Abstract
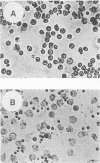
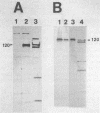
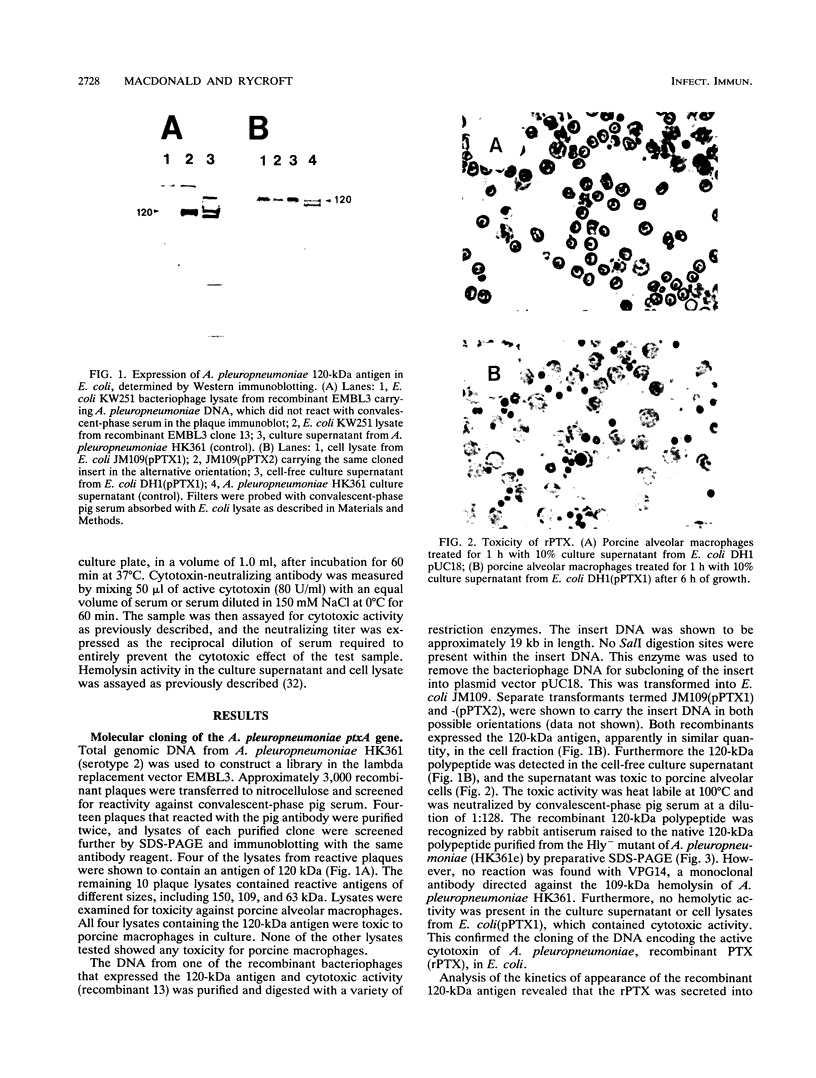
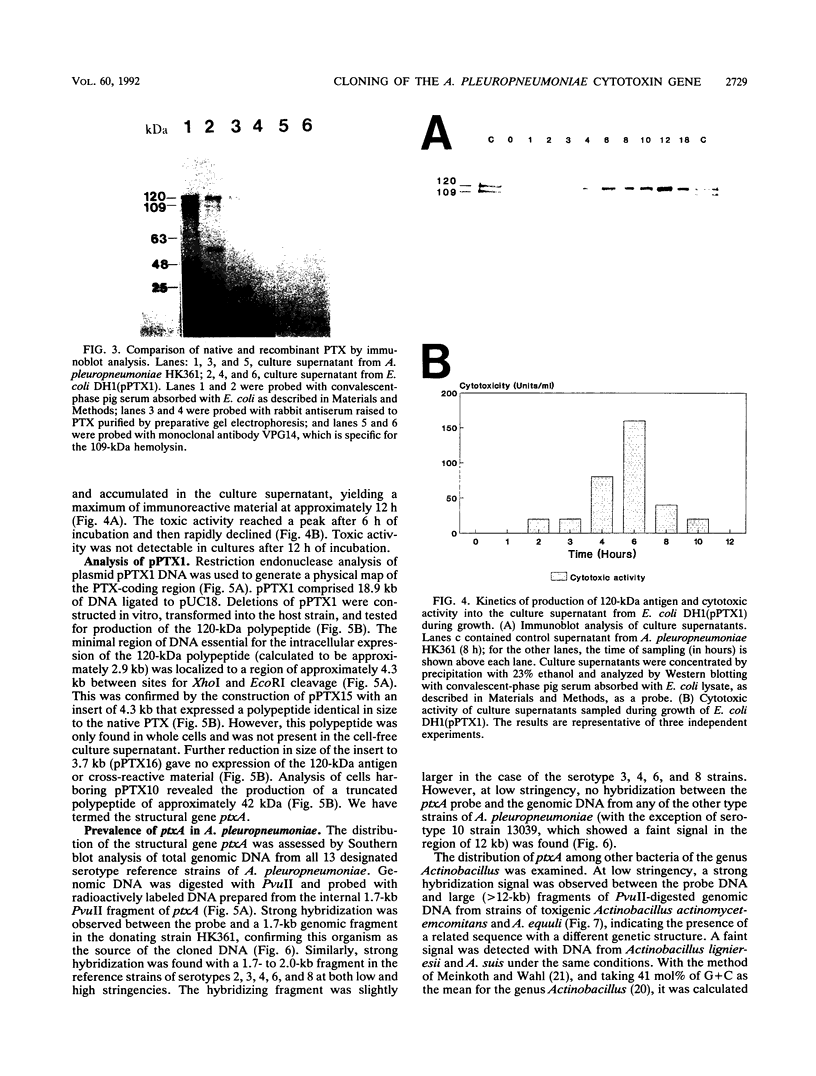
The genetic determinants of the 120-kDa cytotoxin of Actinobacillus pleuropneumoniae serotype 2 were isolated from a lambda DNA library by a plaque immunoblot technique. Expression of the 120-kDa polypeptide was confirmed by Western immunoblot analysis of infected Escherichia coli cell lysates, which were shown to be toxic for porcine alveolar macrophages in vitro. The genetic determinants of the toxin were subcloned into the plasmid vector pUC18. This plasmid (pPTX1) directed the synthesis and secretion of the active 120-kDa cytotoxin in E. coli. The recombinant toxin was indistinguishable from native cytotoxin from A. pleuropneumoniae serotype 2 with respect to molecular size, reaction in Western blot analysis, heat lability, cytotoxic activity, and neutralization by serum antibody. A restriction endonuclease cleavage map of pPTX1 was prepared, and deletion mutants were used to locate the minimal region of DNA required for production of intracellular toxin; this gene was termed ptxA. Southern hybridization analysis with a 1.7-kb PvuII fragment located within the ptxA gene revealed sequences with a high degree of homology in serotype reference strains 2, 3, 4, 6, and 8. Other reference strains did not contain sequences that were recognized by this probe. However, related sequences (greater than 71% homology) were detected in Actinobacillus actinomycetemcomitans and A. equuli. Weak hybridization was observed between the ptxA probe and pLKT5, which carries the lktAC genes of Pasteurella haemolytica, and between the ptxA probe and pAPH1, which carries the structural gene for type II hemolysin from A. pleuropneumoniae. The isolation of the genetic determinants of this cytotoxin will enable investigations of the structure and organization of the ptx DNA region and further analysis of its role in the pathogenesis of pleuropneumonia.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bendixen P. H., Shewen P. E., Rosendal S., Wilkie B. N. Toxicity of Haemophilus pleuropneumoniae for porcine lung macrophages, peripheral blood monocytes, and testicular cells. Infect Immun. 1981 Sep;33(3):673–676. doi: 10.1128/iai.33.3.673-676.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram T. A. Actinobacillus pleuropneumoniae: molecular aspects of virulence and pulmonary injury. Can J Vet Res. 1990 Apr;54 (Suppl):S53–S56. [PubMed] [Google Scholar]
- Bertram T. A. Pathobiology of Acute Pulmonary Lesions in Swine Infected with Haemophilus (Actinobacillus) pleuropneumoniae. Can Vet J. 1988 Jul;29(7):574–577. [PMC free article] [PubMed] [Google Scholar]
- Chang Y. F., Young R., Struck D. K. Cloning and characterization of a hemolysin gene from Actinobacillus (Haemophilus) pleuropneumoniae. DNA. 1989 Nov;8(9):635–647. doi: 10.1089/dna.1.1989.8.635. [DOI] [PubMed] [Google Scholar]
- Chang Y. F., Young R., Struck D. K. The Actinobacillus pleuropneumoniae hemolysin determinant: unlinked appCA and appBD loci flanked by pseudogenes. J Bacteriol. 1991 Aug;173(16):5151–5158. doi: 10.1128/jb.173.16.5151-5158.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Properties of a supercoiled deoxyribonucleic acid-protein relaxation complex and strand specificity of the relaxation event. Biochemistry. 1970 Oct 27;9(22):4428–4440. doi: 10.1021/bi00824a026. [DOI] [PubMed] [Google Scholar]
- Devenish J., Rosendal S. Identification of the heat-labile hemolysin of Actinobacillus pleuropneumoniae serotype 1. Can J Vet Res. 1989 Apr;53(2):251–254. [PMC free article] [PubMed] [Google Scholar]
- Frey J., Deillon J. B., Gygi D., Nicolet J. Identification and partial characterization of the hemolysin (HlyII) of Actinobacillus pleuropneumoniae serotype 2. Vet Microbiol. 1991 Aug 15;28(3):303–312. doi: 10.1016/0378-1135(91)90085-t. [DOI] [PubMed] [Google Scholar]
- Gygi D., Nicolet J., Frey J., Cross M., Koronakis V., Hughes C. Isolation of the Actinobacillus pleuropneumoniae haemolysin gene and the activation and secretion of the prohaemolysin by the HlyC, HlyB and HlyD proteins of Escherichia coli. Mol Microbiol. 1990 Jan;4(1):123–128. doi: 10.1111/j.1365-2958.1990.tb02021.x. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Kamp E. M., Popma J. K., Anakotta J., Smits M. A. Identification of hemolytic and cytotoxic proteins of Actinobacillus pleuropneumoniae by use of monoclonal antibodies. Infect Immun. 1991 Sep;59(9):3079–3085. doi: 10.1128/iai.59.9.3079-3085.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamp E. M., Popma J. K., Van Leengoed L. A. Serotyping of Haemophilus pleuropneumoniae in the Netherlands: with emphasis on heterogeneity within serotype 1 and (proposed) serotype 9. Vet Microbiol. 1987 Mar;13(3):249–257. doi: 10.1016/0378-1135(87)90087-3. [DOI] [PubMed] [Google Scholar]
- Kamp E. M., van Leengoed L. A. Serotype-related differences in production and type of heat-labile hemolysin and heat-labile cytotoxin of Actinobacillus (Haemophilus) pleuropneumoniae. J Clin Microbiol. 1989 Jun;27(6):1187–1191. doi: 10.1128/jcm.27.6.1187-1191.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume K., Nakai T., Sawata A. Interaction between heat-stable hemolytic substance from Haemophilus pleuropneumoniae and porcine pulmonary macrophages in vitro. Infect Immun. 1986 Feb;51(2):563–570. doi: 10.1128/iai.51.2.563-570.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liggett A. D., Harrison L. R., Farrell R. L. Sequential study of lesion development in experimental haemophilus pleuropneumonia. Res Vet Sci. 1987 Mar;42(2):204–212. [PubMed] [Google Scholar]
- Meinkoth J., Wahl G. Hybridization of nucleic acids immobilized on solid supports. Anal Biochem. 1984 May 1;138(2):267–284. doi: 10.1016/0003-2697(84)90808-x. [DOI] [PubMed] [Google Scholar]
- Nielsen R., O'Connor P. J. Serological characterization of 8 Haemophilus pleuropneumoniae strains and proposal of a new serotype: serotype 8. Acta Vet Scand. 1984;25(1):96–106. doi: 10.1186/BF03547283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen R. Serological characterization of Actinobacillus pleuropneumoniae strains and proposal of a new serotype: serotype 12. Acta Vet Scand. 1986;27(3):453–455. doi: 10.1186/BF03548158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen R. Serological characterization of Haemophilus pleuropneumoniae (Actinobacillus pleuropneumoniae) strains and proposal of a new serotype: serotype 10. Acta Vet Scand. 1985;26(4):581–585. doi: 10.1186/BF03546528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen R. Serological characterization of Haemophilus pleuropneumoniae (Actinobacillus pleuropneumoniae) strains and proposal of a new serotype: serotype 9. Acta Vet Scand. 1985;26(4):501–512. doi: 10.1186/BF03546522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen R. Serology of Haemophilus (Actinobacillus) pleuropneumoniae serotype 5 strains: establishment of subtypes a and b. Acta Vet Scand. 1986;27(1):49–58. doi: 10.1186/BF03548558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosendal S., Boyd D. A. Haemophilus pleuropneumoniae serotyping. J Clin Microbiol. 1982 Nov;16(5):840–843. doi: 10.1128/jcm.16.5.840-843.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosendal S., Devenish J., MacInnes J. I., Lumsden J. H., Watson S., Xun H. Evaluation of heat-sensitive, neutrophil-toxic, and hemolytic activity of Haemophilus (Actinobacillus) pleuropneumoniae. Am J Vet Res. 1988 Jul;49(7):1053–1058. [PubMed] [Google Scholar]
- Rycroft A. N., Cullen J. M. Complement resistance in Actinobacillus (Haemophilus) pleuropneumoniae infection of swine. Am J Vet Res. 1990 Sep;51(9):1449–1453. [PubMed] [Google Scholar]
- Rycroft A. N., Williams D., Cullen J. M., Macdonald J. The cytotoxin of Actinobacillus pleuropneumoniae (pleurotoxin) is distinct from the haemolysin and is associated with a 120 kDa polypeptide. J Gen Microbiol. 1991 Mar;137(3):561–568. doi: 10.1099/00221287-137-3-561. [DOI] [PubMed] [Google Scholar]
- Rycroft A. N., Williams D., McCandlish I. A., Taylor D. J. Experimental reproduction of acute lesions of porcine pleuropneumonia with a haemolysin-deficient mutant of Actinobacillus pleuropneumoniae. Vet Rec. 1991 Nov 16;129(20):441–443. doi: 10.1136/vr.129.20.441. [DOI] [PubMed] [Google Scholar]
- Sebunya T. N., Saunders J. R. Haemophilus pleuropneumoniae infection in swine: a review. J Am Vet Med Assoc. 1983 Jun 15;182(12):1331–1337. [PubMed] [Google Scholar]
- Strathdee C. A., Lo R. Y. Cloning, nucleotide sequence, and characterization of genes encoding the secretion function of the Pasteurella haemolytica leukotoxin determinant. J Bacteriol. 1989 Feb;171(2):916–928. doi: 10.1128/jb.171.2.916-928.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. C., Shenker B. J., DiRienzo J. M., Malamud D., Taichman N. S. Extraction and isolation of a leukotoxin from Actinobacillus actinomycetemcomitans with polymyxin B. Infect Immun. 1984 Feb;43(2):700–705. doi: 10.1128/iai.43.2.700-705.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Leengoed L. A., Kamp E. M., Pol J. M. Toxicity of Haemophilus pleuropneumoniae to porcine lung macrophages. Vet Microbiol. 1989 Apr;19(4):337–349. doi: 10.1016/0378-1135(89)90099-0. [DOI] [PubMed] [Google Scholar]
- Welch R. A., Pellett S. Transcriptional organization of the Escherichia coli hemolysin genes. J Bacteriol. 1988 Apr;170(4):1622–1630. doi: 10.1128/jb.170.4.1622-1630.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]