement of 100 lever presses on this night (t14=4.02, p=0.001).
Sucrose training
All animals (NVHL and sham) in group 3 acquired the lever-pressing response for food reinforcement in a single overnight session (Table 1). There was no difference between NVHL and sham animals in the total number of lever presses performed during this session (p= 0.671). A floor effect was evident in this data, which may have obscured any potential differences between the NVHL and sham animals. The causes are unclear, but may include differences in palatability of water vs. sucrose and/or differences in equipment between the two testing sites.
Methamphetamine self-administration
After acquisition of self-administration, one NVHL and one sham animal from group 1 were discontinued from the study due to loss of catheter patency. The remaining animals in groups 1 (water pretrained) and 2 (no pretraining) did not differ in their self-administration of methamphetamine on the first 10 days of FR1 responding (repeated-measures ANOVA; all ps>0.28). Thus, data from all animals were pooled for all subsequent analyses of methamphetamine self-administration.
Fixed-ratio self-administration
NVHL (n=19) and sham (n=13) animals performed at similar stable levels across the 10 daily 1-h sessions of initial FR1 responding (Fig. 2a; p=0.376). Analysis of the dose–response relationship revealed only a significant main effect of dose (F2, 126= 105.6, p<0.001). As shown in Fig. 2b, all animals titrated their responding according to the dose of methamphetamine delivered with each infusion; per session, rats made significantly more lever presses at 0.05 mg/kg/infusion (t8 =9.68, p<0.001) and significantly fewer presses at 0.2 mg/kg/infusion (t8=4.81, p=0.001) compared to the training dose of 0.1 mg/kg/infusion. This dose effect did not interact with either lesion or session (all ps>0.11).
Fig. 2.
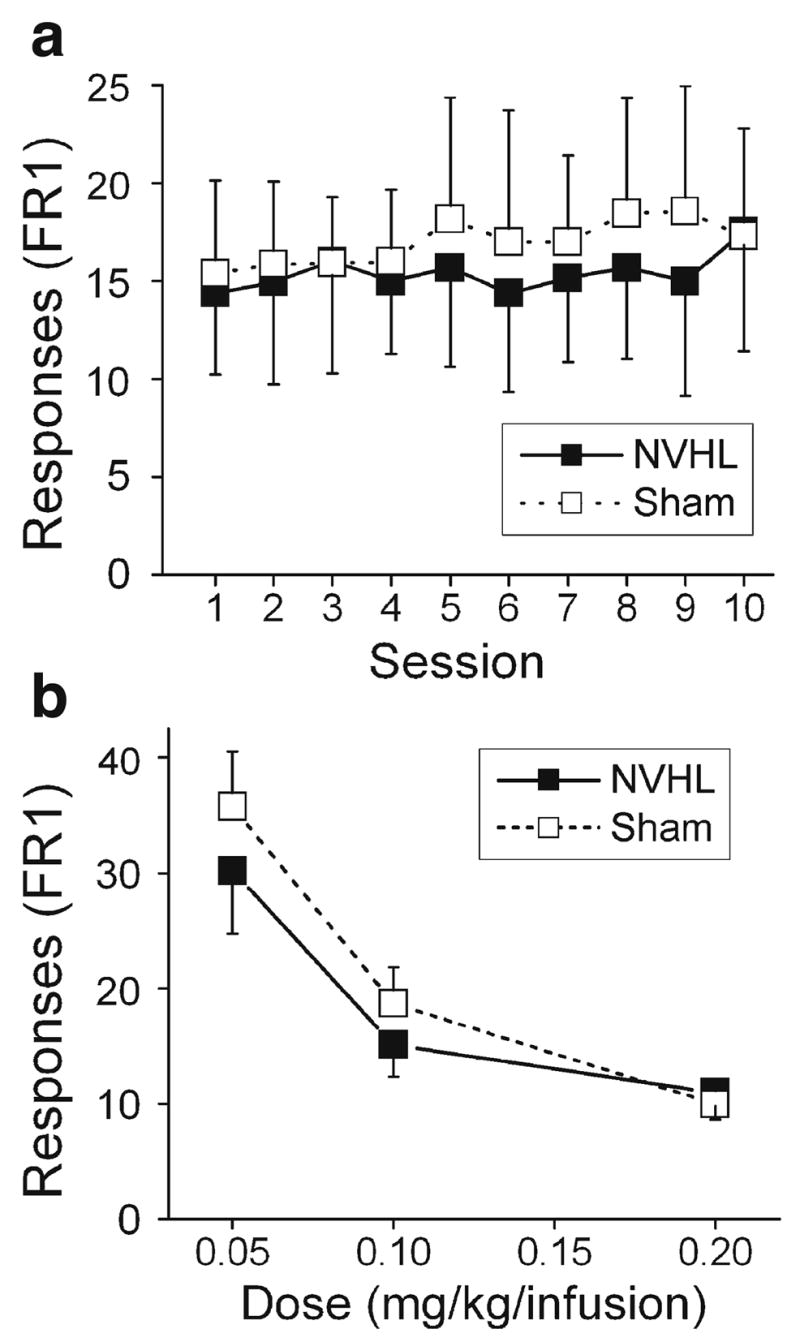
FR self-administration of methamphetamine was similar in NVHL and sham animals across 10 daily sessions (a). The rate of self-administration was dose-dependent in both NVHL and sham animals (b). FR1 fixed-ratio-1. Data in this and all subsequent figures are expressed as mean±SD
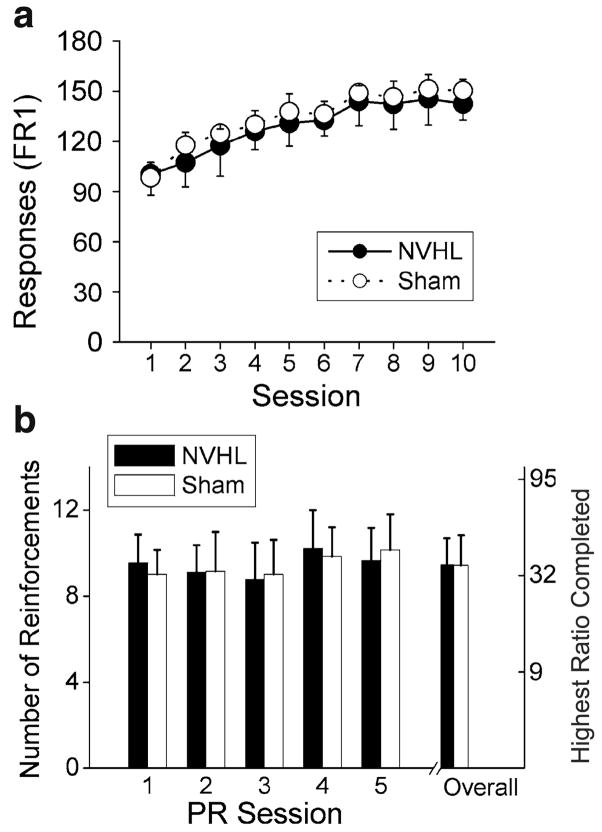
Progressive-ratio self-administration
Across all five sessions, NVHL animals (n=16) earned significantly more infusions (i.e., reached a higher break point) than sham animals (n=12) under the PR schedule of reinforcement (Fig. 3; F1, 26=10.09, p =0.004). All animals appeared to slightly increase responding across the five PR sessions (F4, 104 =16.51, p=0.046). However, this was a main effect of session only and did not interact with lesion status (p=0.12).
Fig. 3.
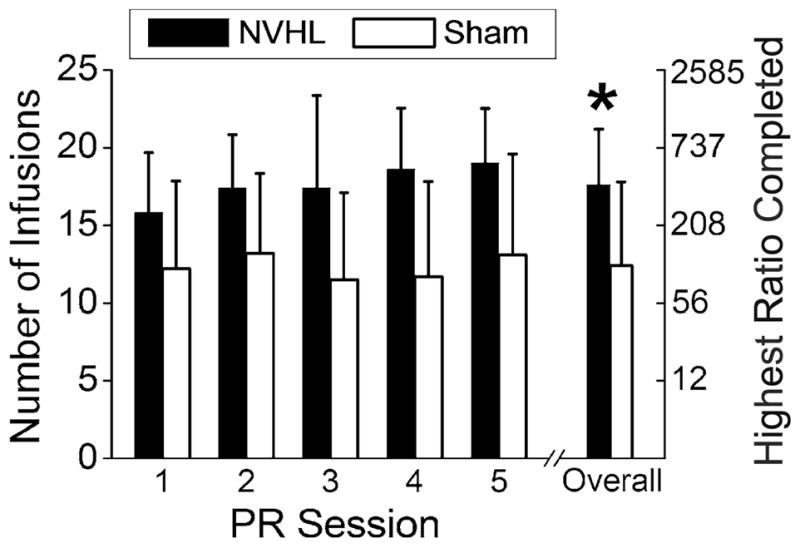
NVHL animals earned more self-administered infusions of methamphetamine and reached higher break points than sham animals when tested under the PR schedule over five daily sessions. Error bars represent SDs. *p<0.01 for number of infusions and highest ratio completed
Sucrose training
Fixed-ratio responding for food
Both NVHL (n=9) and sham-treated (n=7) animals in group 3 increased their responding over the course of the 10 daily sessions of FR1 responding for sucrose pellets (Fig. 4a; F9, 126 =56.16, p< 0.001). However, this effect did not interact with the lesion group (p=0.796), nor was there an overall difference between NVHL and sham animals on the number of lever presses (p=0.220).
Fig. 4.
FR responding for food (sucrose) reinforcement increased across 10 daily sessions for both NVHL and sham animals (a). Similarly, the number of food reinforcements earned under a PR schedule did not differ between NVHL and sham animals (b). Error bars represent SDs
Progressive-ratio responding for food
Overall, the number of reinforcers earned per session fluctuated over the course of the five PR sessions (Fig. 4b; main effect: F4, 56=4.91, p=0.002). Post hoc comparisons revealed that the number of reinforcers (collapsed across lesion group) was significantly higher on the fourth session compared to the second (t15 =2.70, p=0.016) and third sessions (t15 =3.88, p= 0.001). However, the number of reinforcers earned (i.e., break point) was unaffected by lesion group, either overall or in interaction with session (both ps>0.48).
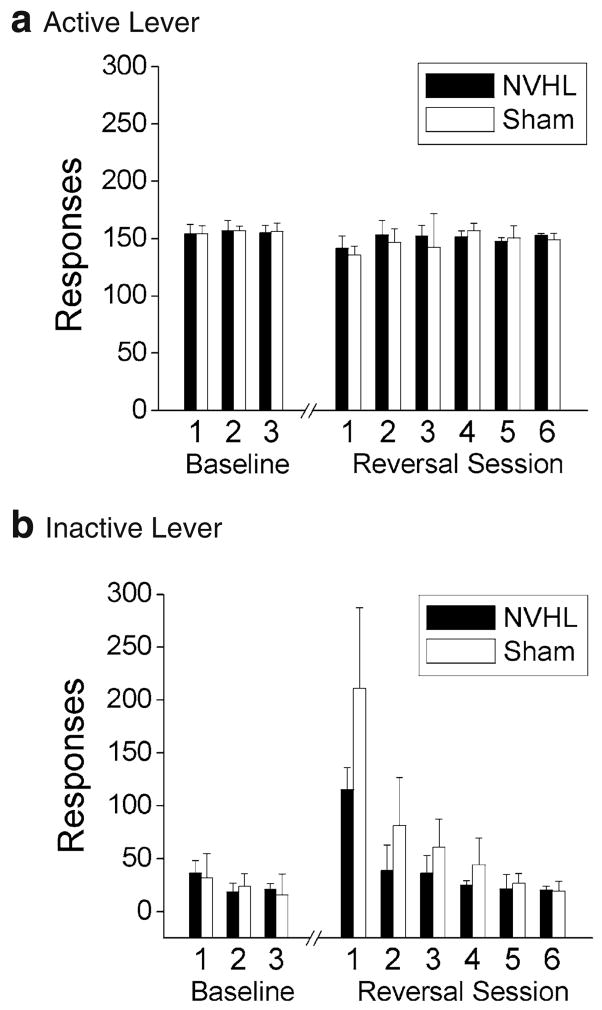
Reversal/perseveration test
After the identification of the preferred lever, NVHL (n=6) and sham (n=7) animals did not differ in the number of days to reach the criterion for stable responding (NVHL=3.5±1.2 days, sham=3.4± 1.1 days; p=0.915). As seen in Fig. 5, all animals responded significantly more on the active (preferred) lever during the last three baseline days of responding at criterion (F1, 11=679.70, p<0.001), and this preference strengthened over the course of these three sessions as responding on the inactive lever declined further (F2, 22=15.82, p<0.001). There were no main effects or interactions involving the lesion group during these baseline days (all ps>0.22). When the active lever was changed to the nonpreferred side, there was again no difference between NVHL and sham animals on the days to reach the new criterion of stable responding (NVHL=4.3±1.4 days, sham=5.3± 2.6 days; p=0.442). Testing was terminated once animals reached criterion on the reversal task; thus, further analysis of reversal performance was limited to the first three reversal sessions, a time period which includes all animals tested in this phase. This analysis revealed a significant three-way interaction between lever, lesion, and session (F2, 22=3.97, p =0.037). Further analysis of responding on the active (previously nonpreferred) lever during reversal sessions (Fig. 5a) indicated that NVHL and sham animals did not differ on this measure, either overall (p=0.316) or across the three reversal sessions (p=0.786). However, NVHL animals responded significantly less often on the inactive (previously preferred) lever than sham animals (Fig. 5b; F1, 11 =8.53, p=0.014), and this difference interacted with session (F2, 22=4.43, p=0.028). Sham animals exhibited higher responding than NVHL animals on the inactive lever on the first reversal session (t11=3.16, p=0.016) but not the second (p=0.067) or third reversal sessions (p=0.076). Thus, as shown in Fig. 5b, sham animals were slower to terminate responding on the inactive lever over the three sessions, indicating slightly more perseveration in the sham-treated animals.
Fig. 5.
NVHL and sham animals displayed strong preferences for the active lever (a) over the inactive lever (b) during baseline FR1 responding for food reinforcement. Upon reversal of the reinforced lever, both NVHL and sham animals quickly reestablished stable responding on the new active lever (a). No evidence was found for perseveration among NVHL animals, but sham-treated animals were slower to terminate their responding on the inactive lever during the first three reversal sessions (b). Error bars represent SDs
Discussion
Animals with a neonatal ventral hippocampus lesion exhibited faster acquisition and enhanced PR self-administration of methamphetamine and faster acquisition of water-reinforced operant responding. However, NVHL animals did not differ from sham-treated animals on FR or PR performance for food reinforcement, suggesting that the enhanced methamphetamine self-administration was not due to lesion-induced changes in general motivation or activity levels. These results extend previous findings (Chambers and Self 2002) by demonstrating enhanced self-administration of a second psychostimulant and differential responding under an additional behavioral schedule (PR) and suggest that this model is useful for investigating the neurobehavioral and neurobiological bases of schizophrenia and substance abuse comorbidity.
Differential effects of NVHL on responding for natural and drug reinforcement
As adults, NVHL animals acquired the initial lever-pressing response for water reinforcement in fewer days than sham-treated animals. Similarly, NVHL animals previously acquired sucrose-reinforced lever pressing faster than shams, suggesting that instrumental learning may be facilitated in NVHL animals (Chambers and Self 2002). This conclusion seems to conflict with reports of impaired cognition in NVHL animals (Chambers et al. 1996; Lipska et al. 2002; Saul et al. 2006); however, these studies assessed spatial learning and working memory, which may recruit different neurobiological substrates than simple instrumental learning (Chambers and Self 2002). Previously, NVHL animals exhibited a higher fluid intake than shams (Le Pen et al. 2002), and a tendency toward polydipsia has also been observed in patients with schizophrenia (Oades and Daniels 1999). However, although our NVHL animals initially reached response criterion in fewer days, they did not display higher water intake than sham-treated animals.
Methamphetamine self-administration acquisition was enhanced in NVHL animals, possibly suggesting an increased vulnerability to addictive behavior (Piazza et al. 1989; Marinelli and White 2000). However, this enhancement of acquisition was limited to animals that were naïve to lever pressing. Given this selectivity, the rapidity of self-administration acquisition in NVHL rats may best be interpreted as a general enhancement of instrumental learning (Chambers and Self 2002), particularly the initial formation of response-reinforcement contingencies. However, our finding that pretrained NVHL animals emitted more responses during the criterion night for methamphet-amine (but not water), and were faster to reach the criterion level of responses for methamphetamine, suggests that this enhancement of instrumental learning may also embed a stronger relationship between drug-taking behavior and reinforcement, possibly leading to increased drug-seeking and an increased vulnerability to addiction (Piazza et al. 2000).
NVHL and sham-treated animals self-administered methamphetamine at similar rates during FR sessions, as previously reported for cocaine (Chambers and Self 2002), implying no change in stable drug-taking behavior once the self-administration response was acquired. Furthermore, NVHL and sham animals were equally sensitive to changes in methamphetamine dose. This was surprising because NVHL rats are behaviorally hyperresponsive to psychostimulants (Lipska et al. 1993) and also because other animals that easily acquire self-administration demonstrate vertical shifts in the dose–response curve for cocaine self-administration (Piazza et al. 2000).
However, NVHL animals did consistently reach higher break points under a PR schedule of methamphetamine self-administration. Thus, NVHL and sham animals demonstrated equal self-administration rates under low-demand FR conditions, even across varying doses, but NVHL animals expended more effort to self-administer the drug when response requirements were progressively increased. Increased PR responding may indicate increased motivation to administer the drug and/or an increase in the drug’s incentive value (Richardson and Roberts 1996). Self-administration under a PR schedule has been suggested to model human addicts’ strong motivation to acquire a drug (Deroche-Gamonet et al. 2004) and may reflect a sensitized incentive value of the drug that is hypothesized to underlie compulsive drug-seeking behaviors (Robinson and Berridge 1993).
The NVHL lesion did not affect PR responding for food, suggesting that NVHL rats did not experience a general increase in the motivation to obtain positive reinforcement. In fact, NVHL rats are less sensitive to reward in conditioned place preference and saccharin consumption tests, an effect which may model the anhedonia observed in patients with schizophrenia (Le Pen et al. 2002). The lack of effect on FR responding for either methamphetamine or food, or on PR responding for food, also suggests that these results cannot be explained by lesion-induced hyperactivity (Wan et al. 1996; Sams-Dodd et al. 1997; Brake et al. 1999).
Finally, although NVHL rats were previously shown to perseverate on a reward-associated lever (Chambers and Self 2002), perseveration is unlikely to explain the enhancement of PR responding for methamphetamine. NVHL animals showed no evidence of perseveration when reinforcement delivery (food) was switched to the previously nonreinforced lever. The generally low levels of responding on the nonreinforced lever also suggest that NVHL rats were not simply exhibiting nonspecific and/or hyperactive responses to reinforcement availability.
Mechanisms of increased drug-seeking behavior in neonatal hippocampal-lesioned rats
Adult lesions of the hippocampus or subiculum inhibit drug self-administration (Caine et al. 2001; Sun and Rebec 2003), suggesting that the enhanced drug-seeking behavior reported in this study is unlikely to arise from damage to the hippocampus per se. Rather, this behavior is likely a result of the developmental nature of the lesion and the subsequent reorganization of hippocampal efferent pathways, particularly to the PFC and nucleus accumbens. PFC pyramidal neurons fire inappropriately in response to dopamine afferent stimulation in adult animals with a neonatal (but not adult) hippocampal lesion, a reversal of the normal filtering process in PFC neurons that may in turn lead to abnormal burst firing of midbrain dopamine cells (O’Donnell et al. 2002). Dopaminergic activation in the PFC mediates cocaine self-administration (McGregor and Roberts 1995; Park et al. 2002), suggesting that disruption of the mesocortical dopamine pathway and of the prefrontal response to dopamine input may lead to alterations in self-administration behavior as observed in this study.
A functional hyperactivity of mesolimbic dopamine systems in NVHL animals has been inferred from hypersensitivity to psychostimulant-induced locomotion (Lipska and Weinberger 2000), implying that NVHL rats may exist in a baseline “sensitized” state. Such an endogenous sensitization process has been hypothesized to underlie psychotic states in schizophrenia (Laruelle 2000). Psychostimulant-sensitized animals reach higher break points than control animals under a PR schedule of drug self-administration (Mendrek et al. 1998; Lorrain et al. 2000; Vezina 2004) similar to the NVHL rats in the present study. Although dopamine release in the nucleus accumbens is not enhanced in NVHL rats (Brake et al. 1999; Lillrank et al. 1999), accumbens neuron properties such as dendritic spine density and immediate early gene expression are altered in both NVHL rats (Lillrank et al. 1996; Bhardwaj et al. 2003; Flores et al., 2005) and psychostimulant-sensitized rats (Robinson and Kolb 1997; Crombag et al. 2002). Activation of dopamine afferent pathways also elicits excessive accumbens neuron firing in NVHL animals, an effect which is likely secondary to the inability of dopamine inputs to suppress PFC firing rates (O’Donnell et al. 2002; Goto and O’Donnell 2002). The abnormal prefrontal control of accumbens neurons is hypothesized to lead to a hypersensitivity to the reinforcing value of addictive drugs, thus facilitating drug-seeking behavior (Chambers et al. 2001; Volkow et al. 2003).
In conclusion, NVHL animals demonstrate an elevated vulnerability to drug-seeking behavior as evident by increased responding under a PR schedule but not a FR schedule, and this effect cannot be easily explained by an increase in general appetitive motivation or activity levels. The NVHL model elicits various behavioral abnormalities that correlate with clinical symptoms of schizophrenia (Lipska and Weinberger 2000), and the present results suggest that the high rates of substance abuse observed among patients (Regier et al. 1990; Selzer and Lieberman 1993) can also be modeled in these animals (Chambers and Self 2002). The neurobiological disruptions produced by the NVHL may confer an additional vulnerability to drug-seeking behavior, making this neurodevelopmental model useful for investigating the common neural and behavioral mechanisms that underlie the dual diagnosis of schizophrenia and addiction.
Acknowledgments
This work was supported by NIH award DA14020 to P.O’D. and an SMCM Faculty Development Grant to A.M.B. We thank Nicole Barnhardt, Katrina Emmerich, Atara Marzouk, Megan MacFarland, Casimira Ruiz, and Ronald Saul for their assistance with histological processing, and Suzanne Asmann and Laurie Warner for their assistance with behavioral testing.
Contributor Information
Anne Marie Brady, Center for Neuropharmacology and Neuroscience, Albany Medical College, Albany, NY, USA; Department of Psychology and Neuroscience Program, St. Mary’s College of Maryland, 18952 E. Fisher Road, St. Mary’s City, MD 20686, USA, e-mail: ambrady@smcm.edu.
Sarah E. McCallum, Department of Psychology and Neuroscience Program, St. Mary’s College of Maryland, 18952 E. Fisher Road, St. Mary’s City, MD 20686, USA
Stanley D. Glick, Department of Psychology and Neuroscience Program, St. Mary’s College of Maryland, 18952 E. Fisher Road, St. Mary’s City, MD 20686, USA
Patricio O’Donnell, Department of Psychology and Neuroscience Program, St. Mary’s College of Maryland, 18952 E. Fisher Road, St. Mary’s City, MD 20686, USA.
References
- Bhardwaj SK, Beaudry G, Quirion R, Levesque D, Srivastava LK. Neonatal ventral hippocampus lesion leads to reductions in nerve growth factor inducible-B mRNA in the prefrontal cortex and increased amphetamine response in the nucleus accumbens and dorsal striatum. Neuroscience. 2003;122:669–676. doi: 10.1016/j.neuroscience.2003.08.016. [DOI] [PubMed] [Google Scholar]
- Blanchard JJ, Brown SA, Horan WP, Sherwood AR. Substance use disorders in schizophrenia: review, integration, and a proposed model. Clin Psychol Rev. 2000;20:207–234. doi: 10.1016/s0272-7358(99)00033-1. [DOI] [PubMed] [Google Scholar]
- Brady AM, Glick SD, O’Donnell P. Selective disruption of nucleus accumbens gating mechanisms in rats behaviorally sensitized to methamphetamine. J Neurosci. 2005;25:6687–6695. doi: 10.1523/JNEUROSCI.0643-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brake WG, Sullivan RM, Flores G, Srivastava LK, Gratton A. Neonatal ventral hippocampal lesions attenuate the nucleus accumbens dopamine response to stress: an electrochemical study in the adult rat. Brain Res. 1999;831:25–32. doi: 10.1016/s0006-8993(99)01477-8. [DOI] [PubMed] [Google Scholar]
- Caine SB, Humby T, Robbins TW, Everitt BJ. Behavioral effects of psychomotor stimulants in rats with dorsal or ventral subiculum lesions: locomotion, cocaine self-administration, and prepulse inhibition of startle. Behav Neurosci. 2001;115:880–894. doi: 10.1037//0735-7044.115.4.880. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Self DW. Motivational responses to natural and drug rewards in rats with neonatal ventral hippocampal lesions: an animal model of dual diagnosis schizophrenia. Neuropsychopharmacology. 2002;27:889–905. doi: 10.1016/S0893-133X(02)00365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Moore J, McEvoy JP, Levin ED. Cognitive effects of neonatal hippocampal lesions in a rat model of schizophrenia. Neuropsychopharmacology. 1996;15:587–594. doi: 10.1016/S0893-133X(96)00132-7. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Krystal JH, Self DW. A neurobiological basis for substance abuse comorbidity in schizophrenia. Biol Psychiatry. 2001;50:71–83. doi: 10.1016/s0006-3223(01)01134-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, Jedynak JP, Redmond K, Robinson TE, Hope BT. Locomotor sensitization to cocaine is associated with increased Fos expression in the accumbens, but not in the caudate. Behav Brain Res. 2002;136:455–462. doi: 10.1016/s0166-4328(02)00196-1. [DOI] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305:1014–1017. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, Goldman D, Weinberger DR. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci U S A. 2001;98:6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores G, Alquicer G, Silva-Gomez AB, Zaldivar G, Stewart J, Quirion R, Srivastava LK. Alterations in dendritic morphology of prefrontal cortical and nucleus accumbens neurons in post-pubertal rats after neonatal excitotoxic lesions of the ventral hippocampus. Neuroscience. 2005;133:463–470. doi: 10.1016/j.neuroscience.2005.02.021. [DOI] [PubMed] [Google Scholar]
- Glick SD, Hinds PA. Differences in amphetamine and morphine sensitivity in lateralized and non-lateralized rats: locomotor activity and drug self-administration. Eur J Pharmacol. 1985;118:239–244. doi: 10.1016/0014-2999(85)90134-7. [DOI] [PubMed] [Google Scholar]
- Goto Y, O’Donnell P. Delayed mesolimbic system alteration in a developmental animal model of schizophrenia. J Neurosci. 2002;22:9070–9077. doi: 10.1523/JNEUROSCI.22-20-09070.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, Grace AA. Alterations in medial prefrontal cortical activity and plasticity in rats with disruption of cortical development. Biol Psychiatry. 2006;60:1259–1267. doi: 10.1016/j.biopsych.2006.05.046. [DOI] [PubMed] [Google Scholar]
- Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, Phillips RL, Kimes AS, Margolin A. Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci U S A. 1996;93:12040–12045. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson ME, Homayoun H, Moghaddam B. NMDA receptor hypofunction produces concomitant firing rate potentiation and burst activity reduction in the prefrontal cortex. Proc Natl Acad Sci U S A. 2004;101:8467–8472. doi: 10.1073/pnas.0308455101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khantzian EJ. The self-medication hypothesis of substance use disorders: a reconsideration and recent applications. Harv Rev Psychiatry. 1997;4:231–244. doi: 10.3109/10673229709030550. [DOI] [PubMed] [Google Scholar]
- Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, Muhammad F, Ely TD, Hoffman JM, Drexler KP. Neural activity related to drug craving in cocaine addiction. Arch Gen Psychiatry. 2001;58:334–341. doi: 10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]
- Koob GF. Drugs of abuse: anatomy, pharmacology and function of reward pathways. Trends Pharmacol Sci. 1992;13:177–184. doi: 10.1016/0165-6147(92)90060-j. [DOI] [PubMed] [Google Scholar]
- Krystal JH, D’Souza DC, Madonick S, Petrakis IL. Toward a rational pharmacotherapy of comorbid substance abuse in schizophrenic patients. Schizophr Res. 1999;35(Suppl):S35–S49. doi: 10.1016/s0920-9964(98)00162-5. [DOI] [PubMed] [Google Scholar]
- Laruelle M. The role of endogenous sensitization in the pathophysiology of schizophrenia: implications from recent brain imaging studies. Brain Res Rev. 2000;31:371–384. doi: 10.1016/s0165-0173(99)00054-5. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Abi-Dargham A, van Dyck CH, Gil R, D’Souza CD, Erdos J, McCance E, Rosenblatt W, Fingado C, Zoghbi SS, Baldwin RM, Seibyl JP, Krystal JH, Charney DS, Innis RB. Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proc Natl Acad Sci U S A. 1996;93:9235–9240. doi: 10.1073/pnas.93.17.9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Pen G, Gaudet L, Mortas P, Mory R, Moreau JL. Deficits in reward sensitivity in a neurodevelopmental rat model of schizophrenia. Psychopharmacology (Berl) 2002;161:434–441. doi: 10.1007/s00213-002-1092-4. [DOI] [PubMed] [Google Scholar]
- Lillrank SM, Lipska BK, Bachus SE, Wood GK, Weinberger DR. Amphetamine-induced c-fos mRNA expression is altered in rats with neonatal ventral hippocampal damage. Synapse. 1996;23:292–301. doi: 10.1002/(SICI)1098-2396(199608)23:4<292::AID-SYN7>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Lillrank SM, Lipska BK, Kolachana BS, Weinberger DR. Attenuated extracellular dopamine levels after stress and amphetamine in the nucleus accumbens of rats with neonatal ventral hippocampal damage. J Neural Transm. 1999;106:183–196. doi: 10.1007/s007020050150. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Weinberger DR. To model a psychiatric disorder in animals: schizophrenia as a reality test. Neuropsychopharmacology. 2000;23:223–239. doi: 10.1016/S0893-133X(00)00137-8. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Jaskiw GE, Weinberger DR. Postpubertal emergence of hyperresponsiveness to stress and to amphetamine after neonatal excitotoxic hippocampal damage: a potential animal model of schizophrenia. Neuropsychopharmacology. 1993;9:67–75. doi: 10.1038/npp.1993.44. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Aultman JM, Verma A, Weinberger DR, Moghaddam B. Neonatal damage of the ventral hippocampus impairs working memory in the rat. Neuropsychopharmacology. 2002;27:47–54. doi: 10.1016/S0893-133X(02)00282-8. [DOI] [PubMed] [Google Scholar]
- Lorrain DS, Arnold GM, Vezina P. Previous exposure to amphetamine increases incentive to obtain the drug: long-lasting effects revealed by the progressive ratio schedule. Behav Brain Res. 2000;107:9–19. doi: 10.1016/s0166-4328(99)00109-6. [DOI] [PubMed] [Google Scholar]
- Marinelli M, White FJ. Enhanced vulnerability to cocaine self-administration is associated with elevated impulse activity of midbrain dopamine neurons. J Neurosci. 2000;20:8876–8885. doi: 10.1523/JNEUROSCI.20-23-08876.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor A, Roberts DC. Effect of medial prefrontal cortex injections of SCH 23390 on intravenous cocaine self-administration under both a fixed and progressive ratio schedule of reinforcement. Behav Brain Res. 1995;67:75–80. doi: 10.1016/0166-4328(94)00106-p. [DOI] [PubMed] [Google Scholar]
- Mendrek A, Blaha CD, Phillips AG. Pre-exposure of rats to amphetamine sensitizes self-administration of this drug under a progressive ratio schedule. Psychopharmacology (Berl) 1998;135:416–422. doi: 10.1007/s002130050530. [DOI] [PubMed] [Google Scholar]
- Mobini S, Chiang TJ, Ho MY, Bradshaw CM, Szabadi E. Comparison of the effects of clozapine, haloperidol, chlorpromazine and D-amphetamine on performance on a time-constrained progressive ratio schedule and on locomotor behaviour in the rat. Psychopharmacology (Berl) 2000;152:47–54. doi: 10.1007/s002130000486. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore H, Jentsch JD, Ghajarnia M, Geyer MA, Grace AA. A neurobehavioral systems analysis of adult rats exposed to methylazoxymethanol acetate on E17: implications for the neuropathology of schizophrenia. Biol Psychiatry. 2006;60:253–264. doi: 10.1016/j.biopsych.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell P, Lewis BL, Weinberger DR, Lipska BK. Neonatal hippocampal damage alters electrophysiological properties of prefrontal cortical neurons in adult rats. Cereb Cortex. 2002;12:975–982. doi: 10.1093/cercor/12.9.975. [DOI] [PubMed] [Google Scholar]
- Oades RD, Daniels R. Subclinical polydipsia and polyuria in young patients with schizophrenia or obsessive-compulsive disorder vs normal controls. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23:1329–1344. doi: 10.1016/s0278-5846(99)00069-x. [DOI] [PubMed] [Google Scholar]
- Park WK, Bari AA, Jey AR, Anderson SM, Spealman RD, Rowlett JK, Pierce RC. Cocaine administered into the medial prefrontal cortex reinstates cocaine-seeking behavior by increasing AMPA receptor-mediated glutamate transmission in the nucleus accumbens. J Neurosci. 2002;22:2916–2925. doi: 10.1523/JNEUROSCI.22-07-02916.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson PE, Robinson TE. Amphetamine-induced time-dependent sensitization of dopamine neurotransmission in the dorsal and ventral striatum: a microdialysis study in behaving rats. Synapse. 1995;19:56–65. doi: 10.1002/syn.890190108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlstein WM, Carter CS, Noll DC, Cohen JD. Relation of prefrontal cortex dysfunction to working memory and symptoms in schizophrenia. Am J Psychiatry. 2001;158:1105–1113. doi: 10.1176/appi.ajp.158.7.1105. [DOI] [PubMed] [Google Scholar]
- Peterson JD, Wolf ME, White FJ. Repeated amphetamine administration decreases D1 dopamine receptor-mediated inhibition of voltage-gated sodium currents in the prefrontal cortex. J Neurosci. 2006;26:3164–3168. doi: 10.1523/JNEUROSCI.2375-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245:1511–1513. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deroche-Gamonent V, Rouge-Pont F, Le Moal M. Vertical shifts in self-administration dose–response functions predict a drug-vulnerable phenotype predisposed to addiction. J Neurosci. 2000;20:4226–4232. doi: 10.1523/JNEUROSCI.20-11-04226.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, Goodwin FK. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA. 1990;264:2511–2518. [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Persistent structural modifications in nucleus accumbens and prefrontal cortex neurons produced by previous experience with amphetamine. J Neurosci. 1997;17:8491–8497. doi: 10.1523/JNEUROSCI.17-21-08491.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction. 2000;95(Suppl 2):S91–S117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- Sams-Dodd F, Lipska BK, Weinberger DR. Neonatal lesions of the rat ventral hippocampus result in hyperlocomotion and deficits in social behaviour in adulthood. Psychopharmacology (Berl) 1997;132:303–310. doi: 10.1007/s002130050349. [DOI] [PubMed] [Google Scholar]
- Saul RD, Wiest MK, Brady AM. Spatial working memory deficits in the neonatal ventral hippocampal lesion rat model of schizophrenia. Program No. 687.3, Abstract Viewer/Itinerary Planner, Society for Neuroscience; Washington DC. 2006. [Google Scholar]
- Selzer JA, Lieberman JA. Schizophrenia and substance abuse. Psychiatr Clin North Am. 1993;16:401–412. [PubMed] [Google Scholar]
- Solinas M, Goldberg SR. Motivational effects of cannabinoids and opioids on food reinforcement depend on simultaneous activation of cannabinoid and opioid systems. Neuropsychopharmacology. 2005;30:2035–2045. doi: 10.1038/sj.npp.1300720. [DOI] [PubMed] [Google Scholar]
- Sun W, Rebec GV. Lidocaine inactivation of ventral subiculum attenuates cocaine-seeking behavior in rats. J Neurosci. 2003;23:10258–10264. doi: 10.1523/JNEUROSCI.23-32-10258.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeszko PR, Goldberg E, Gunduz-Bruce H, Ashtari M, Robinson D, Malhotra AK, Lencz T, Bates J, Crandall DT, Kane JM, Bilder RM. Smaller anterior hippocampal formation volume in antipsychotic-naive patients with first-episode schizophrenia. Am J Psychiatry. 2003;160:2190–2197. doi: 10.1176/appi.ajp.160.12.2190. [DOI] [PubMed] [Google Scholar]
- Trantham H, Szumlinski KK, McFarland K, Kalivas PW, Lavin A. Repeated cocaine administration alters the electrophysiological properties of prefrontal cortical neurons. Neuroscience. 2002;113:749–753. doi: 10.1016/s0306-4522(02)00246-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezina P. Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neurosci Biobehav Rev. 2004;27:827–839. doi: 10.1016/j.neubiorev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. The addicted human brain: insights from imaging studies. J Clin Invest. 2003;111:1444–1451. doi: 10.1172/JCI18533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan RQ, Giovanni A, Kafka SH, Corbett R. Neonatal hippocampal lesions induced hyperresponsiveness to amphetamine: behavioral and in vivo microdialysis studies. Behav Brain Res. 1996;78:211–223. doi: 10.1016/0166-4328(95)00251-0. [DOI] [PubMed] [Google Scholar]
- Weeks JR. Long-term intravenous infusion. In: Myers RD, editor. Methods in psychobiology. Academic; New York: 1972. pp. 155–168. [Google Scholar]
- Weinberger DR. Cell biology of the hippocampal formation in schizophrenia. Biol Psychiatry. 1999;45:395–402. doi: 10.1016/s0006-3223(98)00331-x. [DOI] [PubMed] [Google Scholar]