Abstract
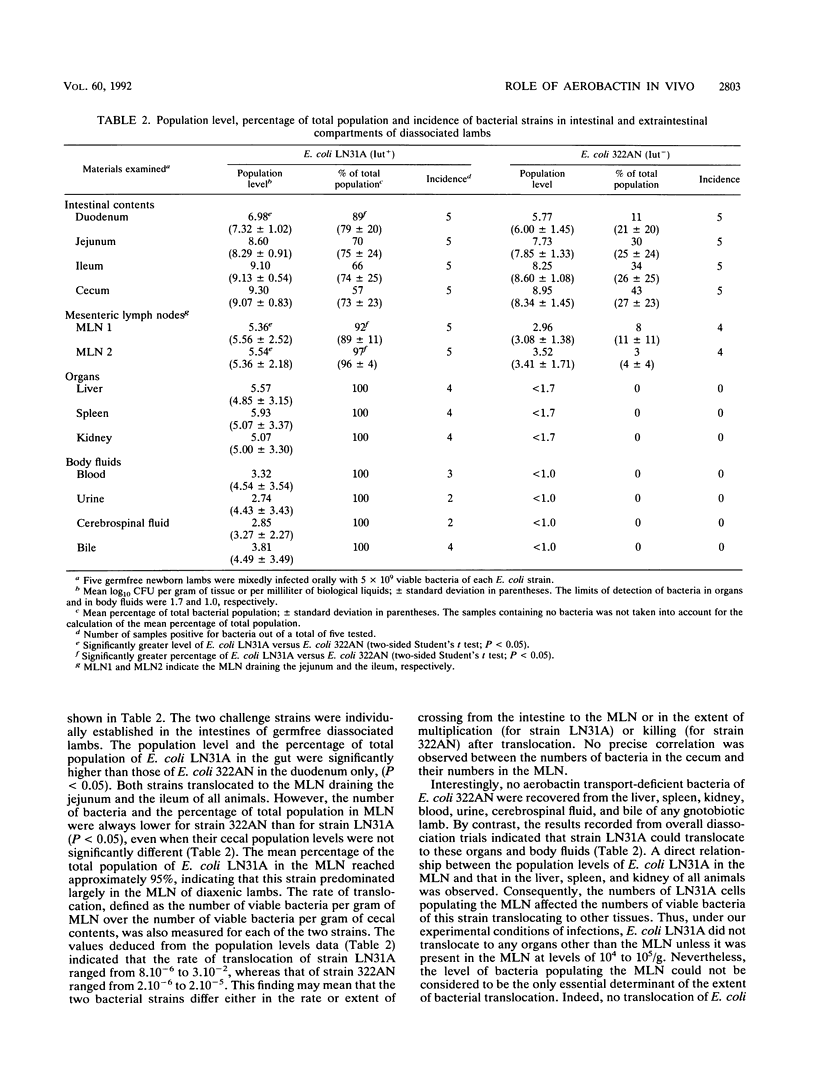
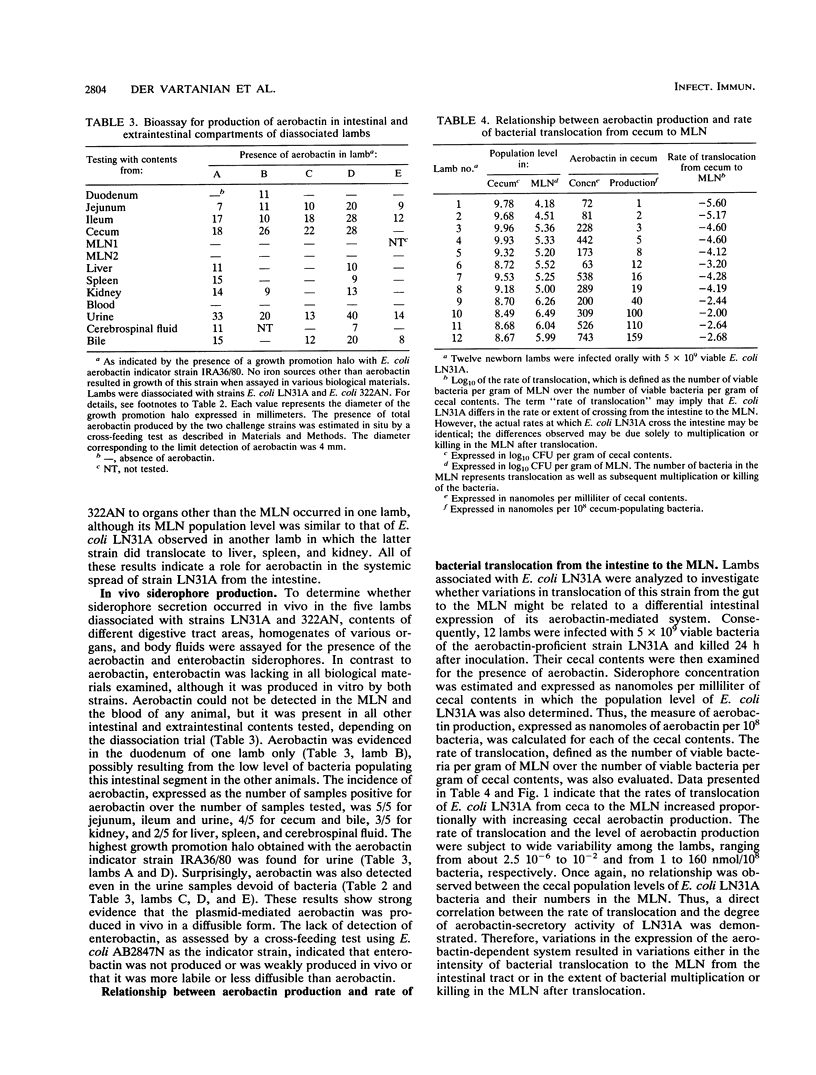
To assess the role of the aerobactin-related system in the virulence of bovine opportunistic Escherichia coli, and to determine the stage(s) of the overall infectious process at which it is acting, germfree lambs were mixedly infected orally with two derivative strains of this bacterium differing in their ability (Iut+) or inability (Iut-) to express a functional aerobactin-mediated iron transport system. The Iut- strain was compared with the Iut+ strain for colonization of the gut, translocation to the mesenteric lymph nodes (MLN), and spread to other organs and to the body fluids of diassociated lambs. The Iut- mutant was found in smaller numbers in the duodenum, suggesting that aerobactin conferred a significant selective advantage for colonization of this intestinal segment. Although the two challenge strains translocated to MLN, the population level in the MLN was always higher for the Iut+ strain. Moreover, experimental infections resulted in recovery of only the Iut+ strain in the organs other than the MLN and in the body fluids. These results indicate a role for aerobactin in promoting systemic spread of the bacteria from the intestine. Direct evidence was obtained that aerobactin secretion occurred in vivo at both intestinal and extraintestinal sites of infection. In contrast to enterobactin, aerobactin was detected in the duodenum, jejunum, ileum, cecum, liver, spleen, kidney, urine, cerebrospinal fluid, and bile. The highest concentration of aerobactin was found in the urine, even when the samples were devoid of infecting bacteria. All of these findings suggest that aerobactin is released in vivo in a diffusible form and that it may be an important step in the production of disease by intestinal opportunistic E. coli.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg R. D. Mechanisms confining indigenous bacteria to the gastrointestinal tract. Am J Clin Nutr. 1980 Nov;33(11 Suppl):2472–2484. doi: 10.1093/ajcn/33.11.2472. [DOI] [PubMed] [Google Scholar]
- Bindereif A., Braun V., Hantke K. The cloacin receptor of ColV-bearing Escherichia coli is part of the Fe3+-aerobactin transport system. J Bacteriol. 1982 Jun;150(3):1472–1475. doi: 10.1128/jb.150.3.1472-1475.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullen J. J., Armstrong J. A. The role of lactoferrin in the bactericidal function of polymorphonuclear leucocytes. Immunology. 1979 Apr;36(4):781–791. [PMC free article] [PubMed] [Google Scholar]
- Bullen J. J. The significance of iron in infection. Rev Infect Dis. 1981 Nov-Dec;3(6):1127–1138. doi: 10.1093/clinids/3.6.1127. [DOI] [PubMed] [Google Scholar]
- Carbonetti N. H., Williams P. H. A cluster of five genes specifying the aerobactin iron uptake system of plasmid ColV-K30. Infect Immun. 1984 Oct;46(1):7–12. doi: 10.1128/iai.46.1.7-12.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contrepois M., Dubourguier H. C., Parodi A. L., Girardeau J. P., Ollier J. L. Septicaemic Escherichia coli and experimental infection of calves. Vet Microbiol. 1986 Jul;12(2):109–118. doi: 10.1016/0378-1135(86)90073-8. [DOI] [PubMed] [Google Scholar]
- Cox T. M., Mazurier J., Spik G., Montreuil J., Peters T. J. Iron binding proteins and influx of iron across the duodenal brush border. Evidence for specific lactotransferrin receptors in the human intestine. Biochim Biophys Acta. 1979 Nov 15;588(1):120–128. doi: 10.1016/0304-4165(79)90377-5. [DOI] [PubMed] [Google Scholar]
- Der Vartanian M. Differences in excretion and efficiency of the aerobactin and enterochelin siderophores in a bovine pathogenic strain of Escherichia coli. Infect Immun. 1988 Feb;56(2):413–418. doi: 10.1128/iai.56.2.413-418.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardeau J. P., Der Vartanian M., Ollier J. L., Contrepois M. CS31A, a new K88-related fimbrial antigen on bovine enterotoxigenic and septicemic Escherichia coli strains. Infect Immun. 1988 Aug;56(8):2180–2188. doi: 10.1128/iai.56.8.2180-2188.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale T. L., Sansonetti P. J., Schad P. A., Austin S., Formal S. B. Characterization of virulence plasmids and plasmid-associated outer membrane proteins in Shigella flexneri, Shigella sonnei, and Escherichia coli. Infect Immun. 1983 Apr;40(1):340–350. doi: 10.1128/iai.40.1.340-350.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haurani F. I., Meyer A., O'Brien R. Production of transferrin by the macrophage. J Reticuloendothel Soc. 1973 Sep;14(3):309–316. [PubMed] [Google Scholar]
- Jacobson S. H., Tullus K., Wretlind B., Brauner A. Aerobactin-mediated uptake of iron by strains of Escherichia coli causing acute pyelonephritis and bacteraemia. J Infect. 1988 Mar;16(2):147–152. doi: 10.1016/s0163-4453(88)93947-3. [DOI] [PubMed] [Google Scholar]
- Johnson J. R. Virulence factors in Escherichia coli urinary tract infection. Clin Microbiol Rev. 1991 Jan;4(1):80–128. doi: 10.1128/cmr.4.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kado C. I., Liu S. T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maneva A. I., Sirakov L. M., Manev V. V. Lactoferrin binding to neutrophilic polymorphonuclear leucocytes. Int J Biochem. 1983;15(7):981–984. doi: 10.1016/0020-711x(83)90179-9. [DOI] [PubMed] [Google Scholar]
- Montgomerie J. Z., Bindereif A., Neilands J. B., Kalmanson G. M., Guze L. B. Association of hydroxamate siderophore (aerobactin) with Escherichia coli isolated from patients with bacteremia. Infect Immun. 1984 Dec;46(3):835–838. doi: 10.1128/iai.46.3.835-838.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassif X., Mazert M. C., Mounier J., Sansonetti P. J. Evaluation with an iuc::Tn10 mutant of the role of aerobactin production in the virulence of Shigella flexneri. Infect Immun. 1987 Sep;55(9):1963–1969. doi: 10.1128/iai.55.9.1963-1969.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riou Y., Gouet P., Dubourguier H. C., Contrepois M., Dardillat C., Lefaivre J. Techniques for obtaining, fistulization and rearing of axenic or gnotoxenic lambs, kids and calves. Ann Rech Vet. 1977;8(1):13–24. [PubMed] [Google Scholar]
- Shand G. H., Anwar H., Kadurugamuwa J., Brown M. R., Silverman S. H., Melling J. In vivo evidence that bacteria in urinary tract infection grow under iron-restricted conditions. Infect Immun. 1985 Apr;48(1):35–39. doi: 10.1128/iai.48.1.35-39.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen E. K., Berg R. D., Deitch E. A. Comparison of translocation rates of various indigenous bacteria from the gastrointestinal tract to the mesenteric lymph node. J Infect Dis. 1988 May;157(5):1032–1038. doi: 10.1093/infdis/157.5.1032. [DOI] [PubMed] [Google Scholar]
- Steffen E. K., Berg R. D. Relationship between cecal population levels of indigenous bacteria and translocation to the mesenteric lymph nodes. Infect Immun. 1983 Mar;39(3):1252–1259. doi: 10.1128/iai.39.3.1252-1259.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg E. D. Iron and infection. Microbiol Rev. 1978 Mar;42(1):45–66. doi: 10.1128/mr.42.1.45-66.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg E. D. Iron withholding: a defense against infection and neoplasia. Physiol Rev. 1984 Jan;64(1):65–102. doi: 10.1152/physrev.1984.64.1.65. [DOI] [PubMed] [Google Scholar]
- Wells C. L., Maddaus M. A., Simmons R. L. Proposed mechanisms for the translocation of intestinal bacteria. Rev Infect Dis. 1988 Sep-Oct;10(5):958–979. doi: 10.1093/clinids/10.5.958. [DOI] [PubMed] [Google Scholar]
- Williams P. H., Carbonetti N. H. Iron, siderophores, and the pursuit of virulence: independence of the aerobactin and enterochelin iron uptake systems in Escherichia coli. Infect Immun. 1986 Mar;51(3):942–947. doi: 10.1128/iai.51.3.942-947.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P. H. Novel iron uptake system specified by ColV plasmids: an important component in the virulence of invasive strains of Escherichia coli. Infect Immun. 1979 Dec;26(3):925–932. doi: 10.1128/iai.26.3.925-932.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P. H., Roberts M. Aerobactin-mediated iron uptake: a virulence determinant in enteropathogenic Escherichia coli? Lancet. 1985 Mar 30;1(8431):763–763. doi: 10.1016/s0140-6736(85)91311-x. [DOI] [PubMed] [Google Scholar]
- de Graaf F. K., Tieze G. A., Wendelaar Bonga S., Stouthamer A. H. Purification and genetic determination of bacteriocin production in Enterobacter cloacae. J Bacteriol. 1968 Feb;95(2):631–640. doi: 10.1128/jb.95.2.631-640.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lorenzo V., Martinez J. L. Aerobactin production as a virulence factor: a reevaluation. Eur J Clin Microbiol Infect Dis. 1988 Oct;7(5):621–629. doi: 10.1007/BF01964239. [DOI] [PubMed] [Google Scholar]


