Abstract
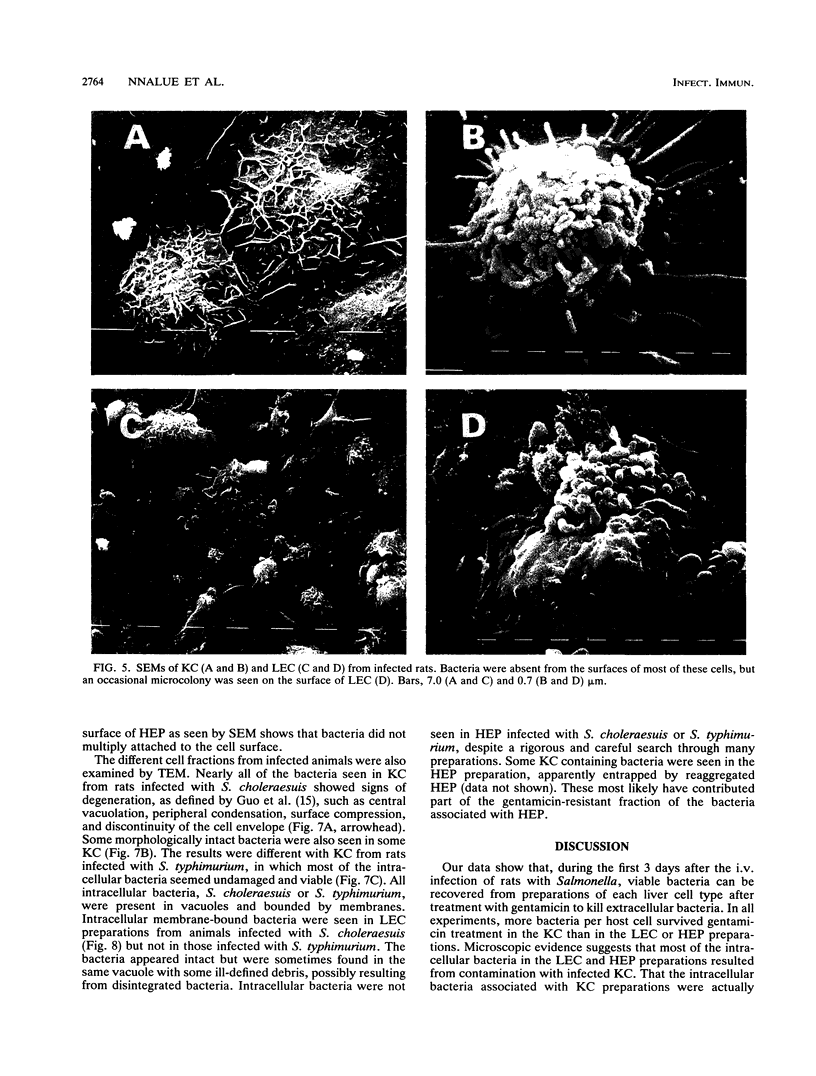
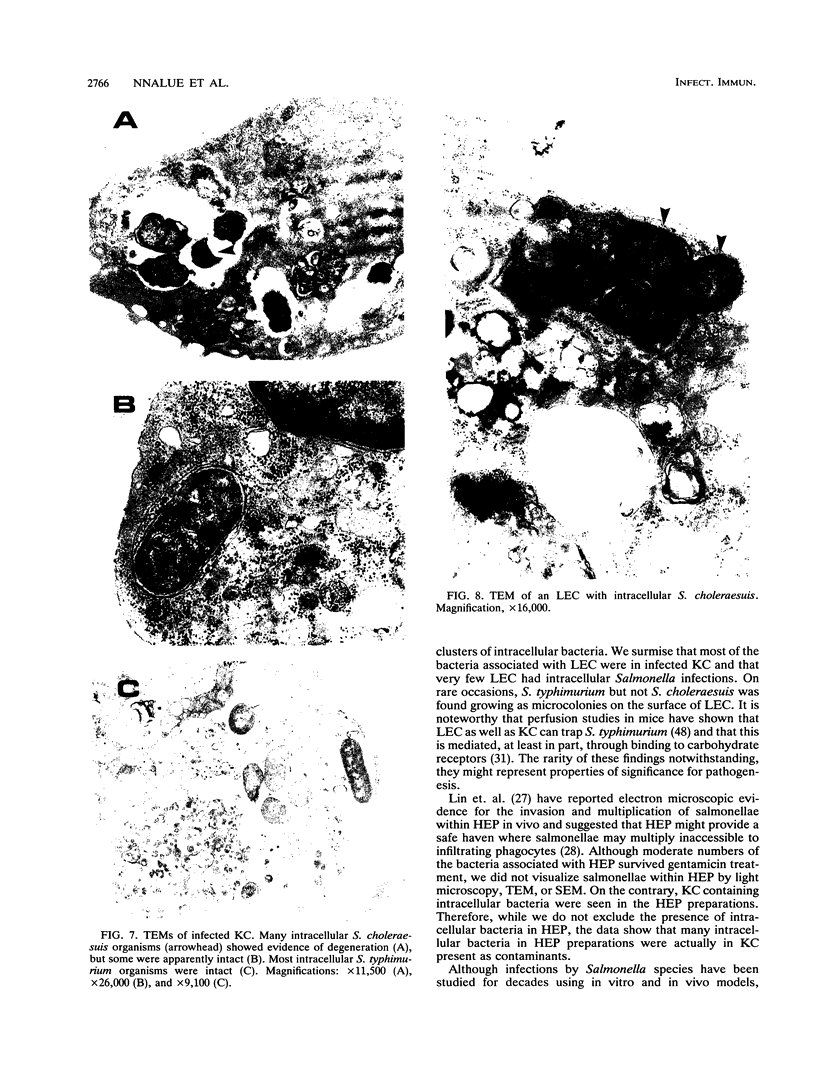
Male Sprague-Dawley rats were inoculated intravenously with Salmonella choleraesuis or Salmonella typhimurium and used over 3 consecutive days to produce highly enriched (greater than 95% homogenous) preparations of Kupffer and mononuclear cells (KC), liver endothelial cells (LEC), and hepatocytes. The methods involved collagenase perfusion of the liver in situ, differential centrifugation of liver cells over a Percoll gradient, and selective attachment of the cells to plastic or to culture dishes coated with collagen. The different cell preparations were then assayed for the number and location, intracellular or extracellular, of associated viable bacteria. Most of the viable bacteria recovered were associated with KC and were mainly intracellular. The intracellular bacteria in KC from rats infected with either bacterial strain increased about 20- to 50-fold over 2 days. Some of the bacteria associated with LEC and in some experiments with hepatocytes also survived treatment with gentamicin and increased in number with time. Intracellular bacteria were readily visualized in KC by light microscopy and transmission electron microscopy. On rare occasions, bacteria were seen within LEC from rats infected with S. choleraesuis but not from those infected with S. typhimurium. Microcolonies of S. typhimurium but not of S. choleraesuis were occasionally found on the surface of some LEC. Bacteria were not seen within or on the surface of hepatocytes by transmission or scanning electron microscopy. The integration of microscopic and viability data suggested that most intracellular S. choleraesuis organisms in KC had been killed whereas most intracellular S. typhimurium organisms were viable.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benjamin W. H., Jr, Hall P., Roberts S. J., Briles D. E. The primary effect of the Ity locus is on the rate of growth of Salmonella typhimurium that are relatively protected from killing. J Immunol. 1990 Apr 15;144(8):3143–3151. [PubMed] [Google Scholar]
- Blomhoff R., Smedsrød B., Eskild W., Granum P. E., Berg T. Preparation of isolated liver endothelial cells and Kupffer cells in high yield by means of an enterotoxin. Exp Cell Res. 1984 Jan;150(1):194–204. doi: 10.1016/0014-4827(84)90714-6. [DOI] [PubMed] [Google Scholar]
- Bouwens L., Wisse E. Proliferation, kinetics, and fate of monocytes in rat liver during a zymosan-induced inflammation. J Leukoc Biol. 1985 May;37(5):531–543. doi: 10.1002/jlb.37.5.531. [DOI] [PubMed] [Google Scholar]
- Briles D. E., Benjamin W., Jr, Posey B., Michalek S. M., McGhee J. R. Independence of macrophage activation and expression of the alleles of the Ity (immunity to typhimurium) locus. Microb Pathog. 1986 Feb;1(1):33–41. doi: 10.1016/0882-4010(86)90029-x. [DOI] [PubMed] [Google Scholar]
- Brown A., Hormaeche C. E. The antibody response to salmonellae in mice and humans studied by immunoblots and ELISA. Microb Pathog. 1989 Jun;6(6):445–454. doi: 10.1016/0882-4010(89)90086-7. [DOI] [PubMed] [Google Scholar]
- Buchmeier N. A., Heffron F. Intracellular survival of wild-type Salmonella typhimurium and macrophage-sensitive mutants in diverse populations of macrophages. Infect Immun. 1989 Jan;57(1):1–7. doi: 10.1128/iai.57.1.1-7.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M., Campbell S. G. Immunity to intracellular bacteria. Vet Immunol Immunopathol. 1982 Jan;3(1-2):5–66. doi: 10.1016/0165-2427(82)90031-9. [DOI] [PubMed] [Google Scholar]
- Collins F. M. Cellular antimicrobial immunity. CRC Crit Rev Microbiol. 1978;7(1):27–91. doi: 10.3109/10408417909101177. [DOI] [PubMed] [Google Scholar]
- Collins F. M. Vaccines and cell-mediated immunity. Bacteriol Rev. 1974 Dec;38(4):371–402. doi: 10.1128/br.38.4.371-402.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap N. E., Benjamin W. H., Jr, McCall R. D., Jr, Tilden A. B., Briles D. E. A 'safe-site' for Salmonella typhimurium is within splenic cells during the early phase of infection in mice. Microb Pathog. 1991 Apr;10(4):297–310. doi: 10.1016/0882-4010(91)90013-z. [DOI] [PubMed] [Google Scholar]
- Edwards C. K., 3rd, Yunger L. M., Lorence R. M., Dantzer R., Kelley K. W. The pituitary gland is required for protection against lethal effects of Salmonella typhimurium. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2274–2277. doi: 10.1073/pnas.88.6.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein T. K., Sultzer B. M. Immunity to Salmonella infection. Adv Exp Med Biol. 1983;162:261–296. doi: 10.1007/978-1-4684-4481-0_26. [DOI] [PubMed] [Google Scholar]
- Friedman R. L., Moon R. J. Hepatic clearance of Salmonella typhimurium in silica-treated mice. Infect Immun. 1977 Jun;16(3):1005–1012. doi: 10.1128/iai.16.3.1005-1012.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y. N., Hsu H. S., Mumaw V. R., Nakoneczna I. Electronmicroscopy studies on the bactericidal action of inflammatory leukocytes in murine salmonellosis. J Med Microbiol. 1986 Mar;21(2):151–159. doi: 10.1099/00222615-21-2-151. [DOI] [PubMed] [Google Scholar]
- Harrington K. A., Hormaeche C. E. Expression of the innate resistance gene Ity in mouse Kupffer cells infected with Salmonella typhimurium in vitro. Microb Pathog. 1986 Jun;1(3):269–274. doi: 10.1016/0882-4010(86)90051-3. [DOI] [PubMed] [Google Scholar]
- Hougen H. P., Jensen E. T. Experimental Salmonella typhimurium infections in rats. III. Transfer of immunity with primed lymphocyte subpopulations. APMIS. 1990 Nov;98(11):1015–1021. [PubMed] [Google Scholar]
- Hougen H. P., Jensen E. T., Klausen B. Experimental Salmonella typhimurium infections in rats. I: Influence of thymus on the course of infection. APMIS. 1989 Sep;97(9):825–832. [PubMed] [Google Scholar]
- Hsu H. S. Pathogenesis and immunity in murine salmonellosis. Microbiol Rev. 1989 Dec;53(4):390–409. doi: 10.1128/mr.53.4.390-409.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantele A., Arvilommi H., Kantele J. M., Rintala L., Mäkelä P. H. Comparison of the human immune response to live oral, killed oral or killed parenteral Salmonella typhi TY21A vaccines. Microb Pathog. 1991 Feb;10(2):117–126. doi: 10.1016/0882-4010(91)90072-i. [DOI] [PubMed] [Google Scholar]
- Knook D. L., Blansjaar N., Sleyster E. C. Isolation and characterization of Kupffer and endothelial cells from the rat liver. Exp Cell Res. 1977 Oct 15;109(2):317–329. doi: 10.1016/0014-4827(77)90011-8. [DOI] [PubMed] [Google Scholar]
- Knook D. L., Sleyster E. C. Separation of Kupffer and endothelial cells of the rat liver by centrifugal elutriation. Exp Cell Res. 1976 May;99(2):444–449. doi: 10.1016/0014-4827(76)90605-4. [DOI] [PubMed] [Google Scholar]
- Kuusi N., Nurminen M., Saxén H., Mäkelä P. H. Immunization with major outer membrane protein (porin) preparations in experimental murine salmonellosis: effect of lipopolysaccharide. Infect Immun. 1981 Nov;34(2):328–332. doi: 10.1128/iai.34.2.328-332.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin D. M., Wong K. H., Reynolds H. Y., Sutton A., Northrup R. S. Vi antigen from Salmonella typhosa and immunity against typhoid fever. 11. Safety and antigenicity in humans. Infect Immun. 1975 Dec;12(6):1290–1294. doi: 10.1128/iai.12.6.1290-1294.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. M., Kaper J. B., Black R. E., Clements M. L. New knowledge on pathogenesis of bacterial enteric infections as applied to vaccine development. Microbiol Rev. 1983 Dec;47(4):510–550. doi: 10.1128/mr.47.4.510-550.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang-Takasaki C. J., Grossman N., Leive L. Salmonellae activate complement differentially via the alternative pathway depending on the structure of their lipopolysaccharide O-antigen. J Immunol. 1983 Apr;130(4):1867–1870. [PubMed] [Google Scholar]
- Lin F. R., Hsu H. S., Mumaw V. R., Nakoneczna I. Intracellular destruction of salmonellae in genetically resistant mice. J Med Microbiol. 1989 Sep;30(1):79–87. doi: 10.1099/00222615-30-1-79. [DOI] [PubMed] [Google Scholar]
- Lin F. R., Wang X. M., Hsu H. S., Mumaw V. R., Nakoneczna I. Electron microscopic studies on the location of bacterial proliferation in the liver in murine salmonellosis. Br J Exp Pathol. 1987 Aug;68(4):539–550. [PMC free article] [PubMed] [Google Scholar]
- Lissner C. R., Swanson R. N., O'Brien A. D. Genetic control of the innate resistance of mice to Salmonella typhimurium: expression of the Ity gene in peritoneal and splenic macrophages isolated in vitro. J Immunol. 1983 Dec;131(6):3006–3013. [PubMed] [Google Scholar]
- Mackaness G. B., Blanden R. V., Collins F. M. Host-parasite relations in mouse typhoid. J Exp Med. 1966 Oct 1;124(4):573–583. doi: 10.1084/jem.124.4.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon R. J., Leunk R. D. Pilus-mediated clearance of Salmonella typhimurium by the perfused mouse liver. Adv Exp Med Biol. 1983;162:251–259. doi: 10.1007/978-1-4684-4481-0_25. [DOI] [PubMed] [Google Scholar]
- Nnalue N. A. Mice vaccinated with a non-virulent, aromatic-dependent mutant of Salmonella choleraesuis die from challenge with its virulent parent but survive challenge with Salmonella typhimurium. J Med Microbiol. 1990 Apr;31(4):225–233. doi: 10.1099/00222615-31-4-225. [DOI] [PubMed] [Google Scholar]
- Nnalue N. A., Stocker B. A. Test of the virulence and live-vaccine efficacy of auxotrophic and galE derivatives of Salmonella choleraesuis. Infect Immun. 1987 Apr;55(4):955–962. doi: 10.1128/iai.55.4.955-962.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nnalue N. A., Stocker B. A. Vaccination route, infectivity and thioglycollate broth administration: effects on live vaccine efficacy of auxotrophic derivatives of Salmonella choleraesuis. Microb Pathog. 1989 Oct;7(4):299–310. doi: 10.1016/0882-4010(89)90048-x. [DOI] [PubMed] [Google Scholar]
- O'Callaghan D., Maskell D., Liew F. Y., Easmon C. S., Dougan G. Characterization of aromatic- and purine-dependent Salmonella typhimurium: attention, persistence, and ability to induce protective immunity in BALB/c mice. Infect Immun. 1988 Feb;56(2):419–423. doi: 10.1128/iai.56.2.419-423.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornellas E. P., Stocker B. A. Relation of lipopolysaccharide character to P1 sensitivity in Salmonella typhimurium. Virology. 1974 Aug;60(2):491–502. doi: 10.1016/0042-6822(74)90343-2. [DOI] [PubMed] [Google Scholar]
- Rabinovitch M. Attachment of modified erythrocytes to phagocytic cells in absence of serum. Proc Soc Exp Biol Med. 1967 Feb;124(2):396–399. doi: 10.3181/00379727-124-31749. [DOI] [PubMed] [Google Scholar]
- Robertsson J. A., Fossum C., Svenson S. B., Lindberg A. A. Salmonella typhimurium infection in calves: specific immune reactivity against O-antigenic polysaccharide detectable in in vitro assays. Infect Immun. 1982 Aug;37(2):728–736. doi: 10.1128/iai.37.2.728-736.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxén H., Reima I., Mäkelä P. H. Alternative complement pathway activation by Salmonella O polysaccharide as a virulence determinant in the mouse. Microb Pathog. 1987 Jan;2(1):15–28. doi: 10.1016/0882-4010(87)90111-2. [DOI] [PubMed] [Google Scholar]
- Shnyra A., Bocharov A., Bochkova N., Spirov V. Large-scale production and cultivation of hepatocytes on Biosilon microcarriers. Artif Organs. 1990 Dec;14(6):421–428. doi: 10.1111/j.1525-1594.1990.tb02998.x. [DOI] [PubMed] [Google Scholar]
- Smedsrød B., Pertoft H. Preparation of pure hepatocytes and reticuloendothelial cells in high yield from a single rat liver by means of Percoll centrifugation and selective adherence. J Leukoc Biol. 1985 Aug;38(2):213–230. doi: 10.1002/jlb.38.2.213. [DOI] [PubMed] [Google Scholar]
- Svenson S. B., Lindberg A. A. Artificial Salmonella vaccines: Salmonella typhimurium O-antigen-specific oligosaccharide-protein conjugates elicit protective antibodies in rabbits and mice. Infect Immun. 1981 May;32(2):490–496. doi: 10.1128/iai.32.2.490-496.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi A. Electron microscope studies of experimental Salmonella infection. I. Penetration into the intestinal epithelium by Salmonella typhimurium. Am J Pathol. 1967 Jan;50(1):109–136. [PMC free article] [PubMed] [Google Scholar]
- Takeuchi A., Sprinz H. Electron-Microscope Studies of Experimental Salmonella Infection in the Preconditioned Guinea Pig: II. Response of the Intestinal Mucosa to the Invasion by Salmonella typhimurium. Am J Pathol. 1967 Jul;51(1):137–161. [PMC free article] [PubMed] [Google Scholar]
- Vladoianu I. R., Chang H. R., Pechère J. C. Expression of host resistance to Salmonella typhi and Salmonella typhimurium: bacterial survival within macrophages of murine and human origin. Microb Pathog. 1990 Feb;8(2):83–90. doi: 10.1016/0882-4010(90)90072-x. [DOI] [PubMed] [Google Scholar]
- Wahdan M. H., Sérié C., Cerisier Y., Sallam S., Germanier R. A controlled field trial of live Salmonella typhi strain Ty 21a oral vaccine against typhoid: three-year results. J Infect Dis. 1982 Mar;145(3):292–295. doi: 10.1093/infdis/145.3.292. [DOI] [PubMed] [Google Scholar]
- Widmann J. J., Cotran R. S., Fahimi H. D. Mononuclear phagocytes (Kupffer cells) and endothelial cells. Identification of two functional cell types in rat liver sinusoids by endogenous peroxidase activity. J Cell Biol. 1972 Jan;52(1):159–170. doi: 10.1083/jcb.52.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dissel J. T., Stikkelbroeck J. J., Michel B. C., Leijh P. C., van Furth R. Salmonella-typhimurium-specific difference in rate of intracellular killing by resident peritoneal macrophages from salmonella-resistant CBA and salmonella-susceptible C57BL/10 mice. J Immunol. 1987 Jun 15;138(12):4428–4434. [PubMed] [Google Scholar]
- van Dissel J. T., Stikkelbroeck J. J., Sluiter W., Leijh P. C., van Furth R. Differences in initial rate of intracellular killing of Salmonella typhimurium by granulocytes of Salmonella-susceptible C57BL/10 mice and Salmonella-resistant CBA mice. J Immunol. 1986 Feb 1;136(3):1074–1080. [PubMed] [Google Scholar]









