Abstract
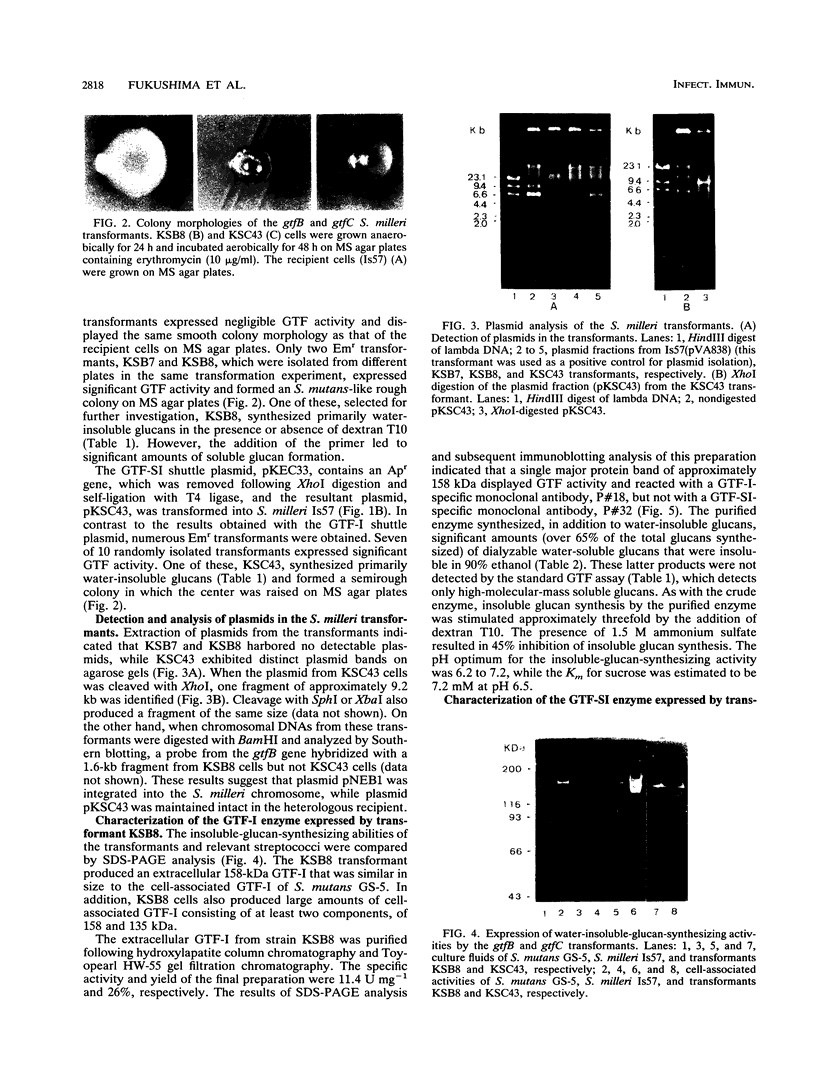
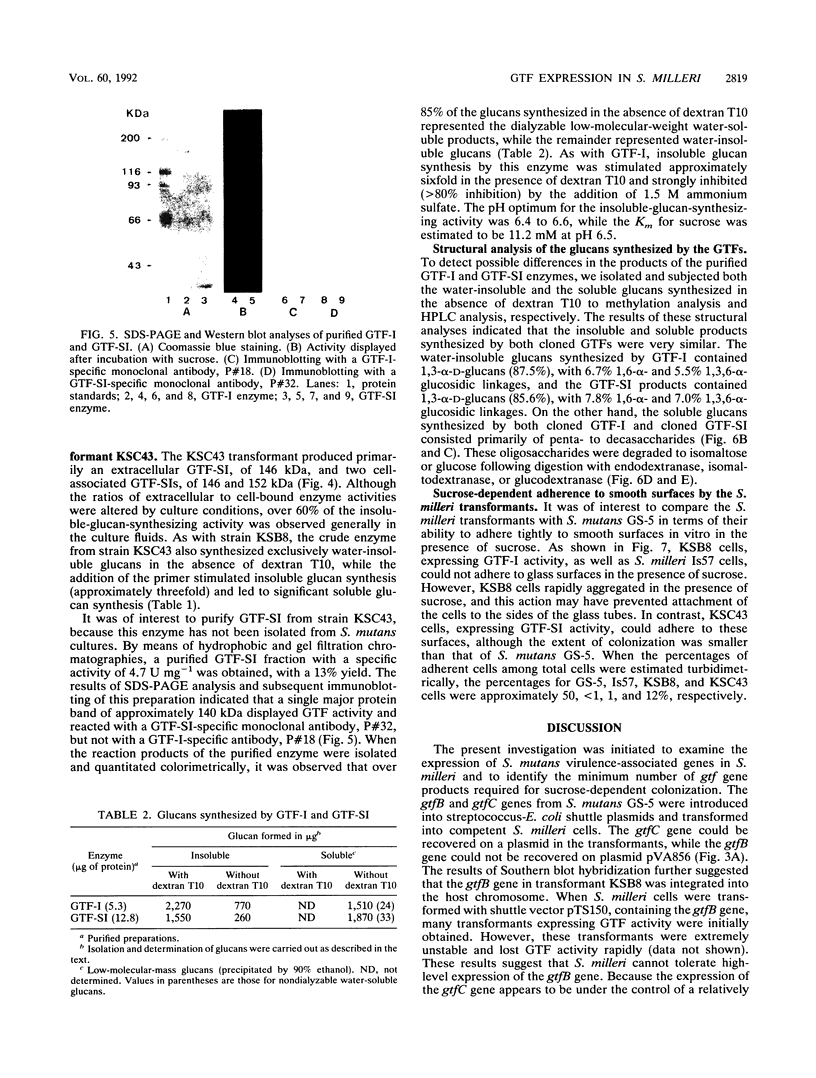
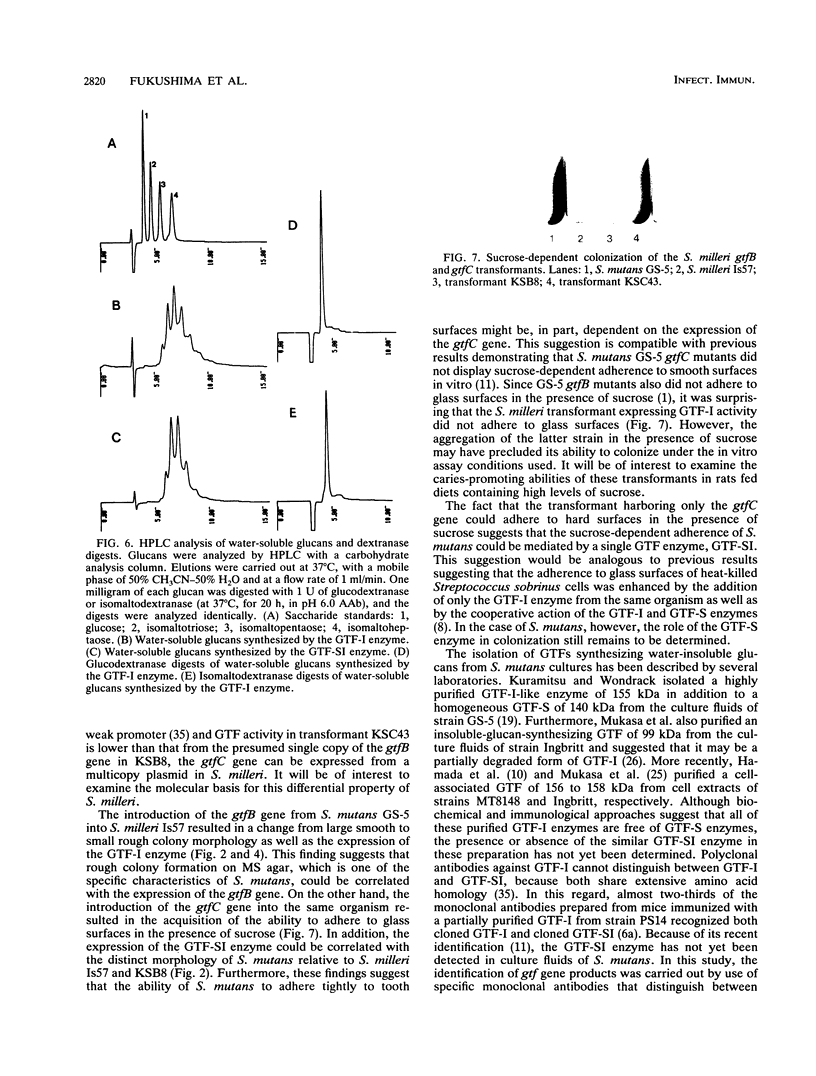
The Streptococcus mutans glucosyltransferase (GTF) genes gtfB and gtfC were ligated into Escherichia coli-streptococcus shuttle plasmids and introduced into Streptococcus milleri. gtfB transformant KSB8 formed an S. mutans-like rough colony on mitis salivarius agar and expressed an extracellular GTF-I, of 158 kDa, and two cell-bound GTF-Is, of 158 and 135 kDa. gtfC transformant KSC43 formed a semirough colony on mitis salivarius agar and expressed primarily an extracellular GTF-SI, of 146 kDa, and two cell-bound GTF-SIs, of 146 and 152 kDa. The extracellular GTFs from KSB8 and KSC43 were purified and characterized. The two types of GTF also reacted specifically with monoclonal antibodies directed against each enzyme. Both enzymes synthesized significant amounts of oligosaccharides, consisting primarily of alpha-1,6-glucosidic linkages, as well as water-insoluble glucans, containing alpha-1,3-glucosidic linkages. Insoluble-glucan-synthesizing activities of both enzymes were stimulated (three- to sixfold) by the addition of dextran T10 and were inhibited in the presence of 1.5 M ammonium sulfate. The Km(s) for sucrose and the optimal pHs were also similar for both enzymes. However, when the transformants were grown in Todd-Hewitt broth supplemented with sucrose, KSC43 cells, expressing GTF-SI activity, adhered to glass surfaces in vitro, while KSB8 cells, expressing GTF-I activity, did not. These results are discussed relative to the potential role of the gtfB and gftC genes in S. mutans cariogenicity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoki H., Shiroza T., Hayakawa M., Sato S., Kuramitsu H. K. Cloning of a Streptococcus mutans glucosyltransferase gene coding for insoluble glucan synthesis. Infect Immun. 1986 Sep;53(3):587–594. doi: 10.1128/iai.53.3.587-594.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba T., Ogawa T., Okahashi N., Yakushiji T., Koga T., Morimoto M., Hamada S. Purification and characterisation of the extracellular D-glucosyltransferase from serotype c Streptococcus mutans. Carbohydr Res. 1986 Dec 15;158:147–155. doi: 10.1016/0008-6215(86)84013-7. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Curtiss R., 3rd Genetic analysis of Streptococcus mutans virulence. Curr Top Microbiol Immunol. 1985;118:253–277. doi: 10.1007/978-3-642-70586-1_14. [DOI] [PubMed] [Google Scholar]
- Ferretti J. J., Gilpin M. L., Russell R. R. Nucleotide sequence of a glucosyltransferase gene from Streptococcus sobrinus MFe28. J Bacteriol. 1987 Sep;169(9):4271–4278. doi: 10.1128/jb.169.9.4271-4278.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima K., Motoda R., Takada K., Ikeda T. Resolution of Streptococcus mutans glycosyltransferases into two components essential to water-insoluble glucan synthesis. FEBS Lett. 1981 Jun 15;128(2):213–216. doi: 10.1016/0014-5793(81)80083-x. [DOI] [PubMed] [Google Scholar]
- Fukushima K., Takada K., Motoda R., Ikeda T. Independence of water-insoluble glucan synthesis and adherence of Streptococcus mutans to smooth surfaces. FEBS Lett. 1982 Nov 29;149(2):299–303. doi: 10.1016/0014-5793(82)81121-6. [DOI] [PubMed] [Google Scholar]
- Gilpin M. L., Russell R. R., Morrissey P. Cloning and expression of two Streptococcus mutans glucosyltransferases in Escherichia coli K-12. Infect Immun. 1985 Aug;49(2):414–416. doi: 10.1128/iai.49.2.414-416.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada S., Horikoshi T., Minami T., Okahashi N., Koga T. Purification and characterization of cell-associated glucosyltransferase synthesizing water-insoluble glucan from serotype c Streptococcus mutans. J Gen Microbiol. 1989 Feb;135(Pt 2):335–344. doi: 10.1099/00221287-135-2-335. [DOI] [PubMed] [Google Scholar]
- Hanada N., Kuramitsu H. K. Isolation and characterization of the Streptococcus mutans gtfC gene, coding for synthesis of both soluble and insoluble glucans. Infect Immun. 1988 Aug;56(8):1999–2005. doi: 10.1128/iai.56.8.1999-2005.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada N., Kuramitsu H. K. Isolation and characterization of the Streptococcus mutans gtfD gene, coding for primer-dependent soluble glucan synthesis. Infect Immun. 1989 Jul;57(7):2079–2085. doi: 10.1128/iai.57.7.2079-2085.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda O., Kato C., Kuramitsu H. K. Nucleotide sequence of the Streptococcus mutans gtfD gene encoding the glucosyltransferase-S enzyme. J Gen Microbiol. 1990 Oct;136(10):2099–2105. doi: 10.1099/00221287-136-10-2099. [DOI] [PubMed] [Google Scholar]
- Inoue M., Koga T., Sato S., Hamada S. Synthesis of adherent insoluble glucan by the concerted action of the two glucosyltransferase components of Streptococcus mutans. FEBS Lett. 1982 Jun 21;143(1):101–104. doi: 10.1016/0014-5793(82)80282-2. [DOI] [PubMed] [Google Scholar]
- Kato C., Kuramitsu H. K. Molecular basis for the association of glucosyltransferases with the cell surface of oral streptococci. FEMS Microbiol Lett. 1991 Apr 15;63(2-3):153–157. doi: 10.1111/j.1574-6968.1991.tb04521.x. [DOI] [PubMed] [Google Scholar]
- Kuramitsu H. K. Characterization of extracellular glucosyltransferase activity of Steptococcus mutans. Infect Immun. 1975 Oct;12(4):738–749. doi: 10.1128/iai.12.4.738-749.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramitsu H. K., Wondrack L. Insoluble glucan synthesis by Streptococcus mutans serotype c strains. Infect Immun. 1983 Nov;42(2):763–770. doi: 10.1128/iai.42.2.763-770.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesche W. J. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986 Dec;50(4):353–380. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrina F. L., Evans R. P., Tobian J. A., Hartley D. L., Clewell D. B., Jones K. R. Novel shuttle plasmid vehicles for Escherichia-Streptococcus transgeneric cloning. Gene. 1983 Nov;25(1):145–150. doi: 10.1016/0378-1119(83)90176-2. [DOI] [PubMed] [Google Scholar]
- Macrina F. L., Jones K. R., Wood P. H. Chimeric streptococcal plasmids and their use as molecular cloning vehicles in Streptococcus sanguis (Challis). J Bacteriol. 1980 Sep;143(3):1425–1435. doi: 10.1128/jb.143.3.1425-1435.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe M. M. Purification and characterization of a primer-independent glucosyltransferase from Streptococcus mutans 6715-13 mutant 27. Infect Immun. 1985 Dec;50(3):771–777. doi: 10.1128/iai.50.3.771-777.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukasa H., Shimamura A., Tsumori H. Purification and characterization of cell-associated glucosyltransferase synthesizing insoluble glucan from Streptococcus mutans serotype c. J Gen Microbiol. 1989 Jul;135(7):2055–2063. doi: 10.1099/00221287-135-7-2055. [DOI] [PubMed] [Google Scholar]
- Mukasa H., Tsumori H., Shimamura A. Isolation and characterization of an extracellular glucosyltransferase synthesizing insoluble glucan from Streptococcus mutans serotype c. Infect Immun. 1985 Sep;49(3):790–796. doi: 10.1128/iai.49.3.790-796.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry D., Wondrack L. M., Kuramitsu H. K. Genetic transformation of putative cariogenic properties in Streptococcus mutans. Infect Immun. 1983 Aug;41(2):722–727. doi: 10.1128/iai.41.2.722-727.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell R. R. Glycosyltransferases of Streptococcus mutans strain Ingbritt. Microbios. 1978;23(93-94):136–146. [PubMed] [Google Scholar]
- Shimamura A., Tsumori H., Mukasa H. Purification and properties of Streptococcus mutans extracellular glucosyltransferase. Biochim Biophys Acta. 1982 Mar 18;702(1):72–80. doi: 10.1016/0167-4838(82)90028-0. [DOI] [PubMed] [Google Scholar]
- Shimamura A., Tsumori H., Mukasa H. Three kinds of extracellular glucosyltransferases from Streptococcus mutans 6715 (serotype g). FEBS Lett. 1983 Jun 27;157(1):79–84. doi: 10.1016/0014-5793(83)81120-x. [DOI] [PubMed] [Google Scholar]
- Shiroza T., Ueda S., Kuramitsu H. K. Sequence analysis of the gtfB gene from Streptococcus mutans. J Bacteriol. 1987 Sep;169(9):4263–4270. doi: 10.1128/jb.169.9.4263-4270.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda S., Kuramitsu H. K. Molecular basis for the spontaneous generation of colonization-defective mutants of Streptococcus mutans. Mol Microbiol. 1988 Jan;2(1):135–140. doi: 10.1111/j.1365-2958.1988.tb00014.x. [DOI] [PubMed] [Google Scholar]
- Ueda S., Shiroza T., Kuramitsu H. K. Sequence analysis of the gtfC gene from Streptococcus mutans GS-5. Gene. 1988 Sep 15;69(1):101–109. doi: 10.1016/0378-1119(88)90382-4. [DOI] [PubMed] [Google Scholar]
- Yamashita Y., Hanada N., Takehara T. Purification of a fourth glucosyltransferase from Streptococcus sobrinus. J Bacteriol. 1989 Nov;171(11):6265–6270. doi: 10.1128/jb.171.11.6265-6270.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]