Abstract
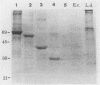
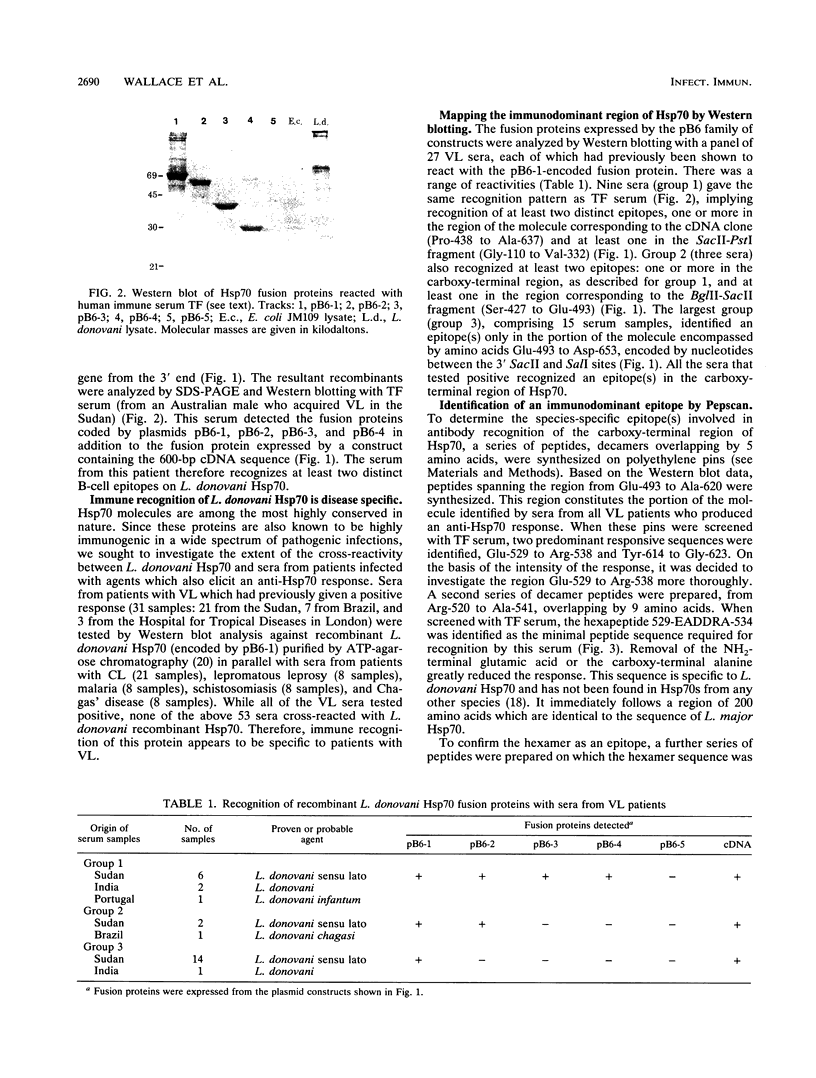
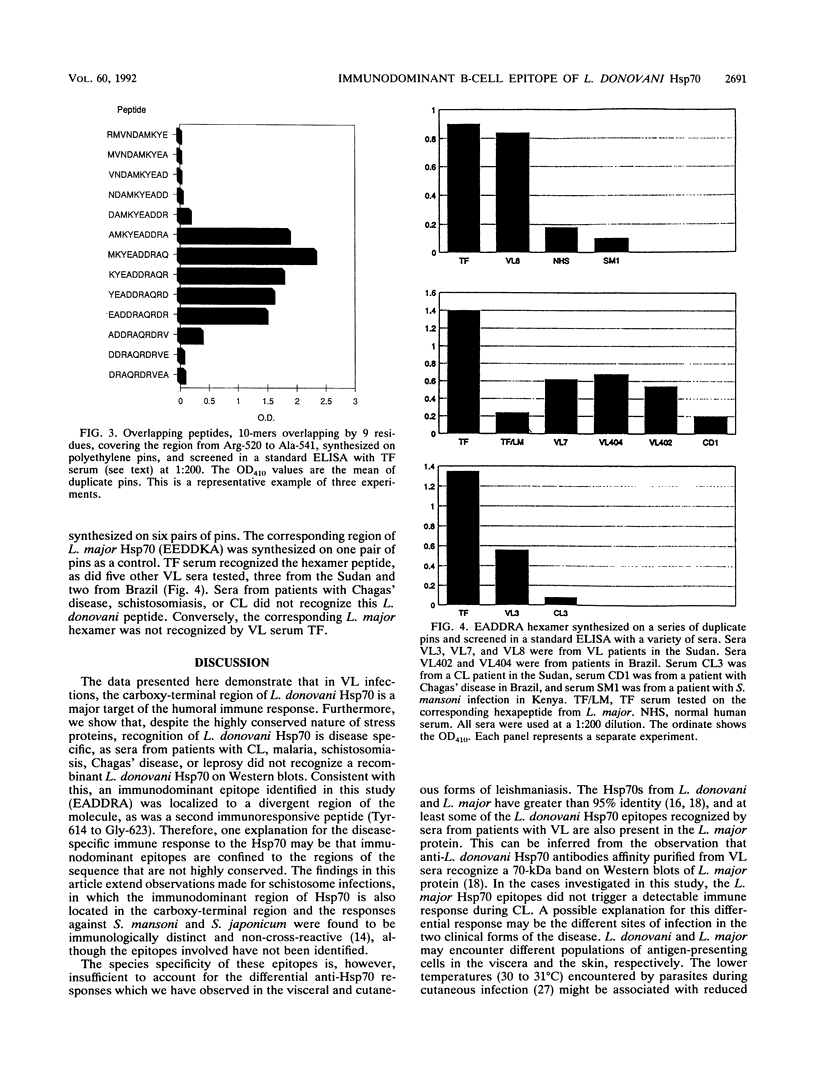
We have shown that a member of the 70-kDa heat shock protein (Hsp70) family is a major target of the humoral immune response during Leishmania donovani infection. A recombinant fusion protein was recognized by sera from 92% (35 of 38) of patients with visceral leishmaniasis, including representatives from each of the major regions where it is endemic. Serological analysis of recombinant Hsp70, expressed by a series of deletion constructs, identified the carboxy-terminal region as the immunodominant site. This region, which is the most evolutionarily divergent part of the molecule, was recognized by all sera from patients with visceral leishmaniasis which exhibited an anti-Hsp70 response. Purified recombinant L. donovani Hsp70 was not recognized by sera from patients with cutaneous leishmaniasis, Chagas' disease, leprosy, malaria, or schistosomiasis. To determine the regions involved in antibody recognition, a series of overlapping peptides were synthesized on polyethylene pins by the Pepscan method, and a hexamer, EADDRA, was identified by the visceral leishmaniasis serum samples as an immunodominant B-cell epitope.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bianco A. E., Favaloro J. M., Burkot T. R., Culvenor J. G., Crewther P. E., Brown G. V., Anders R. F., Coppel R. L., Kemp D. J. A repetitive antigen of Plasmodium falciparum that is homologous to heat shock protein 70 of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8713–8717. doi: 10.1073/pnas.83.22.8713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirico W. J., Waters M. G., Blobel G. 70K heat shock related proteins stimulate protein translocation into microsomes. Nature. 1988 Apr 28;332(6167):805–810. doi: 10.1038/332805a0. [DOI] [PubMed] [Google Scholar]
- Deshaies R. J., Koch B. D., Werner-Washburne M., Craig E. A., Schekman R. A subfamily of stress proteins facilitates translocation of secretory and mitochondrial precursor polypeptides. Nature. 1988 Apr 28;332(6167):800–805. doi: 10.1038/332800a0. [DOI] [PubMed] [Google Scholar]
- Ellis J. Proteins as molecular chaperones. 1987 Jul 30-Aug 5Nature. 328(6129):378–379. doi: 10.1038/328378a0. [DOI] [PubMed] [Google Scholar]
- Engman D. M., Sias S. R., Gabe J. D., Donelson J. E., Dragon E. A. Comparison of HSP70 genes from two strains of Trypanosoma cruzi. Mol Biochem Parasitol. 1989 Dec;37(2):285–287. doi: 10.1016/0166-6851(89)90161-8. [DOI] [PubMed] [Google Scholar]
- Flaherty K. M., DeLuca-Flaherty C., McKay D. B. Three-dimensional structure of the ATPase fragment of a 70K heat-shock cognate protein. Nature. 1990 Aug 16;346(6285):623–628. doi: 10.1038/346623a0. [DOI] [PubMed] [Google Scholar]
- Gaston J. S., Life P. F., Bailey L. C., Bacon P. A. In vitro responses to a 65-kilodalton mycobacterial protein by synovial T cells from inflammatory arthritis patients. J Immunol. 1989 Oct 15;143(8):2494–2500. [PubMed] [Google Scholar]
- Geysen H. M., Meloen R. H., Barteling S. J. Use of peptide synthesis to probe viral antigens for epitopes to a resolution of a single amino acid. Proc Natl Acad Sci U S A. 1984 Jul;81(13):3998–4002. doi: 10.1073/pnas.81.13.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramiccia M., Gradoni L., Pozio E. Leishmania infantum sensu lato as an agent of cutaneous leishmaniasis in Abruzzi region (Italy). Trans R Soc Trop Med Hyg. 1987;81(2):235–237. doi: 10.1016/0035-9203(87)90225-2. [DOI] [PubMed] [Google Scholar]
- Hedstrom R., Culpepper J., Harrison R. A., Agabian N., Newport G. A major immunogen in Schistosoma mansoni infections is homologous to the heat-shock protein Hsp70. J Exp Med. 1987 May 1;165(5):1430–1435. doi: 10.1084/jem.165.5.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedstrom R., Culpepper J., Schinski V., Agabian N., Newport G. Schistosome heat-shock proteins are immunologically distinct host-like antigens. Mol Biochem Parasitol. 1988 Jun;29(2-3):275–282. doi: 10.1016/0166-6851(88)90082-5. [DOI] [PubMed] [Google Scholar]
- Kusukawa N., Yura T., Ueguchi C., Akiyama Y., Ito K. Effects of mutations in heat-shock genes groES and groEL on protein export in Escherichia coli. EMBO J. 1989 Nov;8(11):3517–3521. doi: 10.1002/j.1460-2075.1989.tb08517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. G., Atkinson B. L., Giannini S. H., Van der Ploeg L. H. Structure and expression of the hsp 70 gene family of Leishmania major. Nucleic Acids Res. 1988 Oct 25;16(20):9567–9585. doi: 10.1093/nar/16.20.9567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehn M., Weiser W. Y., Engelhorn S., Gillis S., Remold H. G. IL-4 inhibits H2O2 production and antileishmanial capacity of human cultured monocytes mediated by IFN-gamma. J Immunol. 1989 Nov 1;143(9):3020–3024. [PubMed] [Google Scholar]
- MacFarlane J., Blaxter M. L., Bishop R. P., Miles M. A., Kelly J. M. Identification and characterisation of a Leishmania donovani antigen belonging to the 70-kDa heat-shock protein family. Eur J Biochem. 1990 Jun 20;190(2):377–384. doi: 10.1111/j.1432-1033.1990.tb15586.x. [DOI] [PubMed] [Google Scholar]
- Mattei D., Scherf A., Bensaude O., da Silva L. P. A heat shock-like protein from the human malaria parasite Plasmodium falciparum induces autoantibodies. Eur J Immunol. 1989 Oct;19(10):1823–1828. doi: 10.1002/eji.1830191010. [DOI] [PubMed] [Google Scholar]
- Mehlert A., Young D. B. Biochemical and antigenic characterization of the Mycobacterium tuberculosis 71kD antigen, a member of the 70kD heat-shock protein family. Mol Microbiol. 1989 Feb;3(2):125–130. doi: 10.1111/j.1365-2958.1989.tb01801.x. [DOI] [PubMed] [Google Scholar]
- Munk M. E., Schoel B., Modrow S., Karr R. W., Young R. A., Kaufmann S. H. T lymphocytes from healthy individuals with specificity to self-epitopes shared by the mycobacterial and human 65-kilodalton heat shock protein. J Immunol. 1989 Nov 1;143(9):2844–2849. [PubMed] [Google Scholar]
- Murray H. W., Masur H., Keithly J. S. Cell-mediated immune response in experimental visceral leishmaniasis. I. Correlation between resistance to Leishmania donovani and lymphokine-generating capacity. J Immunol. 1982 Jul;129(1):344–350. [PubMed] [Google Scholar]
- Pelham H. Heat-shock proteins. Coming in from the cold. Nature. 1988 Apr 28;332(6167):776–777. doi: 10.1038/332776a0. [DOI] [PubMed] [Google Scholar]
- Reiner N. E., Ng W., Ma T., McMaster W. R. Kinetics of gamma interferon binding and induction of major histocompatibility complex class II mRNA in Leishmania-infected macrophages. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4330–4334. doi: 10.1073/pnas.85.12.4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Res P. C., Schaar C. G., Breedveld F. C., van Eden W., van Embden J. D., Cohen I. R., de Vries R. R. Synovial fluid T cell reactivity against 65 kD heat shock protein of mycobacteria in early chronic arthritis. Lancet. 1988 Aug 27;2(8609):478–480. doi: 10.1016/s0140-6736(88)90123-7. [DOI] [PubMed] [Google Scholar]
- Rodbard D., Wachslicht-Rodbard H., Rodbard S. Temperature: a critical factor determining localization and natural history of infectious, metabolic, and immunological diseases. Perspect Biol Med. 1980 Spring;23(3):439–474. doi: 10.1353/pbm.1980.0062. [DOI] [PubMed] [Google Scholar]
- Rothstein N. M., Higashi G., Yates J., Rajan T. V. Onchocerca volvulus heat shock protein 70 is a major immunogen in amicrofilaremic individuals from a filariasis-endemic area. Mol Biochem Parasitol. 1989 Mar 15;33(3):229–235. doi: 10.1016/0166-6851(89)90084-4. [DOI] [PubMed] [Google Scholar]
- Searle S., Campos A. J., Coulson R. M., Spithill T. W., Smith D. F. A family of heat shock protein 70-related genes are expressed in the promastigotes of Leishmania major. Nucleic Acids Res. 1989 Jul 11;17(13):5081–5095. doi: 10.1093/nar/17.13.5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkirk M. E., Rutherford P. J., Denham D. a., Partono F., Maizels R. M. Cloned antigen genes of Brugia filarial parasites. Biochem Soc Symp. 1987;53:91–102. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanbuskirk A., Crump B. L., Margoliash E., Pierce S. K. A peptide binding protein having a role in antigen presentation is a member of the HSP70 heat shock family. J Exp Med. 1989 Dec 1;170(6):1799–1809. doi: 10.1084/jem.170.6.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch W. J., Feramisco J. R. Rapid purification of mammalian 70,000-dalton stress proteins: affinity of the proteins for nucleotides. Mol Cell Biol. 1985 Jun;5(6):1229–1237. doi: 10.1128/mcb.5.6.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D., Lathigra R., Hendrix R., Sweetser D., Young R. A. Stress proteins are immune targets in leprosy and tuberculosis. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4267–4270. doi: 10.1073/pnas.85.12.4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Elliott T. J. Stress proteins, infection, and immune surveillance. Cell. 1989 Oct 6;59(1):5–8. doi: 10.1016/0092-8674(89)90861-1. [DOI] [PubMed] [Google Scholar]
- van Eden W., Thole J. E., van der Zee R., Noordzij A., van Embden J. D., Hensen E. J., Cohen I. R. Cloning of the mycobacterial epitope recognized by T lymphocytes in adjuvant arthritis. Nature. 1988 Jan 14;331(6152):171–173. doi: 10.1038/331171a0. [DOI] [PubMed] [Google Scholar]